Abstract
In this chapter, we review existing prevalence estimates for autism spectrum disorders (ASDs) since 2000 and discuss methodological factors impacting the estimation of prevalence and the interpretation of changes in prevalence estimates over time. Possible explanations for an increase in the prevalence of ASD within and across populations are considered. Increases in ASD diagnostic rates cannot currently be attributed to a true increase in the incidence of ASD due to multiple confounding factors. It remains to be seen how changes to diagnostic criteria introduced in the DSM-5 will impact estimates of ASD prevalence going forward.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Epidemiological surveys of autism were first initiated in the mid-1960s in England (Lotter 1966, 1967) and have since been conducted in over 20 countries. In this chapter, we provide a comprehensive review of the findings and methodological features of published epidemiological surveys about the prevalence of autism spectrum disorders (ASDsFootnote 1) . This chapter builds upon previous reviews (Elsabbagh et al. 2012; Fombonne 2003a, 2005; Fombonne et al. 2011; French et al. 2013; Hill et al. 2014; Williams et al. 2006) and includes the results of pertinent studies since published. The specific questions addressed are: (1) What is the range of prevalence estimates for ASDs?, and (2) How should the time trends observed in the current prevalence rates of ASDs be interpreted?
2.1.1 Study Design and Methodological Issues
Epidemiologists use several measures of disease occurrence including incidence, cumulative incidence, and prevalence . Prevalence is a measure used in cross-sectional surveys (in which there is no passage of time) and reflects the proportion of subjects in a given population who suffer from the disease at that point in time. Most epidemiological studies of ASDs have assessed prevalence (point prevalence or period prevalence) as a cross-sectional approach is more appropriate for disorders where timing of diagnosis lags behind the onset of symptoms and is likely to be influenced by a range of factors unrelated to risk. In designing a prevalence study, three elements are critical: case definition, case identification (or case ascertainment), and case evaluation methods (Fombonne 2007) .
2.1.1.1 Case Definition
The definition and diagnostic criteria of autism has changed over time. Starting with Kanner’s definition of autism (1943) , case definitions have progressively broadened to include criteria proposed by Rutter (1970) , and subsequently the International Classification of Diseases, ninth revision (ICD-9; World Health Organization 1977); the Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM-III; American Psychiatric Association 1980; DSM-III-R, American Psychiatric Association 1987), until two recent nosographies were adopted worldwide; ICD-10 (World Health Organization 1992) and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV, American Psychiatric Association 1994; DSM-IV-TR, American Psychiatric Association 2000).
Early diagnostic criteria reflected the more qualitatively severe behavioral phenotypes, usually associated with severe delays in language and cognitive skills. In the 1980s less severe forms of autism were recognized, either as a qualifier for autism occurring without intellectual disability (i.e., high-functioning autism), or as separate diagnostic categories (e.g., pervasive developmental disorders not otherwise specified [PDD-NOS], or ASD). Asperger’s disorder appeared in the 1990s, with unclear validity, particularly with respect to its differentiation from high-functioning autism. Some ASD subtypes that were described in DSM-III subsequently disappeared (e.g., Autism-Residual State); however, other nomenclatures have since added new diagnostic categories, such as “atypical autism” and “PDD unspecified” (ICD-10).
The changes now occurring with the introduction of Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5; American Psychiatric Association 2013), may impact prevalence estimates in the future. DSM-5 proposes a single new category of ASDs , conceptually equivalent to the previous diagnostic class of PDDs. However, fewer diagnostic criteria have been retained that are combined in two clusters of social communication deficits, and restricted patterns of behaviors and interests. The removal of the loosely defined PDD-NOS that was in DSM-IV-TR (American Psychiatric Association 2000) will likely increase the specificity of the ASD diagnostic category, and the removal of Asperger disorder as a separate category is consistent with the research that has generally failed to provide evidence for the discriminant validity of this diagnostic concept vis-à-vis the forms of autistic disorder that are not associated with severe language impairments or intellectual deficits.
The impact of DSM-5 changes remains to be fully assessed in the context of epidemiological surveys. Two recent population-based surveys have addressed this issue. Maenner and colleagues (2014) retrospectively applied the new diagnostic criteria to a previously obtained population-based sample from the Centers for Disease Control and Prevention (CDC) 2006 and 2008 surveillance years. They found that 81.2 % of children classified as having ASD according to DSM-IV-TR (American Psychiatric Association 2000) also met DSM-5 criteria (American Psychiatric Association 2013), resulting in a DSM-5 based prevalence of 100/10,000—an estimate lower than the CDC 2006 and 2008 estimates. In addition, 304 children met DSM-5 but not DSM-IV-TR. In a similar study, Kim and colleagues (2014) reported that 92 % of children with ASD according to DSM-IV-TR also met DSM-5 criteria. However, when DSM-5 ASD and social communication disorder (SCD; a new diagnostic category in DSM-5) were considered together, there was no significant change in the prevalence estimate (Kim et al. 2014). It is important to note that new diagnostic information required in DSM-5 (e.g., emphasis on sensory processing deficits) is generally not available in prior studies, leading to potentially biased estimates. Additionally, previous studies are often constrained in sampling children with a DSM-IV PDD diagnosis and cannot therefore accurately estimate the proportion of children who did not meet the criteria for DSM-IV yet would have met those for DSM-5.
While there is currently high reliability overall regarding diagnosis of ASDs and commonality of concepts across experts, differences still persist between nomenclatures about the terminology and operationalized criteria of ASDs. It is unclear to what extent the changing nomenclature of ASDs plays a role in prevalence estimates described in epidemiological studies. More studies are on their way that will provide further examination of the impact on prevalence estimates of narrowing the ASD definition in DSM-5 .
2.1.1.2 Case Identification/Ascertainment
When a population is identified for a survey, different strategies are employed to find individuals matching the study’s case definition. Some studies rely solely on service provider databases (Chien et al. 2011; Croen et al. 2002; Davidovitch et al. 2013) , special education databases (Fombonne et al. 2006; Gurney et al. 2003; Lazoff et al. 2010; Maenner and Durkin 2010) , or national registers (Al-Farsi et al. 2011; Parner et al. 2012; Samadi et al. 2011) for case identification. These studies have the common limitation of relying on a population group that was readily accessible, rather than sampling from the population at large. As a result, individuals with the disorder who are not in contact with services are not included as cases, leading to an underestimation of prevalence. This limitation is particularly problematic in communities with recognized limitations in available services.
Other investigations have relied on a multistage approach to identify cases in underlying populations (Centers for Disease Control and Prevention 2012; Idring et al. 2012; Kim et al. 2011) . In these studies’ first screening stage, a wide net is cast to identify subjects possibly affected with ASD, with the final diagnostic status being determined at subsequent stages. This process often consists of sending letters or screeners to school and health professionals, searching for the possible cases of autism. Few such investigations rely on systematic sampling techniques that would ensure a near complete coverage of the target population, and screening often varies substantially in ascertainment of all relevant data sources. Additionally, surveyed areas often differ in terms of specific educational or health care systems available, and inclusion information sent often varies in reliability and validity. Finally, uneven participation rates in the screening stage can lead to variation in the screening efficiency of surveys.
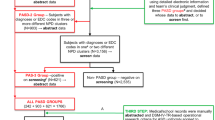
To illustrate how differential participation in the screening stage affect prevalence estimates, two hypothetical scenarios are illustrated in Fig. 2.1, both of which are based on a true ASD prevalence of 150/10,000 and a sensitivity of 100 % for the screening process and total accuracy in the diagnostic confirmation. In Scenario A, we assume 60 % participation for ASD and non-ASD cases in the first screening stage, resulting in 90 participating ASD cases that screen positive. With 70 % participation for both ASD and non-ASD cases in the diagnostic stage, we would identify and confirm 63 ASD cases in the second phase. Weighting back phase 2 data, we would obtain an unbiased prevalence estimate of 1.5 % (or 150/10,000) in this scenario. In Scenario B, we also assume 60 % overall participation, but with an 80 % participation rate for ASD cases, reflecting a scenario in which individuals with ASD are more likely to participate in the first screening stage than non-ASD cases. Thus, with the same participation rates in the first screening (60 %) and final diagnostic stages (70 %), we identify 84 ASD cases and calculate a biased prevalence estimate of 2 % (200/10,000), an estimate that is 0.5 % higher than true prevalence. The bias arises for two reasons: (1) participation in screening is associated with case status (here, with ASD cases more likely to participate than non-cases); and (2) as investigators typically have no such information, weights used for prevalence estimation were not adjusted correspondingly, resulting in the upward bias.
Assuming a true ASD prevalence of 150/10,000 and a sensitivity of 100% for the screening process and total accuracy in the diagnostic confirmation, weighting back phase 2 data results in an unbiased prevalence estimate when caseness is unrelated to participation in screening (Scenario A), but when participation in screening is more likely for ASD cases than for non-cases (Scenario B), prevalence will be overestimated (see discussion in text)
It is also possible that individuals with ASD participate less than non-cases, which would result in underestimates of prevalence. For example, Posserud and colleagues (2010) reported the ASD prevalence of 72/10,000 in their identified sample and estimated a prevalence of 128/10,000 in no responders (based on teacher ratings during the screening phase), indicating increased refusal rates among those with more ASD symptoms. Unfortunately, few studies have been able to estimate the extent to which willingness or refusal to participate is associated with final caseness, so it is not known what effect differential participation rates at different phases in population surveys may have on prevalence estimates.
The sensitivity of the screening methodology is difficult to gauge in autism surveys, as the proportion of children truly affected with the disorder but not identified in the screening stage (false negatives) remains generally unmeasured. Few studies provided an estimate of the reliability of the screening procedure. The usual approach, which consists of randomly sampling screen-negative subjects to adjust estimates, has not been generally used, mainly due to the relatively low frequency of ASD, which makes such a strategy both imprecise and costly.
As an example, the surveys conducted by US CDC (2007a, 2007b, 2009, 2012, 2014) rely, for case ascertainment, on scrutinizing educational and medical records. Children not accessing such services cannot be identified. Although some recent surveys that systematically screen the normal school population might detect a large pool of unidentified cases (Kim et al. 2011) , it remains to be seen if this applies to most populations and requires change in sampling approaches for surveying autism. Of note, the CDC methodology identifies ASD cases without prior official ASD diagnosis (21 % of identified cases in 2008; Centers for Disease Control and Prevention 2012), suggesting that underidentification is a widespread phenomenon.
Since more recent prevalence studies suggest that autism can no longer be regarded as rare, screening for false negatives may become a more common strategy. Currently, however, prevalence estimates must be understood as underestimates of “true” prevalence rates, with the magnitude of this underestimation unknown in each survey.
2.1.1.3 Case Evaluation
When the screening phase is completed, subjects identified as positive go through a more in-depth diagnostic evaluation to confirm case status. Similar considerations about methodological variability across studies apply in more intensive assessment phases. The information used to determine diagnosis usually involves a combination of data from informants (parents, teachers, pediatricians, other health professionals, etc.) and data sources (medical records, educational sources), with a direct assessment of the person with autism being offered in some but not all studies. When subjects are directly examined, assessments typically use various diagnostic instruments, ranging from a typical unstructured examination by a clinical expert (but without demonstrated psychometric properties) to the use of batteries of standardized measures by trained research staff. The Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994) and/or the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000) have been increasingly used in the most recent surveys (Table 2.1) .
Obviously, surveys of large populations, such as those conducted by the CDC (2007a, 2007b, 2009, 2012) or in national registers (Idring et al. 2012) , cannot include direct diagnostic assessment of all subjects by researchers. However, investigators generally improve the accuracy of caseness determinations by undertaking, on a randomly selected subsample, a more complete diagnostic workup (Rice et al. 2007) . The CDC surveys have established a methodology for surveys of large populations based on the screening of the population using multiple data sources, standardized records abstraction, and systematic review and scoring of the data gathered in the screening phase. In the less obvious cases, this information is combined with input from experienced clinicians with known reliability and validity. This methodology is adequate for large samples, and is likely to be used in the future for surveillance efforts.
2.2 Systematic Review of Prevalence Estimates
2.2.1 Search Strategies
Keeping in mind the range and limitations of case definition, identification, and evaluation methods employed in epidemiological surveys, we present the results of epidemiological reports conducted since 2000 in Table 2.1. These reports were identified from previous reviews of epidemiological surveys (Elsabbagh et al. 2012; Fombonne 2003b, 2003a, 2005, 2009a; Fombonne et al. 2011; French et al. 2013; Williams et al. 2006) and through systematic searches using major scientific literature databases (Medline, PsycINFO, Embase, PubMed). Where multiple surveys based on the same or overlapping populations were evident, the publication listed is the most detailed and comprehensive account. For example, surveys conducted by the CDC (2007a, 2007b, 2009, 2012, 2014) as part of the autism and developmental disabilities monitoring (ADDM) network are each included in the table, although additional accounts for individual states are available elsewhere (Nicholas et al. 2008; Pinborough-Zimmerman et al. 2012; Rice et al. 2010; Zahorodny et al. 2014) .
2.2.2 Inclusion and Exclusion Criteria
The following criteria were set to select epidemiological surveys included in Table 2.1:
-
The full article was published in English.
-
The minimum population was 5000.
-
The survey included independent validation of caseness by professionals. In addition, surveys that imposed further non-ASD criteria were excluded.
-
The following information categories were included or could be ascertained based on information from the survey: country and area where the survey was conducted, size of the population for which the prevalence estimate was ascertained, age range of participants, number of children affected, diagnostic criteria used in case definition, and prevalence estimate (number per 10,000). Where available, we also report the proportion of subjects with intelligence quotient (IQ) within the normal range and gender ratios.
2.2.3 Prevalence Estimates for Combined ASDs since 2000
The results of the 53 surveys that estimated the prevalence of the whole spectrum of ASDs are summarized in Table 2.1. All selected surveys were published since 2000, with the majority (55 %) published in 2009 or later. The studies were performed in 18 different countries (including 14 in the UK and 12 in the USA, of which 5 were conducted by the CDC). Sample sizes ranged from 5007 to 4.5 million (median: 58,654; mean: 346,776). Ages of the surveyed populations ranged from 0 to 98 (median: 8; mean: 9). One study was specifically conducted on adults and provided the only estimate (98.2/10,000) thus far available for adults (Brugha et al. 2011) . Two surveys focusing on toddlers (Nygren et al. 2012) and preschoolers (Nicholas et al. 2009) provided estimates of approximately 80 per 10,000. In the 50 remaining surveys, the average median age was 8.23 years (SD = 2.8) .
The diagnostic criteria used in 53 studies reflected the reliance on modern diagnostic schemes (11 studies used ICD-10, 25 the DSM-III, DSM-IV, or DSM-IV-TR; both schemes being used simultaneously in 9 studies). Assessments were often performed with standardized diagnostic measures (i.e., ADI-R and ADOS). In 26 studies where IQ measures were reported, the proportion of subjects within the normal IQ range varied from 0 to 100 % (median: 55.4 %; mean: 53.9 %), a proportion that reflects the lesser association, or lack thereof, between intellectual impairment and milder forms of ASDs. Overrepresentation of males was seen in the 47 studies reporting gender ratios, with male/female ratio ranging from 1.8:1 to 15.7:1 (median: 4.5:1; mean: 4.9:1).
There was a 189-fold variation in ASD prevalence, ranging from 1.4/10,000 to 264/10,000 (see Fig. 2.2). There was also substantial variation in confidence interval width, reflecting variation in sample sizes and consequently in each study’s precision (range: 0.5–146; mean interval width: 22.4). However, some consistency in ASD prevalence is found in the center of this distribution, with a median rate of 61.9/10,000 and a mean rate of 68.9/10,000 (interquartile range: 44.2–84.0/10,000). Prevalence was negatively associated with sample size (Kendall’s tau: − 0.23, p = 0.01), with small-scale studies reporting higher prevalence.
Prevalence estimates for ASD since 2000 (per 10,000 with 95 % confidence intervals; also see Table 2.1). The dashed vertical line denotes the mean prevalence of 69/10,000 across all 53 surveys
There was also a significant positive correlation between ASD prevalence estimates and publication year (Kendall’s tau: 0.26, p = 0.007), with higher rates in more recent surveys. Eight studies since 2000 reported ASD prevalence estimates higher than 100/10,000 (Baird et al. 2006; Centers for Disease Control and Prevention 2012; Idring et al. 2012; Kawamura et al. 2008; Kim et al. 2011; Ouellette-Kuntz et al. 2006; Saemundsen et al. 2013) . Baird et al. (2006) and Kim et al. (2011) both employed proactive case finding techniques, relying on multiple and repeated screening phases, involving both different informants at each phase and surveying the same cohorts at different ages, which certainly enhanced the sensitivity of case identification. Multisource active surveillance techniques, as employed in the Stockholm Youth Cohort (Idring et al. 2012) and by the CDC’s ADDM Network (2012,, 2014), also improve identification of individuals with ASD. The most recent CDC prevalence estimate of 147 per 10,000 reflects the highest estimate to date across all of the previous ADDM Network reports (Centers for Disease Control and Prevention 2014).
Overall, results of recent surveys agree that an average figure of 69/10,000 can be used as the current estimate for the spectrum of ASDs. The convergence of estimates around 60 to 90 per 10,000 for all ASDs combined, conducted in different regions and countries by different teams, is striking especially when derived from studies with improved methodology. The prevalence figure of 69/10,000 (equivalent to 6.9/1000 or.69 %) translates into 1 child out of 145 with an ASD diagnosis . This estimate is now the best current estimate for the ASD prevalence. However, it represents an average and conservative figure, and substantial variability exists between studies and within studies, across sites or areas.
2.3 Time Trends in Prevalence and Their Interpretation
The debate on the hypothesis of a secular increase in rates of autism has been obscured by a lack of clarity in the measures of disease occurrence. As noted previously, it is crucial to differentiate prevalence from incidence, since only incidence rates can be used for causal research, and prevalence and incidence will increase when case definition is broadened or case ascertainment is improved. Moreover, epidemiological surveys of ASDs possess unique design features that could account almost entirely for between-study variation in prevalence estimates, making time trends even more difficult to gauge. Time trends in prevalence estimates can therefore only be evaluated in investigations that hold methodological parameters under strict control over time. Such requirements must be considered when reviewing evidence for a secular increase in rates of ASDs, or testing for the “epidemic” hypothesis.
The epidemic hypothesis emerged in the 1990s when, in most countries, increasing numbers were diagnosed with ASDs leading to an upward trend in children registered in service providers’ databases that was paralleled by higher prevalence rates in epidemiological surveys. These trends were interpreted as evidence that the actual population incidence of ASDs was increasing. However, because methodological factors contribute to variability in prevalence estimates, these must be considered before concluding that there is a true rise in the number of children diagnosed with ASDs and include the following:
2.3.1 Use of Referral Statistics
Increasing numbers of children referred to specialist services or known to special education registers have been taken as evidence for increased ASD incidence. Such upward trends have been seen in many different countries (Gurney et al. 2003; Lotter 1966; Shattuck 2006; Taylor et al. 1999) , all occurring in the late 1980s and early 1990s. However, trends over time in referred samples are confounded by referral patterns, availability of services, heightened public awareness, decreasing age at diagnosis, and changes over time in diagnostic concepts and practices.
As an illustration, Fig. 2.3 contrasts two methods for surveying ASD using hypothetical data: one based on sampling from the total population, and the other relying solely on service access counts. Here, assuming a constant incidence and prevalence of 100/10,000 between Time 1 and Time 2 (meaning there is no epidemic), population surveys at two time points result in prevalence estimates that are not only accurate but also stable over time, showing no prevalence change in the target population. However, if prevalence is estimated based only on service access counts where the number of ASD individuals accessing services increases from 20 to 60 % over time, prevalence would be underestimated at both time points, yet would appear to rise 200 % while the underlying true incidence and prevalence remained stable. Such a pattern of results was recently reported based on special education data in Wisconsin (Maenner and Durkin 2010) , in which ASD prevalence rates were stable between 2002 and 2008 in school districts with initially high baseline prevalence rates (≈ 120/10,000), whereas school districts with low baseline rates experienced significant increases in prevalence (e.g., in one district rates rose from 5 to 70/10,000; corresponding to a 1300 % increase in 6 years). Failure to control for these confounding factors was obvious in previous reports (Fombonne 2001) , including widely quoted reports from California Developmental Database Services (California Department of Developmental Services 2003).
Assuming a constant incidence and prevalence of 100/10,000 between Time 1 and Time 2 (meaning there is no “epidemic”), prevalence estimates that rely solely on service access counts not only underestimate the true prevalence but may also create the illusion of rising prevalence over time (see discussion in text)
Additionally, the decreasing age at diagnosis results in itself to increasing numbers of young children being identified in official statistics (Wazana et al. 2007) or referred to specialist medical and educational services. Earlier identification of children from the prevalence pool may therefore result in increased service activity that may lead to a misperception by professionals of an epidemic.
2.3.2 Diagnostic Substitution
Another possible explanation for increased prevalence in a diagnostic category is that children presenting with the same developmental disability may receive one particular diagnosis initially and another diagnosis subsequently. Such diagnostic substitution (or switching) may occur when diagnostic categories become increasingly familiar to health professionals and/or when access to better services is ensured by using a new diagnostic category.
The strongest evidence of diagnostic substitution contributing to ASD prevalence increase was shown in a complex analysis of Department of Education Data in 50 US states (Shattuck 2006) , indicating that a relatively high proportion of children previously diagnosed with mental retardation (MR) were subsequently identified as having ASD. Shattuck showed that the odds of having ASD increased by 1.21 during 1994–2003 while the odds of having learning disability (LD) (odds ratio [OR] = 0.98) and MR (OR = 0.97) decreased. Shattuck (2006) further demonstrated that the growing ASD prevalence was directly associated with decreasing prevalence of LD and MR within states, and that a significant downward deflection in the historical trajectories of LD and MR occurred when ASD became reported in the USA as an independent category in 1993–1994.
Using individual level data, a newer study reexamined the hypothesis of diagnostic substitution in the California department of developmental services dataset (King and Bearman 2009) and showed that 24 % of the increase in caseload was attributable to diagnostic substitution (from MR to ASD). It is important to keep in mind that other types of diagnostic substitution are likely to have occurred as well for milder forms of ASD. For example, children currently diagnosed with Asperger’s disorder may be previously diagnosed with other psychiatric diagnoses (i.e., obsessive-compulsive disorder, school phobia, social anxiety, etc.) in clinical settings before the developmental nature of their condition was fully recognized (Fombonne 2009b) .
2.3.3 Cross-Sectional Variability in Epidemiological Surveys
Evidence that method factors could account for most of the variability in published prevalence estimates comes from a direct comparison of eight recent surveys conducted in the UK and the USA (Fombonne 2005). In each country, four surveys were conducted around the same year and with similar age groups. As there is no reason to expect large variations in between-area differences in rates, prevalence estimates should therefore be comparable within each country. However, there was a 6-fold variation in rates for the UK surveys, and a 14-fold variation in the US rates. In each set of studies, high rates were found when intensive population-based screening techniques were employed, whereas lower rates were found in studies relying on passive administrative methods for case finding. Since no passage of time was involved, the magnitude of these gradients in rates is likely to reflect methodological differences.
Even more convincing evidence comes from the most recent survey by the CDC on 363,749 children aged 8 in 2010, where an average prevalence of 147/10,000 was reported across 11 US states (Centers for Disease Control and Prevention 2014). One striking finding in this report is the almost four-fold variation in prevalence rates by state (range: 57–219 per 10,000; see Fig. 2.4). Across individual states, Alabama had the lowest rate of 57/10,000, whereas New Jersey had the highest rate of 219/10,000 (Centers for Disease Control and Prevention 2014). Estimated ASD prevalence was significantly lower in states that had access to health data sources only compared to that of the states where educational data was also available (97.7 versus 149 out of 10,000, respectively), a factor that is consistently associated with higher prevalence rates in the ADDM Network. It would be surprising if there were truly this much inherent state-to-state variability in the number of children with autism in the USA. Thus, these differences likely reflect ascertainment variability across sites in a study that was otherwise performed with the same methods, at the same time, on children of the same age, and within the same country.
2.3.4 Repeated Surveys in Defined Geographical Areas
Repeated surveys, using the same methodology and conducted in the same geographical area at different time-points, can potentially yield useful information on time trends if methods are kept relatively constant. The Göteborg studies (Gillberg 1984; Gillberg et al. 1991) provided three prevalence estimates that increased over a short period of time from 4.0 (1980) to 6.6 (1984) to 9.5/10,000 (1988), the gradient being even steeper in urban areas only (Gillberg et al. 1991). However, comparison of these rates is not straightforward, as different age groups were included in each survey. Furthermore, increased prevalence was associated with improved detection among those with intellectual delays in the second survey, and with improved detection of cases born to immigrant parents in the third survey, suggesting that migration into the area could be a key explanation. Taken in conjunction with a change in local services and a progressive broadening of the autism definition over time (Gillberg et al. 1991), findings provide weak evidence for increased autism incidence. Similarly, studies conducted in Japan at different points in time in Toyota (Kawamura et al. 2008) and Yokohama (Honda et al. 2005; Honda et al. 1996) showed rises in prevalence rates that their authors interpreted as reflecting the effect of both improved population screening of preschoolers and a broadening of diagnostic concepts and criteria.
Two separate surveys of children born between 1992 and 1995, and between 1996 and 1998 in Staffordshire, UK (Chakrabarti and Fombonne 2001, 2005) , were performed with rigorously identical methods for case definition and case identification. The prevalence for combined ASDs was comparable and not statistically different in the two surveys (Chakrabarti and Fombonne 2005), suggesting no upward trend in overall rates of ASDs, at least during the short time interval between studies.
2.3.5 Birth Cohorts
In large surveys encompassing wide age ranges, increasing prevalence among most recent birth cohorts could be interpreted as indicating a secular increase in ASD incidence, provided that alternative explanations can be confidently eliminated. This analysis was used in two large French surveys (Fombonne and Du Mazaubrun 1992; Fombonne et al. 1997) . The surveys included birth cohorts from 1972 to 1985 (735,000 children, 389 of whom had autism). When pooling the data of both surveys, age-specific rates showed no upward trend (Fombonne et al. 1997).
However, data assessing birth cohorts can be problematic, as illustrated in Fig. 2.5, which shows an increase in the prevalence of ASD by year of birth across three hypothetical successive birth cohorts (a cohort effect; Fig. 2.5a). Within each birth cohort, followed longitudinally, prevalence increases as children age (Fig. 2.5b): for children in the 2000 birth cohort, based on previous ASD prevalence estimates, at age 6 prevalence is 20/10,000, whereas at age 12, we may expect prevalence of 80/10,000 for the same birth cohort. Increasing prevalence rates with age within birth cohorts is unlikely to reflect the onset of ASD in later childhood and early adolescence. It is more likely that observed increases in prevalence reflect underdiagnosis in the preschool years as well as changes in public awareness, service availability, and diagnostic concepts and practices.
Using hypothetical data, Figure 5a illustrates rising prevalence rates among 6-, 8-, and 12-year-old children across three different birth cohorts. Prevalence rates also increase within birth cohorts as they age (Figure 5b), potentially coinciding with changes in patterns of referral, service availability, public awareness, and diagnostic concepts and practices (see discussion in text)
As an example, an analysis of special educational data from Minnesota showed a 16-fold increase in children identified with ASD from 1991–1992 to 2001–2002 (Gurney et al. 2003) . However, during the same time period, an increase of 50 % was observed for all disability categories (except severe intellectual deficiency); especially for the category including attention-deficit/hyperactivity disorder (ADHD). The large sample size allowed the authors to assess age, period, and cohort effects. Prevalence increased regularly in successive birth cohorts; for example, among 7-year-olds, prevalence rose from 18/10,000 among those born in 1989, to 29/10,000 among those born in 1991, to 55/10,000 in those born in 1993. Within the same birth cohorts, age effects were also apparent since for children born in 1989 the prevalence rose with age from 13/10,000 at age 6, to 21/10,000 at age 9, and 33/10,000 at age 11. As argued by Gurney et al. (2003), this pattern is not consistent with the natural etiology of ASD, which first manifests in early childhood. Gurney et al.’s analysis also showed a marked period effect, where rates started to increase in all ages and birth cohorts in the 1990s. The authors noted that this phenomenon coincided closely with the inclusion of ASDs in the federal Individuals with Disabilities Educational Act in the USA. A similar interpretation of upward trends had been put forward by Croen and colleagues (2002) in their analysis of the California department of developmental services data, and by Shattuck (2006) in his analysis of trends in US Department of Education data.
2.4 Conclusions
Epidemiological surveys of ASDs pose substantial challenges to researchers seeking to measure rates of ASD, particularly given the range of case definition, case identification, and case evaluation methods employed across surveys. However, from recent studies, a best estimate of (69/10,000) (equivalences = 6.9/1,000 or 0.69 % or 1 child in about 145 children) can be derived for the prevalence of ASD. Currently, the recent upward trend in rates of prevalence cannot be directly attributed to an increase in the incidence of the disorder, or to an epidemic of autism. Although power to detect time trends is seriously limited in existing datasets, there is good evidence that changes in diagnostic criteria and practices, policies for special education, service availability, and awareness of ASDs in both the lay and professional public may be responsible for increasing the prevalence over time. It is also noteworthy that the rise in number of children diagnosed occurred concurrently in many countries in the 1990s, when services for children with ASD also expanded significantly. Statistical power may also be a significant limitation in most investigations; thus, variations of small magnitude in ASD incidence may be undetected or should be interpreted with caution.
Nonetheless, the possibility that a true increase in the incidence of ASDs has also partially contributed to the upward trend in prevalence rates cannot, and should not, be completely eliminated based on the available data. To assess whether the incidence has increased, methodological factors that account for an important proportion of the variability in rates must be stringently controlled for. New survey methods have been developed for use in multinational comparisons; ongoing surveillance programs are currently underway and will soon provide more meaningful data to evaluate this hypothesis. Additionally, it remains to be seen how changes to diagnostic criteria introduced in the DSM-5 will impact the ASD prevalence estimates going forward. Meanwhile, the available prevalence figures carry straightforward implications for current and future needs in services and early educational intervention programs.
Notes
- 1.
Autism spectrum disorder (ASD) is the modern term that replaces the former pervasive developmental delay (PDD).
References
Al-Farsi YM, Al-Sharbati MM, Al-Farsi OA, Al-Shafaee MS, Brooks DR, Waly MI (2011) Brief report: prevalence of autistic spectrum disorders in the Sultanate of Oman. J Autism Dev Disord 41(6):821–825. doi:10.1007/s10803-010-1094-8
American Psychiatric Association (1980) Diagnostic and statistical manual of mental disorders, 3rd ed. American Psychiatric Association, Washington, DC
American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders, (3rd ed, revision). American Psychiatric Association, Washington, DC
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Association, Washington, DC
American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed, text revision). American Psychiatric Association, Washington, DC doi:10.1176/appi.books.9780890423349.
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Publishing., Washington, DC
Baird G, Charman T, Baron-Cohen S, Cox A, Swettenham J, Wheelwright S, Drew A (2000) A screening instrument for autism at 18 months of age: a 6-year follow-up study. J Am Acad Child Adolesc Psychiatry 39(6):694–702. doi:10.1097/00004583-200006000-00007
Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T (2006) Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the special needs and autism project (SNAP). Lancet 368(9531):210–215. doi:10.1016/S0140-6736(06)69041-7
Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J et al (2011) Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry 68(5):459–465. doi:10.1001/archgenpsychiatry.2011.38
California Department of Developmental Services (2003) Autistic spectrum disorders: Changes in the California caseload-an update 1999 through 2002. Retrieved from http://www.dds.ca.gov/Autism/docs/AutismReport2003.pdf
Centers for Disease Control and Prevention (2007a) Prevalence of autism spectrum disorders- autism and developmental disabilities monitoring network, six sites, United States, 2000. Morb Mortal Wkly Rep Surveillance Summaries, 56(1):1–11
Centers for Disease Control and Prevention (2007b) Prevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, 14 Sites, United States, 2002. Morb Mortal Wkly Rep Surveillance Summaries, 56(SS01):12–28
Centers for Disease Control and Prevention (2009) Prevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, United States, 2006. Morb Mortal Wkly Rep Surveillance Summaries, 58(SS10):1–20
Centers for Disease Control and Prevention (2012) Prevalence of autism spectrum disorders- autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morb Mortal Wkly Rep Surveillance Summaries, 61(3):1–19
Centers for Disease Control and Prevention (2014) Prevalence of autism spectrum disorders among children aged 8 years- autism and developmental disabilities monitoring network, 11 Sites, United States, 2010. Morb Mortal Wkly Rep Surveillance Summaries, 63(2):1–22
Chakrabarti S, Fombonne É (2001) Pervasive developmental disorders in preschool children. J Am Med Assoc 285(24):3093–3099. doi:10.1001/jama.285.24.3093
Chakrabarti S, Fombonne É (2005) Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 162(6):1133–1141. doi:10.1176/appi.ajp.162.6.1133
Chien IC, Lin CH, Chou YJ, Chou P (2011) Prevalence and incidence of autism spectrum disorders among national health insurance enrollees in Taiwan from 1996 to 2005. J Child Neurol 26(7):830–834. doi:10.1177/0883073810393964
Croen LA, Grether JK, Hoogstrate J, Selvin S (2002) The changing prevalence of autism in California. J Autism Dev Disord 32(3):207–215
Croen LA, Najjar DV, Fireman B, Grether JK (2007) Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 161(4):334–340. doi:10.1001/archpedi.161.4.334
Davidovitch M, Hemo B, Manning-Courtney P, Fombonne É (2013) Prevalence and incidence of autism spectrum disorder in an Israeli population. J Autism Dev Disord 43(4):785–793. doi:10.1007/s10803-012-1611-z
Ellefsen A, Kampmann H, Billstedt E, Gillberg IC, Gillberg C (2007) Autism in the Faroe Islands: an epidemiological study. J Autism Dev Disord 37(3):437–444. doi:10.1007/s10803-006-0178-y
Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C et al (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3):160–179. doi:10.1002/aur.239
Fombonne É (2001) Is there an epidemic of autism? Pediatrics 107(2):411–412
Fombonne É (2003a) Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 33(4):365–382
Fombonne É (2003b) The prevalence of autism. J Am Med Assoc 289(1):87–89. doi:10.1001/jama.289.1.87
Fombonne É (2005) Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry 66(Suppl 10):3–8
Fombonne É (2007) Epidemiology. In Martin A, Volkmar F (eds) Lewisʼs child and adolescent psychiatry: a comprehensive textbook (4 ed). Philadelphia, Lippincott Williams & Wilkins, pp. 150–171
Fombonne É (2009a) Epidemiology of pervasive developmental disorders. Pediatr Res 65(6):591–598. doi:10.1203/PDR.0b013e31819e7203
Fombonne É (2009b) Commentary: on King and Bearman. Int J Epidemiol 38(5):1241–1242. doi:10.1093/ije/dyp259
Fombonne É, Du Mazaubrun C (1992) Prevalence of infantile autism in four French regions. Soc Psychiatry Psychiatr Epidemiol 27(4):203–210. doi:10.1016/S0890-8567(09)66566-7
Fombonne É, Du Mazaubrun C, Cans C, Grandjean H (1997) Autism and associated medical disorders in a French epidemiological survey. J Am Acad Child Adolesc Psychiatry 36(11):1561–1569. doi:10.1016/S0890-8567(09)66566-7
Fombonne É, Zakarian R, Bennett A, Meng L, McLean-Heywood D (2006) Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics 118(1):e139–e150. doi:10.1542/peds.2005-2993
Fombonne É, Quirke S, Hagen A (2011) Epidemiology of pervasive developmental disorders. In Amaral DG, Dawson G, Geschwind DH (eds) Autism spectrum disorders. New York, Oxford University Press,(pp. 90–111)
French L, Bertone A, Hyde K, Fombonne É (2013) Epidemiology of autism spectrum disorders. In Buxbaum JD, Hof PR (eds) The neuroscience of autism spectrum disorders.Oxford, Oxford University Press, pp 3–24
Gillberg C (1984) Infantile autism and other childhood psychoses in a Swedish urban region. Epidemiological aspects. J Child Psychol Psychiatry 25(1):35–43. doi:10.1111/j.1469-7610.1984.tb01717.x
Gillberg C, Steffenburg S, Schaumann H (1991) Is autism more common now than 10 years ago? Br J Psychiatry 158:403–409.
Gurney JG, Fritz MS, Ness KK, Sievers P, Newschaffer CJ, Shapiro EG (2003) Analysis of prevalence trends of autism spectrum disorder in Minnesota. Arch Pediatrics Adolesc Med 157(7):622–627. doi:10.1001/archpedi.157.7.622
Hill AP, Zuckerman KE, Fombonne É (2014) Epidemiology of Autism Spectrum Disorders. In Handbook of Autism and Pervasive Developmental Disorders. Hoboken, Wiley, pp 57–96
Honda H, Shimizu Y, Misumi K, Niimi M, Ohashi Y (1996) Cumulative incidence and prevalence of childhood autism in children in Japan. Br J Psychiatry 169(2):228–235
Honda H, Shimizu Y, Rutter M (2005) No effect of MMR withdrawal on the incidence of autism: a total population study. J Child Psychol Psychiatry 46(6):572–579. doi:10.1111/j.1469-7610.2005.01425.x
Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E et al (2012) Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS ONE 7(7):e41280. doi:10.1371/journal.pone.0041280
Kanner L (1943). Autistic disturbances of affective contact. Nerv child 2(3):217–250
Kawamura Y, Takahashi O, Ishii T (2008) Reevaluating the incidence of pervasive developmental disorders: impact of elevated rates of detection through implementation of an integrated system of screening in Toyota, Japan. Psychiatry Clin Neurosci 62(2):152–159. doi:10.1111/j.1440-1819.2008.01748.x
Kim YS, Leventhal BL, Koh Y-J, Fombonne É, Laska E, Lim E-C et al (2011) Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 168(9):904–912. doi:10.1176/appi.ajp.2011.10101532
Kim YS, Fombonne É, Koh Y-J, Kim S-J, Cheon K-A, Leventhal BL (2014) A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry 53(5):500–508. doi:10.1016/j.jaac.2013.12.021
King M, Bearman P (2009) Diagnostic change and the increased prevalence of autism. Int J Epidemiol 38(5):1224–1234. doi:10.1093/ije/dyp261
Lauritsen MB, Pedersen CB, Mortensen PB (2004) The incidence and prevalence of pervasive developmental disorders: a Danish population-based study. Psychol Med 34(7):1339–1346.
Lazoff T, Zhong L, Piperni T, Fombonne É (2010) Prevalence of pervasive developmental disorders among children at the English montreal school board. Can J Psychiatry 55(11):715–720.
Leonard H, Glasson E, Nassar N, Whitehouse A, Bebbington A, Bourke J et al (2011) Autism and intellectual disability are differentially related to sociodemographic background at birth. PLoS ONE 6(3):e17875. doi:10.1371/journal.pone.0017875
Lord C, Rutter M, Couteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24(5):659–685. doi:10.1007/BF02172145
Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC et al (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30(3):205–223. doi:10.1023/A:1005592401947
Lotter V (1966) Epidemiology of autistic conditions in young children. Soc Psychiatry 1(3):124–135. doi:10.1007/bf00584048
Lotter V (1967) Epidemiology of autistic conditions in young children. Soc Psychiatry 1(4):163–173. doi:10.1007/BF00578950
Maenner MJ, Durkin MS (2010) Trends in the prevalence of autism on the basis of special education data. Pediatrics 126(5):e1018–e1025. doi:10.1542/peds.2010-1023
Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA et al (2014) Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry 71(3):292–300. doi:10.1001/jamapsychiatry.2013.3893
Nassar N, Dixon G, Bourke J, Bower C, Glasson E, de Klerk N, Leonard H (2009) Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol 38(5):1245–1254. doi:10.1093/ije/dyp260
Nicholas JS, Charles JM, Carpenter LA, King LB, Jenner W, Spratt EG (2008) Prevalence and characteristics of children with autism-spectrum disorders. Ann Epidemiol 18(2):130–136. doi:10.1016/j.annepidem.2007.10.013
Nicholas JS, Carpenter LA, King LB, Jenner W, Charles JM (2009) Autism spectrum disorders in preschool-aged children: prevalence and comparison to a school-aged population. Ann Epidemiol 19(11):808–814. doi:10.1016/j.annepidem.2009.04.005
Nygren G, Cederlund M, Sandberg E, Gillstedt F, Arvidsson T, Carina Gillberg I et al (2012) The prevalence of autism spectrum disorders in toddlers: a population study of 2-year-old Swedish children. J Autism Dev Disord 42(7):1491–1497. doi:10.1007/s10803-011-1391-x
Ouellette-Kuntz H, Coo H, Yu CT, Chudley AE, Noonan A, Breitenbach M et al (2006) Prevalence of pervasive developmental disorders in two Canadian providences. J Policy Pract Intellect Disabil 3(3):164–172
Parner ET, Thorsen P, Dixon G, de Klerk N, Leonard H, Nassar N et al (2011) A comparison of autism prevalence trends in Denmark and Western Australia. J Autism Dev Disord 41(12):1601–1608. doi:10.1007/s10803-011-1186-0
Parner ET, Baron-Cohen S, Lauritsen MB, Jørgensen M, Schieve LA, Yeargin-Allsopp M, Obel C (2012) Parental age and autism spectrum disorders. Ann Epidemiol 22(3):143–150. doi:10.1016/j.annepidem.2011.12.006
Pinborough-Zimmerman J, Bakian AV, Fombonne É, Bilder D, Taylor J, McMahon WM (2012) Changes in the administrative prevalence of autism spectrum disorders: contribution of special education and health from 2002-2008. J Autism Dev Disord 42(4):521–530. doi:10.1007/s10803-011-1265-2
Posserud M, Lundervold AJ, Lie SA, Gillberg C (2010) The prevalence of autism spectrum disorders: impact of diagnostic instrument and non-response bias. Soc Psychiatry Psychiatr Epidemiol 45(3):319–327. doi:10.1007/s00127-009-0087-4
Rice CE, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS, ADDM Network (2007) A public health collaboration for the surveillance of autism spectrum disorders. Paediatr Perinat Epidemiol 21(2):179–190. doi:10.1111/j.1365-3016.2007.00801.x
Rice C, Nicholas J, Baio J, Pettygrove S, Lee L-C, Van Naarden Braun K et al (2010) Changes in autism spectrum disorder prevalence in 4 areas of the United States. Disabil Health J 3(3):186–201. doi:10.1016/j.dhjo.2009.10.008
Rutter M (1970) Autistic children: infancy to adulthood. Semin Psychiatry 2(4):435–450
Saemundsen E, Magnusson P, Georgsdóttir I, Egilsson E, Rafnsson V (2013) Prevalence of autism spectrum disorders in an Icelandic birth cohort. BMJ Open 3(6). doi:10.1136/bmjopen-2013-002748
Samadi SA, Mahmoodizadeh A, McConkey R (2011) A national study of the prevalence of autism among five-year-old children in Iran. Autism 16(1):5–14. doi:10.1177/1362361311407091
Shattuck PT (2006) The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics 117(4):1028–1037. doi:10.1542/peds.2005-1516
Taylor B, Miller E, Farrington CP, Petropoulos MC, Favot-Mayaud I, Li J, Waight PA (1999) Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet 353(9169):2026–2029.
Wazana A, Bresnahan M, Kline J (2007) The autism epidemic: fact or artifact? J Am Acad Child Adolesc Psychiatry 46(6):721–730
Williams JG, Higgins JPT, Brayne CEG (2006) Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child 91(1):8–15. doi:10.1136/adc.2004.062083
Windham GC, Anderson MC, Croen LA, Smith KS, Collins J, Grether JK (2011) Birth prevalence of autism spectrum disorders in the San Francisco Bay area by demographic and ascertainment source characteristics. J Autism Dev Disord 41(10):1362–1372. doi:10.1007/s10803-010-1160-2
World Health Organization (1977) International Statistical Classification of Diseases and Related Health Problems, Ninth Revision. World Health Organization, Geneva, Switzerland
World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. World Health Organization, Geneva, Switzerland
Zahorodny W, Shenouda J, Howell S, Rosato NS, Peng B, Mehta U (2014) Increasing autism prevalence in metropolitan New Jersey. Autism 18(2):117–126. doi:10.1177/1362361312463977
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Presmanes Hill, A., Zuckerman, K., Fombonne, E. (2015). Epidemiology of Autism Spectrum Disorders. In: Robinson-Agramonte, M. (eds) Translational Approaches to Autism Spectrum Disorder. Springer, Cham. https://doi.org/10.1007/978-3-319-16321-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-16321-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16320-8
Online ISBN: 978-3-319-16321-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)