Abstract
This study examined changes in the administrative prevalence of autism spectrum disorders (ASD) in Utah children from 2002 to 2008 by record source (school and health), age (four, six, and eight), and special education classification. Prevalence increased 100% with 1 in 77 children aged eight identified with ASD by 2008. Across study years and age groups rates were higher when health and school data were combined with a greater proportion of cases ascertained from health. The proportion of children with both a health ASD diagnosis and a special education autism classification did not significantly change. Most children with an ASD health diagnosis did not have an autism special education classification. Findings highlight the growing health and educational impact of ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Once thought to be a rare condition, autism and related spectrum disorders have recently emerged as relatively common childhood neurodevelopmental disorders. In the United States (US), the current autism spectrum disorder (ASD) prevalence rate reported by the Centers for Disease Control and Prevention (CDC) in children aged eight was 1 in 110 children, reflecting a significant increase (57%) in the ten US sites that tracked prevalence changes from 2002 to 2006 (CDC 2009). ASD prevalence based on education administrative data from the US Department of Education, Office of Special Education and Programs is significantly lower than rates reported by the CDC, however, the proportion of children in special education with an autism classification nearly doubled from 2.3% in the 2002–2003 school year to 4.4% in 2007–2008 (Office of Special Education Programs 2008a, 2008b). The question remains whether similar prevalence increases are found in other US based ASD data sets such as individual state administrative registries with access to single and multiple administrative data sources.
Several reasons for increased ASD prevalence have been proposed. They include shifts in provider diagnostic patterns (Bishop et al. 2008; Grether et al. 2009; King and Bearman 2009; Nassar et al. 2009), increased awareness (Barbaresi et al. 2005; Fombonne et al. 2006; Kogan et al. 2009), and changes in administrative diagnostic criteria or program eligibility requirements (Fombonne 2009; Gurney et al. 2003; Kielinen et al. 2000; Newschaffer et al. 2005; Shattuck 2006). Two prominent changes are the broadening of the ASD diagnostic criteria in 1994 by the American Psychiatric Association (2000) and the addition of an autism special education classification with mandated implementation by the 1992–1993 school-year.
Variability across ASD epidemiology studies further complicates the interpretation of rising prevalence rates. Methods and data sources used for ASD case ascertainment and definition vary and impede clear comparisons across studies. Increased availability and access to more data sources has been correlated with higher ASD prevalence in previous studies (CDC 2007; CDC 2009). Conversely, prevalence has been consistently lower in studies in which case ascertainment has been based on health-only or special education-only administrative records (CDC 2007; CDC 2009; Fombonne 2001; Laidler 2005; Newschaffer et al. 2005; Pinborough-Zimmerman et al. 2010). Demographic factors such as population age also influence prevalence rates. Rates in children between 5 and 10 years of age are generally higher than in other age groups (Kogan et al. 2009; Yeargin-Allsopp et al. 2003).
Here, we report on the prevalence of ASD using multiple administrative datasets (health and education) spanning a 6 year time period in the same geographic region and age groups. The primary aims are to quantify changes in the measured ASD administrative prevalence (ASD health diagnosis and/or special education autism classification) and to examine how the completeness of surveillance and the magnitude of prevalence estimates vary when relying on administrative data from single versus multiple data sources and age groups. Our hypotheses are (1) that with minimal changes to the health and special education ASD diagnostic/classification criteria since the early 1990’s, changes in the ASD administrative prevalence will begin to slow or plateau; (2) ASD administrative prevalence rates will be significantly lower when derived from one data source (health versus school), and lower in the youngest age group compared to older age groups; (3) with dramatic increases observed nationally in the proportion of children in special education with an autism classification, the proportion of total ASD cases identified from special education administrative data will significantly increase; and (4) with greater provider awareness and efforts to improve early diagnosis and access to services, the proportion of children with both an ASD health diagnosis and special education autism classification will significantly increase.
Method
Target Population
This study was conducted using ASD cases identified from the population of all children aged eight living in one of the three most densely populated counties in Utah (Davis, Salt Lake, and Utah) in study years (SY) 2002 (n = 26,213), 2006 (n = 29,494), and 2008 (n = 33,757). Additionally, ASD cases were identified from the same geographic region from the population of children aged four and six in SY 2006 (n = 33,955 and n = 32,801, respectively) and SY 2008 (n = 35,803 and n = 34,368, respectively). Growth in the target population was non-linear which likely was due to a number of factors. Utah was the fourth fastest growing state from 1990 through 2000 (Governor’s Office of Planning and Budget 2001) and historically has had the highest fertility rate and largest family size in the US (Hamilton et al. 2009; U.S. Census Bureau 2009). Approval was obtained from the institutional review board at the University of Utah.
Case Ascertainment
Ascertainment of records was facilitated by Utah law requiring providers to report ASD cases upon request to the Utah Department of Health or their agent. Cases were identified from administrative child records obtained from multiple electronic datasets including all major public education and health sources. Children receiving special education services were identified through a contractual agreement with the Utah State Office of Education. Special education data was comprised of lists of all students receiving special services by school and district as described by an Individualized Education Program (IEP) as mandated by Individuals with Disability Education Act (IDEA). We reduced the special education data to produce unduplicated lists of children receiving special education eligibility by year along with their primary classification. Children were counted as cases if they received Autism special education eligibility as their primary classification during a given study year.
Health sources included the Utah Department of Health’s direct service programs, private and public clinics, child disability diagnostic centers, mental health centers and hospitals, and individual providers specializing in services for children with disabilities. Health sources were requested to provide birth date, gender, race/ethnicity, residential address, and date of service information from billing records containing at least one of approximately 200 International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes (ICD 1988) for ASD, and related developmental disabilities and medical conditions such as intellectual disability, language delay, Fragile X, etc.
Data linkages across all data sources were conducted utilizing a deterministic method using SAS software, version 9.2 (SAS Institute 2008) to obtain unduplicated counts from which overall ASD prevalence rates were determined. First, children were assigned unique identifiers based on a combination of parts of their first name, last name and date of birth. Next, we produced a master list containing all ASD cases from each source. This list was hand-checked for accidental duplicates based on errors in the unique identifier. After hand-cleaning of the data, the master list was transposed to create a database containing one entry per child. Residential address and/or school address were verified for study year and records were then de-identified for further analysis. In children with both a school and residential address, we did not identify any cases in which children received educational services outside their county of residence.
Administrative ASD case definitions
ASD cases were assigned to at least one of the following groups: (1) a special education autism classification (a special education disability classification of autism as defined by IDEA), (2) a health diagnosis of ASD (a previously documented ICD-9 ASD diagnosis–299.00, 299.80, 299.90–by a qualified provider), and (3) a special education autism classification plus a health ASD diagnosis.
Analyses
Outcome variables of interest were ASD prevalence rates by administrative classification (a special education autism classification and/or an ASD health diagnostic code), study year (SY; 2002, 2006, and 2008), age group (children aged four, six, and eight), and gender. Period prevalence estimates were calculated using as the denominator the number of same-aged children residing in the three county surveillance area according to the Utah Department of Health’s Center for Health Data (Utah Department of Health 2010). Prevalence results were reported per 1,000 children. Normal approximation to the binomial distribution was used to calculate 95% confidence intervals for prevalence rates. Chi-square tests were used to test for differences in prevalence rates across years and across age groups and to test for differences in special education classification within and across years and age groups. Prevalence rate ratios and percentage change were calculated to investigate trends in prevalence across years within age groups. Changes in the contribution of ascertainment source type to overall ASD prevalence was found by counting the number of ASD administrative cases derived from special education classification-only, health-only diagnosis, and both. Changes in ascertainment source contribution to ASD prevalence within age groups across years were tested using Welch’s t-tests of proportions. Statistical analyses were conducted in SAS software, version 9.2 (SAS Institute 2008), and an alpha level of .05 was used for all statistical tests.
Results
Overall Prevalence Trends
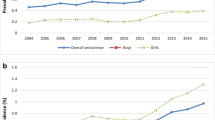
ASD administrative prevalence per 1,000 children aged four, six, and eight in the three county surveillance region in Utah by year, sex, and record ascertainment source are shown in Fig. 1. In children aged eight prevalence increased from 6.5 (CI = 5.5-7.5) per 1000 in SY 2002, to 10.2 per 1000 (CI = 9.1–11.3) in SY 2006 (χ 2(1, N = 56,179) = 21.4, p = < .0001), and to 13.0 per 1000 (CI = 11.8–14.2) in SY 2008 (χ 2(1, N = 60,026) = 61.3, p = < .0001). In children aged 6 years prevalence significantly increased from 9.8 per 1000 (CI = 8.7–10.9) in SY 2006 to 12.2 (11.0–13.4) in study year 2008 (χ 2(1, N = 67,909) = 8.5, p = .004). The overall ASD administrative prevalence in children aged 4 years was 7.6 (CI = 6.7–8.5) in SY 2006. Prevalence increased to 8.2 (7.3–9.1) per 1000 in SY 2008 however this increase was not statistically significant (χ 2(1, N = 70,308) = 0.9, p = .36). Rate ratio and percentage of prevalence change by age group, source and gender are shown in Table 1. The greatest increase in prevalence (130.4%; χ 2(1, N = 28,976) = 16.5, p = < .0001) was found within the female group aged eight from SY 2002 to SY 2008.
The overall prevalence rates for children aged six and eight within the same SY were not significantly different in SY 2006 (χ 2(1, N = 62,918) = 0.37, p = .54) or SY 2008 (χ 2(1, N = 68,428) = 0.97, p = .32). Prevalence in the same SY was significantly lower for children aged four compared with children aged eight in SY 2006 (χ 2(1, N = 64,007) = 12.6, p = .0004) and SY 2008 (χ 2(1, N = 69,738) = 39.0, p < .0001) and children aged four compared with children aged six in SY 2006 (χ 2(1, N = 67,335) = 9.1, p = .0025) and SY 2008 (χ 2(1, N = 70,882) = 27.7, p < .0001).
Prevalence by Ascertainment Source
Prevalence rates for each age group varied as a function of ascertainment source with rates generally higher when both health and school ascertainment sources were used (Fig. 1). Prevalence rates based on health records alone were significantly higher than prevalence rates based on school records alone for children aged eight across all study years (SY 2002 versus SY 2006[χ 2(1, N = 55,952) = 16.8, p < .0001], SY 2006 vs. SY 2008 [χ 2(1, N = 63,080) = 17.4, p < .0001], and SY 2002 vs. SY 2008 [χ 2(1, N = 59,728) = 24.8, p < .0001])).
The proportion of ASD cases by age, year, and source type is displayed in Fig. 2. Of the total ASD administrative cases, on average, 49% of children aged eight, 59% of children aged six, and 81% of children aged four were only captured by a health diagnosis. The proportion of ASD cases aged four, six and eight captured by health-only sources did not significantly vary by year. Similarly, the proportion of ASD cases captured by both health and special education sources did not vary as a function of year. The proportion of total ASD administrative cases captured only by a special education autism classification, however, did significantly increase in children aged four and six from SY 2006 to SY 2008 (χ 2 (1, N = 593) = 6.6, p = .01, and χ 2(1, N = 834) = 11.9, p = .0006, respectively) and children aged eight from SY 2002 to SY 2006 (χ 2(1, N = 554) = 4.8, p = .03).
Proportion of ASD cases identified by source type (school-only, health-only, and both health and school) by year and age group. The * indicates a statistically significant change (at α = .05) in the proportion of ASD cases captured by the school-only source between two study years. The significant change is between the year containing the * and the proceeding study year
School Special Education Services and an Autism Classification
A large proportion of children (20–50%) across age groups and study years with an ASD health diagnosis did not receive any special education services (Table 2). The proportion of children with an ASD diagnosis with an autism special education exceptionality ranged from 8% in children aged four in SY 2008 to 43% of children aged eight in SY 2002. As shown in Table 3, children aged eight with an ASD health diagnosis were not significantly more likely to receive an autism special education classification than an alternative special education classification in SY 2002 (χ 2(1, N = 152) = 1.68, p = .20) and SY 2006 (χ 2(1, N = 241) = .04, p = .85). In SY 2008, children aged six with an ASD health diagnosis were significantly more likely to receive non-ASD special education classification than ASD special education classification (χ 2(1, N = 353) = 5.26, p = .02), and children aged eight were about as likely to receive ASD than non-ASD special education classification (χ 2(1, N = 359) = 3.58, p = .06). Children aged four with an ASD health diagnosis, however, were significantly less likely to receive an autism special education classification in SY 2006 (χ 2(1, N = 244) = 33.24, p < .0001) and SY 2008 (χ 2(1, N = 263) = 76.94, p < .0001).
Discussion
Several key findings emerge from this study. First, with the significant increases in ASD prevalence in young children aged six and eight, this study provides little or no evidence that the ASD administrative rates are slowing in our state. The measured administrative prevalence doubled from 2002 to 2008 in children aged eight with the best estimate of ASD risk to Utah children now 1 in 77. ASD rates in males rose to above 2% in children aged eight in 2008 with a 2.3 fold increase in females. Prevalence rates in children aged six significantly increased from SY 2006 to SY 2008 by 24.5% but no significant change in prevalence was found in children aged four during the same time period.
Two published studies conducted outside of the US investigated the positive predictive value of an ASD diagnosis contained in a record on receiving a secondary ASD diagnosis through an alternative surveillance approach. In Great Britain a diagnosis of pervasive developmental disorders was confirmed 92.5% of the time after independent expert record reviews (Fombonne et al. 2004). Similarly Danish researchers (Lauritsen et al. 2010) found a slightly higher rate at 94%. A preliminary multisite US analysis of the CDC’s Autism and Developmental Disabilities Monitoring Network data for study years 2002 and 2006 obtained through personal correspondence with the CDC showed that of the total number of children abstracted with a previous ASD diagnosis and/or autism education classification, between 89.3% (2006) to 93.2% (2002) were classified as an ASD case using the network surveillance methodology. The question yet to be answered is whether or not the positive predictive value of a recorded diagnosis of ASD will remain high should administrative prevalence rates continue to rise.
In light of the magnitude of increases in our administrative prevalence it is important to note that the true ASD prevalence in our state is still unknown. Use of administrative data in isolation as used in this study raises the potential for both false positives and negatives. Even though study findings are higher than current national rates, we are concerned that our 2008 prevalence findings may be actually an underestimate of prevalence in our state. The CDC’s record review system has been found to be conservative in determining ASD case status (Avchen et al. 2010). A number of researchers have made the point that surveillance methods that rely on a previous clinical diagnosis only or on single administrative datasets potentially under estimates the number of children with ASD in the population (Barbaresi et al. 2005; CDC 2007; CDC 2009; Laidler 2005; Newschaffer et al. 2005; Pinborough-Zimmerman et al. 2010; Shattuck 2006). With this in mind efforts are underway to validate Utah’s administrative prevalence in a subset of our surveillance population using the CDC’s surveillance approach which includes an expert review of abstracted charts for the identification of previously undiagnosed children meeting study ASD case definitions (Van Naarden Braun et al. 2007) and verification of the ASD diagnosis in the record. Additionally the CDC’s surveillance method gives us the ability to capture important phenotypic information missing in administrative datasets.
The consistency of our 2002 and 2006 administrative ASD prevalence rates in children aged eight (6.5 per 1,000 and 10.2 per 1,000 respectively) compared with those reported using CDC’s MADDSP in study year 2002 (6.6 per 1,000; CDC 2007) and four of the six sites with access to school and health records in SY 2006 (CDC 2009) give us confidence that our 2008 rates are likely not an over estimate of prevalence. Interestingly, the rise in administrative prevalence found in this study among children aged eight from SY 2002 to SY 2006 mirrored the 57% average increase reported by the CDC in ten US sites (Utah excluded) using the MADDSP surveillance approach during the same study years (CDC 2009).
With national and local efforts aimed at improving the identification of children with ASD at younger ages, an encouraging finding was that no significant difference was found within the same study year in the measured ASD administrative prevalence between children aged six and eight. Conversely, prevalence in children aged four was significantly lower than both prevalence in children aged six and eight in the same study year suggesting continued efforts are still needed to improve early diagnosis of ASD. Since the average age of diagnosis in the US exceeds 4 years of age (CDC 2009), the invariable prevalence in the 4 year old population in SY 2006 and SY 2008 along with the lower prevalence of this age group compared to 6 years old from the same study year may reflect slow improvements in the identification of ASD among young children in our study area.
Understanding the severity of ASD and co-morbid intellectual disability may provide additional insight into changes in ASD prevalence by age, study year, and gender. The relatively higher proportion of children in the older age groups being ascertained, in particular, females suggests that providers have made improvements in diagnosing females and children with milder forms of ASD either with or without intellectual impairment. Unfortunately very few measures of severity of ASD are captured by the administrative datasets used for this study with the exception of health data diagnostic codes and those specifically for autism (299.00) and other specified pervasive developmental disorder (299.80 and 299.90). However, an ad-hoc analysis of ASD diagnostic codes by year and age group showed no discernable pattern emerging. Across age groups and study years the proportion of ASD cases identified from health sources with only a 299.80 ICD code ranged from 27% to 36% while cases with only a 299.00 ICD code ranged from 31% to 42%. Similarly cases with greater than one ASD ICD-9 code ranged from 28% to 32%.
The American Psychiatric Association (APA) reported that distinctions among the ASD subcategories are inconsistent over time and may vary across regions (APA 2010). In the APA’s proposed changes to the Diagnostic and Statistical Manual (DSM) V, 299.00 and 299.80 have been collapsed into a single category with the inclusion of clinical specifiers such as severity. The addition of a severity level scale may improve our ability in the future to capture and understand changes in prevalence related to severity.
A second major finding of this study was, as we hypothesized, that ASD administrative prevalence rates are significantly lower when derived from only a single data source, and rates are lowest in the youngest age group. The proportion of overall cases derived from health versus school administrative data varied significantly by age group, and although the proportion of children aged 4 and 6 ascertained by school-only sources increased between 2006 and 2008, a higher proportion of children were consistently captured at health than school sources across years and age groups. Not surprisingly, preschool-age children were predominately captured by health data.
Like other states, Utah is experiencing significant increases in the number of children receiving services under an autism special education classification (Office of Special Education Programs 2008a, 2008b). Statewide, the proportion of children aged eight in special education with an autism classification more than doubled from 2002 (2%) to 2008 (5%). The number of students with two other classification categories–communication disorders and developmental disabilities—has also risen; yet, enrollment has dropped for students qualifying under intellectual disabilities, specific learning disabilities, and behavior disorders exceptionalities. Shattuck (2006) suggested that changes in autism prevalence may reflect diagnostic substitution for those who previously may have qualified under intellectual and learning disability categories. Although not able to address Shattuck’s hypothesis directly, the findings of this study may yield important information on special education’s role on increases in overall ASD administrative prevalence.
We found that special education’s contribution to overall prevalence was measurable, but small compared to the proportion of health administrative cases for a number of reasons. Health sources consistently diagnose ASD almost twice as frequently as special education sources assign an autism classification. Second, the growth in the number of children with autism special education classification parallels the growth in ASD health diagnoses but a large gap remains between the administrative prevalence based on health versus special education datasets. Third, without special education autism classification data, the overall ASD administrative prevalence in children aged eight in our region would have been reduced only by 12% in 2002 and 17% in 2008. Contrary to our third hypothesis, the majority of children with a health ASD diagnosis were not receiving school special education services under an autism classification. While one-third of children aged eight with a health ASD diagnosis were receiving services under an autism special education classification, another one-third of children aged six and eight with a health ASD diagnosis were not receiving any type of special education services. It is unknown if these children were in regular public education programs, home-schooled, or private school which is a limitation of this study. In addition, we do not have any information concerning the cognitive functioning in this group of children. Lastly, contrary to our fourth hypothesis, the proportion of children with both an ASD health diagnosis and autism classification did not significantly change over time for children aged six and eight.
It is not totally clear why healthcare data would show greater prevalence and increase than special education administrative data. Nor do we know the reasons for ongoing discordance between children with ASD health diagnosis that do not receive a school autism classification. School practitioners are not required to use the health criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 2000) to identify children whom qualify for an autism special education classification. Rather, school teams assign a special education classification based on the US Code of Federal Regulations (CFR; National Archives and Records Administration 2010). Both DSM and CFR guidelines emphasize deficits in three general domains: social interaction, communication, and restrictive/stereotypical behaviors. Distinctions between DSM criteria and CFR classification vary across states (MacFarlane and Kanaya 2009), however, federal code requires that students eligible for special education services receive only one classification. Utah State Board of Education special education rules state that an autism classification is used if autism adversely affects the student’s educational performance, necessitates special education and related services, and is recognized by the IEP team as the student’s primary disability even in the presence of other disabling conditions such as emotional disturbance or intellectual disability (Utah State Board of Education, 2010). Furthermore, as part of the US Individuals with Disability Act, schools by law are required to educate pupils with disabilities and other special needs in the least restrictive environment that is appropriate to the individual pupil’s needs. As children with disabilities, such as ASD, must have the opportunity to be educated with non-disabled peers to the greatest extent appropriate, pupils with ASD may not require special education services and may be receiving instruction and necessary accommodations in a general education classroom. These students would not be captured in a special education administrative dataset. So in other words, like elsewhere in the US, our school practitioner’s operational definitions differ from DSM criteria, educators are able to only assign one primary special education classification, and special education data does not capture pupils with ASD for whom the regular classroom has been deemed the least restrictive environment.
Our study’s findings have important policy implications. Given disparities between the proportion of children with an ASD health diagnosis and special education autism classifications shown by this study, the potential exists for families and providers to become disgruntled or confused by differences in special education classifications and health diagnoses. Baird (1999) reported that disputes between parents of pupils with autism and school district programs represent the fastest growing and most expensive area of litigation in special education. Eleven US states (excluding Utah) now require that a pediatrician, clinician, or ASD diagnosis be part of the education evaluation (MacFarlane and Kanaya 2009). With Utah’s per public funding in education ranked last in the nation and high special education costs for children with an autism classification (Center for Special Education Finance 2003), it could be particularly helpful for health providers, upon appropriate parental consent, to provide diagnostic and prognostic information to the education team. This communication is particularly critical for general education personnel who may be educating students with ASD that are not eligible for an autism classification yet need accommodations in the general education setting. To improve communication between health and education, Utah education teams are currently developing autism specific guidelines for health care providers that outline the type of information that may be most beneficial for the schools to receive. Health provider awareness of laws for special education is key for physicians who are caring for families and children in a medical home. Cross health and education collaboration on providing specialized autism training could further understanding of the distinctions between an ASD diagnosis and special education autism classification and may improve overall communication.
Several additional limitations of this study should be noted. First, individual child records were not reviewed to substantiate an ASD diagnosis through an independent review process. Race/ethnicity could not be captured across all data sources and were thus not available for analysis. Next, US healthcare providers face numerous challenges getting reimbursed for ASD-related services. It is common for insurance companies to have exemptions specific to autism. Providers subsequently may choose not to use ASD diagnostic codes in health administrative datasets despite the presence of an ASD and these children would not have been captured in this study. Measuring trends in co-morbid diagnostic codes of cases and non-cases in future studies may provide insight into this potential trend. With lack of healthcare coverage or the potential for non-health insurance reimbursement, the only option for some US families may be to obtain autism related services through the public schools making special education data crucial to US administrative prevalence estimates. Regional variations in service availability through health and education sources are likely to exist across the US so our findings may not apply to other regions in the US.
Limitations of this study are balanced with a number of strengths. Surveillance data was examined across multiple administrative datasets which allowed for the matching of individual children across sources. As we have shown, access to both health and education datasets results in a more accurate estimate of ASD administrative prevalence in our community. This surveillance method demonstrates a cost effective means of monitoring changes in ASD prevalence over time. Data from our surveillance activities assist in program planning and provide a potential mechanism to study long-term ASD trends. The identification of disparities in prevalence rates as a function of data source (health versus school) may support efforts to improve communication and understanding of ASD diagnosis and classification requirements across sources.
Reasons for such marked changes in ASD prevalence in Utah remain unclear. The magnitude of this measure necessitates an investigation as to whether or not it represents a true rise in risk for the development of an ASD in Utah. In the present study, there is no definitive way to measure the portions of the increase that can be attributed to improved ASD awareness, diagnostic changes, or service mandates. It remains possible that in addition to these factors there is a true increase in incidence due to a yet unknown environmental risk mechanism. Regardless, increases in ASD prevalence of the magnitude we document in this study place severe burdens on public health and education services in our state.
References
U.S. Census Bureau. (2009). 2009 American community survey. Table R1105. Retrieved October 15, 2010, from http://factfinder.census.gov/home/saff/main.html?_lang=en.
American Psychiatric Association. (2000). Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association.
American Psychiatric Association. (2010). DSM-5: The Furture of psychiatric diagnosis. Retrieved from https://www.dsm5.org/Pages/Default.aspx.
Avchen, R. N., Wiggins, L.D., Devine, O., Van Naarden Braun, K., Rice, C., Hobson, N. C., et al. (2010). Evaluation of a records-review surveillance system used to determine the prevalence of autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(2), 227–236.
Baird, M. M. (1999). Legal issues in autism. In Proceedings of the 20th National Institute on Legal Issues of Educating Individuals with Disabilities. Alexandria, VA: LRP Publications.
Barbaresi, W. J., Katusic, S. K., Colligan, R. C., Weaver, A. L., & Jacobsen, S. J. (2005). The incidence of autism in Olmsted County, Minnesota, 1976–1997: Results from a population-based study. Archives of Pediatrics and Adolescent Medicine, 159(1), 37–44.
Bishop, D. V., Whitehouse, A. J., Watt, H. J., & Line, E. A. (2008). Autism and diagnostic substitution: evidence from a study of adults with a history of developmental language disorder. Developmental Medicine and Child Neurology, 50(5), 341–345.
Center for Special Education Finance. (2003). Total expenditures for students with disabilities, 1999–2000: Spending variation by disability. American Institutes for Research.
Centers for Disease Control and Prevention. (2007). Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveillance summaries, 56(1), 12–28.
Centers for Disease Control and Prevention. (2009). Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveillance summaries, 58(10), 1–20.
Fombonne, E. (2001). Is there an epidemic of autism? Pediatrics, 107(2), 411–412.
Fombonne, E. (2009). Epidemiology of pervasive developmental disorders. Pediatric Research, 65(6), 591–598.
Fombonne, E., Heavey, L., Smeeth, L., Rodrigues, L. C., Cook, C., Smith, P. G., et al. (2004). Validation of the diagnosis of autism in general practitioner records. BMC Public Health, 4, 5.
Fombonne, E., Zakarian, R., Bennett, A., Meng, L., & McLean-Heywood, D. (2006). Pervasive developmental disorders in Montreal, Quebec, Canada: Prevalence and links with immunizations. Pediatrics, 118(1), e139–e150.
Governor’s Office of Planning and Budget. (2001). Top ten Utah census 2000. Retrieved October 18, 2010, from http://governor.utah.gov/dea/Census2000Data/TopTen.PDF.
Grether, J. K., Rosen, N. J., Smith, K. S., & Croen, L. A. (2009). Investigation of shifts in autism reporting in the California department of developmental services. Journal of Autism and Developmental Disorders, 39(10), 1412–1419.
Gurney, J. G., Fritz, M. S., Ness, K. K., Sievers, P., Newschaffer, C. J., & Shapiro, E. G. (2003). Analysis of prevalence trends of autism spectrum disorder in Minnesota. Archives of Pediatrics and Adolescent Medicine, 157(7), 622–627.
Hamilton, B. E., Martin, J. A., & Ventura, S. J. (2009). Utah has the country’s highest fertility rate in the country. National vital statistics reports 57 (7). Retrieved January 20, 2010, from http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf.
International Classification of Diseases. (1988). Clinical modifications (9th ed.). Washington, DC: Public Health Service.
Kielinen, M., Linna, S. L., & Moilanen, I. (2000). Autism in Northern Finland. European Child and Adolescent Psychiatry, 9(3), 162–167.
King, M., & Bearman, P. (2009). Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology, 38(5), 1224–1234.
Kogan, M. D., Blumberg, S. J., Schieve, L. A., Boyle, C. A., Perrin, J. M., Ghandour, R. M., et al. (2009). Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics, 124(5), 1395–1403.
Laidler, J. R. (2005). US Department of Education data on “autism” are not reliable for tracking autism prevalence. Pediatrics, 116(1), e120–e124.
Lauritsen, M., Jorgensen, M., Madsen, K., Lemcke, S., Toft, S., Grove, J., et al. (2010). Validity of childhood autism in the Danish psychiatric central register: Findings from a chorot sample born 1990–1999. Journal of Autism and Developmental Disorders, 40(2), 139–148.
MacFarlane, J. R., & Kanaya, T. (2009). What does it mean to be autistic? Inter-state variation in special education criteria for autism services. Journal of Child and Family Studies, 18(6), 662–669.
Nassar, N., Dixon, G., Bourke, J., Bower, C., Glasson, E., de Klerk, N., et al. (2009). Autism spectrum disorders in young children: Effect of changes in diagnostic practices. International Journal of Epidemiology, 38(5), 1245–1254.
National Archives and Records Administration. (2010). Code of federal regulations. Retrieved from http://www.access.gpo.gov/cgi-bin/cfrassemble.cgi?title=200034.
Newschaffer, C. J., Falb, M. D., & Gurney, J. G. (2005). National autism prevalence trends from United States special education data. Pediatrics, 115(3), e277–e282.
Office of Special Education Programs. (2008). Part B –trend data report for states and outlying areas, 2003-04 through 2007-08. Retrieved from https://www.ideadata.org/default.asp.
Office of Special Education Programs. (2008). Profiles of parts b and c programs in states and outlying areas. Retrieved from https://www.ideadata.org/default.asp.
Pinborough-Zimmerman, J., Bilder, D., Satterfield, R., Hossain, S., & McMahon, W. (2010). The impact of surveillance method and record source on autism prevalence: collaboration with Utah maternal and child health programs. Maternal and Child Health Journal, 14(3), 392–400.
SAS Institute. (2008). SAS version 9.2. Cary North Carolina, USA: SAS Institute.
Shattuck, P. T. (2006). Diagnostic substitution and changing autism prevalence. Pediatrics, 117(4), 1438–1439.
Utah Department of Health. (2010). Center for health data, indicator-based information system for public health. Retrieved September 1, 2010, from http://ibis.helath.utah.gov.
Utah State Board of Education. (2010). Special education rules. Retrieved October 18, 2010, from http://www.schools.utah.gov/sars/DOCS/law/finalrules.aspx.
Van Naarden Braun, K., Pettygrove, S., Daniels, J., Miller, L., Nicholas, J., Baio, J., et al. (2007). Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveillance summaries, 56(1), 29–40.
Yeargin-Allsopp, M., Rice, C., Karapurkar, T., Doernberg, N., Boyle, C., & Murphy, C. (2003). Prevalence of autism in a US metropolitan area. The journal of the American Medical Association, 289(1), 49–55.
Acknowledgments
This work was partially supported by funding from the Utah Department of Health and Utah State Office of Education. Special thanks to Marc Babitz, Paul Carbone, Nan Gray, Harper Randall, Robert Satterfield and Nan Streeter.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinborough-Zimmerman, J., Bakian, A.V., Fombonne, E. et al. Changes in the Administrative Prevalence of Autism Spectrum Disorders: Contribution of Special Education and Health from 2002–2008. J Autism Dev Disord 42, 521–530 (2012). https://doi.org/10.1007/s10803-011-1265-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-011-1265-2