Abstract
An immune deviation toward Th2-type immunity is involved in the pathogenesis of allergic asthma and rhinitis. Allergic inflammation is characterized by upregulation of Th2-type cytokines (the so-called Th2 polarization), whereas there is a downregulation of Th1-type immune response and related cytokines like interferon-γ (IFN-γ). The latter is a strong inducer of enzyme indoleamine 2,3-dioxygenase (IDO), which degrades the essential amino acid tryptophan as part of an antiproliferative strategy of immunocompetent cells to halt the growth of infected and malignant cells. Tryptophan metabolism may also play a relevant role in the pathophysiology of allergic disorders.
In patients with pollen allergy, raised serum tryptophan concentrations were observed compared to healthy blood donors. Moreover, the higher baseline tryptophan concentrations were associated with poor response to specific immunotherapy. It turned out that the increase of tryptophan concentrations in patients with pollen allergy only exists outside pollen season, but not in season. Interestingly, there was only a minor alteration of the kynurenine to tryptophan ratio (Kyn/Trp, an index of tryptophan breakdown), which is used as an estimate of IDO activity.
The reason for the higher tryptophan concentrations in patients with pollen allergy outside season remains obscure. With this respect, specific interaction of nitric oxide (NO.) with IDO could be important, because an enhanced formation of NO. has been reported in patients with asthma and allergic rhinitis: exhaled breath NO. is increased in asthma versus healthy controls, and serum nitrite concentrations were found to be higher in allergic patients out of pollen season than in season. Importantly, NO. slows down the expression and activity of the heme enzyme IDO. So, the higher tryptophan levels could be explained when IDO activity was suppressed by NO.. As a consequence, inhibitors of inducible NO. synthase (iNOS) should be reconsidered as candidates for antiallergic therapy out of season that may decrease the production of NO. and thus abrogate the arrest of IDO.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Allergy
- Atopy
- Cross-regulation
- Indoleamine 2,3-dioxygenase
- Interferon-gamma
- Kynurenine to tryptophan ratio
- Neopterin
- Nitric oxide
- Th2-type immunity
- Tryptophan 2,3-dioxygenase
3.1 Tryptophan
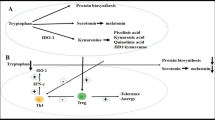
L-Tryptophan is an essential amino acid that is required for protein biosynthesis and also serves as precursor of several metabolites in humans. Absorbed tryptophan circulates in its free form or bound to albumin in the peripheral blood stream. Only in its free form, it can cross the blood-brain barrier. There are three different biosynthetic pathways in which tryptophan is metabolized: (i) the formation of kynurenine derivates, which represents the major route; (ii) the generation of serotonin, a neurotransmitter and precursor of melatonin (Schroecksnadel et al. 2006; Chen and Guillemin 2009); and (iii) the biosynthesis of proteins (Fig. 3.1).
The three different ways of tryptophan usage. (i) The first one represents the major route of tryptophan breakdown. The rate-limiting enzymes indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO) convert the essential amino acid tryptophan into kynurenine. This metabolite is precursor of several metabolites. (ii) The second pathway is the conversion via tryptophan 5-hydroxylase (T5H) and followed by decarboxylation to the neurotransmitter serotonin (5-hydroxytryptamine). Furthermore, tryptophan is required for protein biosynthesis (iii)
To generate kynurenine, tryptophan is oxidized by a cleavage of the indole ring moiety, which is achieved either by tryptophan 2,3-dioxygenase (TDO), indoleamine 2,3-dioxygenase 1 (IDO-1), or IDO-2. TDO is primarily expressed in the liver and is inducible by tryptophan or corticosteroids (Badawy 2013). IDO-1 is induced by various inflammatory cytokines, with this respect the most prominent one being interferon-γ (IFN-γ), and is expressed in numerous cells as macrophages, microglia, neurons, and astrocytes, but also epithelial cells and fibroblasts (Guillemin et al. 2007). The recently discovered IDO-2 possesses similar activities to IDO-1 but differs in its expression pattern, substrate specificity, and signaling pathways (Chen and Guillemin 2009).
IDO-1 plays an essential role within the immune response and could even serve as a biomarker for the inflammation status in human. It has been discovered that IDO-1 inhibits immune cell and pathogen proliferation by the depletion of tryptophan and/or by the production of bioactive catabolites (Samelson-Jones and Yeh 2006). In addition, tryptophan breakdown products such as kynurenine, 3-hydroxyanthranilic acid, and quinolinic acid may negatively affect neurological functions, while other metabolites such as kynurenic acid can be neuroprotective (Heyes et al. 1992; Klein et al. 2013; Sas et al. 2007).
The second metabolic pathway is the generation of the neurotransmitter 5-hydroxytryptamine (serotonin) via the enzyme tryptophan 5-hydroxylase (T5H). In a first step, 5-hydroxytryptophan is formed, which is converted to serotonin under the influence of 5-hydroxytryptophan decarboxylases that require pyridoxal phosphate as a cofactor. In the case of insufficient tryptophan availability, serotonin production is diminished, which may cause neuropsychiatric symptoms like depression or other mood disorders (Widner et al. 2002). The third pathway represents tryptophan as a component of proteins.
3.2 Tryptophan and Its Influence on the Immune System
Significant alterations of serum tryptophan concentrations were observed in pregnant women (Schröcksnadel et al. 1996). Tryptophan concentrations declined with the duration of pregnancy and correlated inversely with neopterin concentrations. Data indicated that IDO activity was involved in the tryptophan metabolism. Thereafter, it was found that IDO activation is an important aspect in the establishment of immunotolerance against the fetus (Munn et al. 1998) and thus that tryptophan metabolism is strongly involved in immunomodulation (Mellor and Munn 2004). Great attention was paid to the estimation of tryptophan breakdown as a biomarker in various immune pathologies such as infections, autoimmune and neurodegenerative disorders, and allergy (Schroecksnadel et al. 2006; Widner et al. 2000a, b; Raitala et al. 2006; Kositz et al. 2008). In the human immune system, various cell types play an important role to protect the integrity of the organism from invaders. The efficient host defense against pathogens is achieved through the thorough coordination of the innate and adaptive immune system. Once an antigen is present in the body, it has to be recognized by T cells, which identify the antigen in cooperation with antigen-presenting cells. The recognition sites include the T-cell receptor and the major histocompatibility complex. Furthermore, the signal cascades involve several binding proteins such as the protein ligand B7 and the cluster of differentiation 28 (CD28), which provide co-stimulatory signals to T cells (Balakrishnan and Adams 1995). The activation of different subsets of T-helper (Th) cells characterizes different immune responses. T cells can differentiate into a variety of effector subsets, including the classical Th1- and Th2-type cells, the recently defined Th17-type cells, the Th9 subset that control the growth and activation of mast cells, the follicular helper T (Tfh) cells that are responsible for the B-cell maturation responses (Zhou et al. 2009), and the regulatory T cell (Treg). The decision for differentiation is mostly driven by cytokines that are expressed in the microenvironment. Also, the interaction strength between the T-cell antigen receptor and the antigen can influence the direction of differentiation (Zhou et al. 2009).
Signaling by the arylhydrocarbon receptor (AHR) is thought to be involved in T-cell differentiation. AHR is a cytosolic receptor, which translocates into the nucleus after ligand binding and dimerizes with the AHR nuclear translocator (ARNT) to act as a transcription factor for various genes including the cytochrome P450 (CYP) enzymes (Van Voorhis et al. 2013). The AHR is known as a sensor to the outside environment that modulates the immune system in response to toxins. However, AHR signaling is activated not only after toxic exposures but also by endogenous compounds like the tryptophan catabolite kynurenine, which leads to the activation of several CYP isoenzymes and other metabolizing enzymes such as glutathione S-transferase Ya (GSTYa) or aldehyde-3-dehydrogenase (ALDH-3) (Van Voorhis et al. 2013). Via AHR, a toxin can also elicit an inflammatory response with the induction of Treg, where the IDO pathway and its metabolites are involved (Van Voorhis et al. 2013).
3.3 Types of Immune Response
Th1-type immune reaction is crucial in the pathogenesis of several inflammatory disorders like cardiovascular diseases, autoimmune syndromes, malignant tumor diseases, and neurodegenerative disorders (Asehnoune et al. 2004; Romagnani 2004; Schroecksnadel et al. 2007). Th1-type cells are characterized by the production of typical Th1-type cytokines like IFN-γ and are involved in the cellular immune response against pathogens and malignant cells. In the opposite, Th2-type cells predominate in allergic reactions and asthma (Romagnani 2004), interleukin-4 (IL-4), interleukin-5, and interleukin-13 representing prominent cytokines released from Th2-type cells. They mediate antibody responses, especially immunoglobulin E (IgE) production (Barth et al. 2003), and control helminthes infections (Zhou et al. 2009). Th17-type cells represent another type of T-helper cells, which modulate immune responses. They are supposed to combine innate and adaptive immunity (Yu and Gaffen 2008); produce IL-17A, IL-17F, and IL-22; and play important roles against extracellular bacteria or fungi. Regulatory T cells (Tregs) are involved in the maintenance of immunological self-tolerance (Hori et al. 2003) and limit potential collateral tissue damage (Zhou et al. 2009). Tregs are characterized by expression of forkhead transcription factor box p3 (Foxp3). There are two subgroups of Tregs: the naturally occurring Tregs (nTreg) and the induced Tregs (iTreg). Both cell types play a role in the maintenance of self-tolerance and the prevention of autoimmunity.
During the Th1-type immune reaction, the most prominent immune inductor IFN-γ is secreted by activated T lymphocytes and natural killer (NK) cells to initiate antimicrobial and antitumoral defense mechanisms (Romagnani 2006). Thereby, IFN-γ induces various biochemical pathways such as the activation of GTP-cyclohydrolase I (GTP-CH1) and IDO. This includes also the high output of reactive oxygen species (ROS) by human macrophages or monocytes (Nathan et al. 1983) and the induction of the inducible nitric oxide synthase (iNOS) and of several other immune effector pathways (Widner et al. 2000a; Werner et al. 1991).
The activation of GTP-CH1 by IFN-γ leads to the production of the pteridine derivatives neopterin and 5,6,7,8-tetrahydrobiopterin (BH4). BH4 is the essential cofactor for several monooxygenases including iNOS and is formed in various cells of several species upon exposure to proinflammatory stimuli. Upon stimulation, these cells produce NO. in a high rate. However, the production of BH4 involves 6-pyruvoyltetrahydropterin (PTPS), an enzyme, which is of low activity in human and primate macrophages and dendritic cells. As a result of this biochemical peculiarity, human and primate monocyte-derived cells produce high amounts of neopterin at the expense of BH4. In the absence of sufficient amounts of BH4, also the proper function of enzyme iNOS and in this way NO. output are diminished (Werner et al. 1990; Andrew and Mayer 1999). In contrast, human fibroblasts or endothelial cells preferentially produce BH4, and thus, also NO. is formed.
Neopterin is a stable biomarker of immune activation, which can be easily determined in body fluids like blood, urine, and cerebrospinal fluid (CSF) (Fuchs et al. 1992; Murr et al. 2002). Because of the common immunostimulatory background, neopterin production and tryptophan breakdown are not only induced in parallel in vitro (Weiss et al. 1999) but also in patients (Schroecksnadel et al. 2006). Several in vivo studies confirm the association between altered neopterin concentrations and tryptophan breakdown rates, as detected in serum samples of patients with infectious diseases, like HIV, gynecological cancer, malignant tumors, cardiovascular disease, neurodegenerative disorders, or diseases associated with normal aging processes (Schroecksnadel et al. 2005a, 2006; Fuchs et al. 1988, 1990, 1991, 2009; De Rosa et al. 2011; Pedersen et al. 2011; Wirleitner et al. 2003). Both pathways turned out to represent robust and strongly predictive immune activation biomarkers.
Neopterin concentrations can be measured with commercially available ELISA. Usually, tryptophan and kynurenine concentrations are measured with high-performance liquid chromatography (HPLC), and IDO activity can be estimated by the ratio of kynurenine to tryptophan (Kyn/Trp) concentrations.
In vitro, high neopterin output by activated human monocyte-derived macrophages has been shown to be associated with a strong release of hydrogen peroxide (H2O2) (Nathan 1986). In line with this observation, higher neopterin concentrations in, e.g., patients with coronary artery disease were found to concur with low concentrations of serum antioxidants (Murr et al. 2009). This fact implies that neopterin concentrations can also serve as sensitive indirect marker of oxidative stress during immune activation (Murr et al. 1999).
3.3.1 Allergy
In the past few years, the incidence of allergy and asthma has increased drastically. Allergy and asthma are among the most common chronic diseases in the world. Currently, more than 130 million people are affected by asthma and the numbers are steadily growing. Interestingly, in developing countries, there is a lower prevalence of allergic diseases. Environmental factors, for example, more indoor allergens, pollution, changes in diet, or breastfeeding, could be the reason for these increasing atopic diseases. However, there are still little relations and evidences, which demonstrate definitive risk factors. A link between lifestyle, habits, and the development of allergy might exist, but the connection is still heavily discussed (Fuchs 2012). However, childhood infections seem to have a protective effect for the development of atopy and allergic diseases in the later life. A higher allergic sensitization occurs often in newborn, but less in children from large families and those who attend daily day care (Strachan 1989; Krämer et al. 1999; Yazdanbakhsh et al. 2002). These results suggest that a frequent contact with infections could have protective effects on the children (Strachan 1989).
The main explanatory theories for the increase of atopic diseases are altered hygienic conditions (Strachan 1989) and human nutrition. Nowadays, there exist improved sanitation and living conditions, vaccinations, and antimicrobial therapies, and most people have less contact to microbes. Immune stimulations by microbes are considered to be necessary to hinder the consolidation of the atopic responder type, as was concluded from the hygiene hypothesis (Liu and Murphy 2003). Furthermore, human nutrition has considerably changed. Food preservation and sterilization reduces the microbial exposure, and pasteurization has replaced drying and fermentation (Fuchs 2012; Yazdanbakhsh et al. 2002; Isolauri et al. 2004). The food preservatives have become more and more popular because of the globalization, as food is shipped and offered all over the world and needs to be conserved over a long time. Many of the commonly used preservatives are antioxidative substances, which can inhibit the oxidation of the food components (Gostner et al. 2014).
Recently, major attention is payed to the role of the human innate immune system as it was shown to be strongly activated during allergic responses. Antigen-presenting cells can absorb the allergen and initiate the signal transduction for T-cell development within the Th2-type immune response direction. Th2-type cell activation leads to IL-4, IL-5, and IL-13 cytokine expression. These cytokines can interact with their receptors to stimulate allergen-specific IgE production. Furthermore, this cytokine production leads to the accumulation of a high number of eosinophils and mast cells and boosts inflammation in the body (Holt et al. 1999). Immune cells start to produce large amounts of cytokines, chemotactic factors, or free radicals, which leads in the end to enhanced vascular permeability and persistent inflammation (Ciprandi et al. 2011a). High amounts of IgE circulate in the blood and bind to the high affinity IgE receptor (FcεRI) of mast cells or basophiles to activate histamine release, which is the main inductor of an allergic disease (Brown et al. 2008). At this time point, the sensitization to a specific allergen is stored. If this antigen is present at another time, it can bind to the IgE of mast cells and activate several cascades like vasodilation, mucous secretion, and nerve stimulation of muscle contraction (Zaknun et al. 2012). However, not every Th2-type response is characterized by IgE production.
It has been argued that a decreased exposure to pathogens in early childhood may result in an insufficient stimulation of Th1-type cells, which leads to a diminished capability to counterbalance the expansion of Th2-type cells and thus results in a predisposition to allergy (Yazdanbakhsh et al. 2002). High IgE levels may indicate atopy, which underlies allergic diseases as asthma, rhinoconjunctivitis, and eczema. It is well accepted that Th1- and Th2-type cytokines cross-regulate each other (Romagnani 2004). Allergic inflammation is characterized by the upregulation of Th2-type cytokines and downregulation of Th1-type cytokines such as IFN-γ. Von Bubnoff and Bieber described the IDO pathway as one of the central pathways in allergy development. IDO activity not only is crucial during pregnancy, chronic inflammation, tumorigenesis, and infections but also influences the inflammatory state of atopy or allergy (von Bubnoff and Bieber 2012).
An in vitro study in human peripheral blood mononuclear cells (PBMC) further confirmed this observation that typical Th2-type cytokines, IL-4 and IL-10, can counteract IFN-γ- and Th1-mediated pathways, when the effects of the different cytokines on neopterin formation and tryptophan breakdown were compared (Weiss et al. 1999). After IL-4 or IL-10 exposure, a lower stimulatory effect of IFN-γ was observed, which resulted in a diminished tryptophan breakdown rate and lower neopterin levels, whereas Th1-type cytokine IL-12 had the opposite effect of co-stimulating both biochemical pathways. Thus, exposure of PBMC to Th2-type cytokines was reflected by higher tryptophan concentrations in culture supernatants, because the breakdown of the amino acid was suppressed.
Allergic rhinitis is associated with the dysfunction of T-cell responses, where the antigen induces mast cell activation by allergen-specific IgE. Severe allergic rhinitis has a huge impact on the health-related quality of life and/or work, which can result in a significant individual burden. Furthermore, allergic rhinitis and asthma are often comorbid diseases. Frequent treatments with aspirin, anti-inflammatory agents, or antibiotics can inhibit Th1-type immune response and strengthen the development of Th2-type responses and cause allergic symptoms in the case of a concomitant phenomenon (Kuo et al. 2013). The activated cells release large amounts of proinflammatory cytokines, which induce inflammatory cell enhancement. It may lead to a persistent inflammation of the nasal mucosa, which is the main relevant pathophysiological feature in allergic rhinitis (Ciprandi et al. 2011a). Interestingly, treatment of PBMC with aspirin or salicylic acid had a similar effect on neopterin production and tryptophan breakdown as compared with Th2-type cytokines, where both were suppressed (Schroecksnadel et al. 2005b).
These results are in line with the hypothesis that allergy results from a shift of Th1- toward Th2-type immunity. Inhibition of IFN-γ and as a result also of IDO decreases the Th1-type immune response.
Besides typical nasal symptoms like itching, sneezing, rhinorrhea, or obstruction (Bousquet et al. 2008), many allergic rhinitis patients exhibit also nonnasal symptoms as behavioral changes like tiredness, somnolence, depression, apathy, and impaired attention, which can reduce the quality of life (Juniper and Guyatt 1991; Ciprandi et al. 2011b). This fact supports the hypothesis that the tryptophan pathway and as a result also serotonin production play an important role in allergy.
Allergen-specific immunotherapy (AIT) is widely used to treat asthma and allergic rhinitis and to modify the disease development. AIT is typically used, when medication or environmental changes cannot control asthma or allergic rhinitis symptoms. There are two ways of desensitization procedures, the subcutaneous immunotherapy (SCIT), where the allergens are injected subcutaneous to the patients, also known as “allergy shots.” In contrast, the sublingual immunotherapy (SLIT) provides the allergen as drops to the sublingual area for local absorption. The outcome of both treatments seems to be equal (Saporta 2012), although some studies claimed that SCIT might have better results (Mungan et al. 1999). SCIT is a well-established method, which has been used for many decades, and furthermore, it is well tolerated (Saporta 2012). SLIT is also a very old method, which is not well established in the USA, but in Europe it is still a commonly used treatment. SLIT seems to be a safer method for treatment of children (André et al. 2000).
3.4 Tryptophan in Allergy
Induction of IFN-γ leads to the activation of downstream biochemical pathways like tryptophan breakdown by IDO. IDO activity is drastically enhanced during the proinflammatory Th1-type immune response and contributes to the pathogen defense by deprivation of the essential amino acid tryptophan. Furthermore, ROS and reactive nitrogen species (RNS) are produced in high amounts, and these are commonly known to interference with target cells or pathogens by oxidation and/or nitration of vital cellular structures. For the balance of immune responses, Th1 and Th2 responses can cross-regulate each other (Romagnani 2004, 2006). This can be achieved by the activation of redox-sensitive signaling cascades, where oxidative conditions support Th1-type development, while excess of antioxidant compounds “antioxidant stress” can lead to a shift toward allergic Th2-type immune responses (Murr et al. 2005; Poljsak and Milisav 2012). IDO is widely accepted for its role in infection, pregnancy, autoimmunity, and neoplasia, but also the control of allergic inflammation was attributed to the enzyme (von Bubnoff and Bieber 2012).
Higher serum tryptophan concentrations were observed in adult patients with pollen allergy compared to healthy blood donors (Kositz et al. 2008). In this study, 44 patients with allergic rhinitis were examined before and after SCIT and compared to 38 healthy controls. In atopics, higher tryptophan levels in comparison to healthy blood donors were noted, but there were no differences in kynurenine concentrations. Also Kyn/Trp was only slightly, but not significantly, lower in atopics, and serum neopterin levels tended to be at the upper limit of the normal levels. Interestingly, higher levels of tryptophan were preferentially observed in nonresponders to SCIT. Thus, tryptophan concentrations could help to predict the outcome of SCIT.
A further study confirmed the higher tryptophan levels in patients with pollen allergy, but this observation was made only off pollen season but not in pollen season (Ciprandi et al. 2010). Notably, also the higher tryptophan levels observed in the first study (Kositz et al. 2008) were measured in patients before they received desensitization therapy and were thus off pollen season. Patients with pollen allergy seem to have a distinct IDO activity pattern with higher tryptophan levels due to a less breakdown out of season. However, tryptophan levels decline closer to normal values in spring; when under allergen exposure, tryptophan breakdown becomes initiated. In regard to this observation, the higher tryptophan levels during winter could represent a consequence of the chronic Th2-type immune response in summer due to counter-regulation.
However, any possible (primary or additional) role of TDO activation should not be disregarded.
Another substrate of IDO, serotonin, was found to be higher in patients with pollen allergy compared to outside of pollen season (Ciprandi et al. 2011b). Interestingly, low serotonin levels in allergic rhinitis patients in season upon pollen allergen exposure were strongly related with behavioral impairment, as was assessed by quality of life questionnaires, and thus, serotonin can serve as a biomarker of behavioral symptoms during allergic response (Ciprandi et al. 2011b). As in other clinical inflammatory conditions, tryptophan availability is strongly involved in the pathogenesis of mood disorders and depression (Widner et al. 2002). Abnormal tryptophan concentrations may be involved in the development of neuropsychiatric symptoms, while serotonin production is decreased (Widner et al. 2000a) or may be also above normal.
3.4.1 Nitric Oxide: Nitrite
The reason for the higher tryptophan concentrations in patients outside of pollen season still remains obscure. Specific interactions of NO. with IDO could be very important in this circumstance (Ciprandi and Fuchs 2013). There are several reports in the literature that exhaled breath from patients with allergic rhinitis or asthma contained higher NO. levels compared to healthy controls (Stewart and Katial 2012). Likewise, serum nitrite concentrations were found to be higher in allergic rhinitis patients compared to healthy controls, and again, this was apparent off pollen season rather than during pollen season (Ciprandi et al. 2011a).
These observations seem to provide a link between tryptophan breakdown and the formation of NO., which has been demonstrated earlier to inhibit the expression and function of IDO (Thomas et al. 1994) (Fig. 3.2). Thus, when NO. formation is increased, an inhibition of IDO becomes more likely, and as a consequence, tryptophan concentration would increase (Ciprandi et al. 2010). The increase of tryptophan in atopics out of season can be explained by a suppression of IDO activity through the enhanced availability of NO. (Gostner et al. 2014). Importantly, no inhibitory activity on GTP-CH1, the key enzyme for neopterin production, is known for NO..This would agree with the independent development of tryptophan and neopterin concentration in patients with allergic rhinitis, e.g., mast cells can produce IFN-γ and stimulate the production of neopterin in monocyte-derived macrophages or dendritic cells, while NO. formation starts in endothelial cells and IDO activity becomes arrested by the presence of NO. (Ciprandi and Fuchs 2013).
Interferon-γ (IFN-γ) expression leads to the induction of GTP-cyclohydrolase I (GTP-CH1), which produces out of guanosine triphosphate (GTP) 7,8-dihydroneopterin triphosphate. In human macrophages and dendritic cells, the enzyme 6-pyruvoyltetrahydropterin (PTPS) is lacking, and neopterin is produced. In all other cell types PTPS forms 5,6,7,8-tetrahydrobiopterin (BH4). BH4 serves as a cofactor for inducible nitric oxide synthase (iNOS) to produce nitric oxide (NO.). High levels of NO. can inhibit IDO activation and as a result inhibit tryptophan breakdown. If BH4 is not available, iNOS produces superoxide (O2 .−) instead of NO..
iNOS inhibitors have already been considered as candidates for an antiallergic therapy (Hesslinger et al. 2009) without considering their influence on IDO. iNOS inhibitors may abrogate the IDO arrest, by diminishing NO. production. Still, treatment with iNOS inhibitors should be more effective outside of pollen season, than during season (Ciprandi and Fuchs 2013). IDO and iNOS are both induced by IFN-γ. iNOS inhibitors block NO. production and thereby can promote IDO activity. However, the interference of NO. and IDO could be cell specific. In stimulated monocyte-derived cells, ROS are concomitantly produced with NO. and give rise to the cell-toxic peroxynitrite (ONOO–), whereas in other cells, because of the absence of superoxide anion (O2 .–), NO. is not oxidized and exerts its inhibitory effect on IDO. This can be explained with an excess of antioxidants, which can stabilize the iNOS cofactor BH4 to guarantee high NO. production. Furthermore, other NOS enzymes that are not induced by IFN-γ can continue to produce NO. and inhibit IDO.
3.4.2 Nitric Oxide and Tryptophan Metabolism
NO. is a classical messenger for several biological processes, which include vasodilatation (Allen et al. 2009), neurotransmission (Bult et al. 1990; Garthwaite 2008), macrophage-mediated cytotoxicity (Marletta et al. 1988), gastrointestinal smooth muscle relaxation (Bult et al. 1990), and bronchodilation (Lindeman et al. 1995). NO. synthases produce NO. by the oxidation of L-arginine and formation of the by-product, L-citrulline (McNeill and Channon 2012). There are three isoforms of NOS: (1) neuronal NO. synthase (nNOS) is involved in the regulation of autonomic functions in cardiovascular diseases; (2) iNOS has effects on vascular functions under conditions of sepsis and is a potent mediator of inflammation; (3) endothelial NO. synthase (eNOS) acts in vascular diseases such as atherosclerosis, hypertension, and ischaemia-reperfusion. All of them can be responsible for abnormalities in endothelial functions. nNOS and eNOS are both calcium dependent, while iNOS works calcium independently (Moncada 1999). Furthermore, the first two NOS isoforms are constitutively expressed, while iNOS seems to be active only during immune responses (Tsutsui et al. 2009).
NO. synthesis is commonly not only cell specific, but also the environment in which the cells, organs, or the whole organisms that are experienced at the time of the production site is important for the activity of the three different NOS (Villanueva and Giulivi 2010). Several cross talks have been described for NO. and IDO. For example, tryptophan and the tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid can inhibit iNOS at the expression and catalytic level (Samelson-Jones and Yeh 2006). Furthermore, during the immune response, NO. is an important regulator of the enzyme IDO. NO. inhibits IDO by preventing both the expression and the activity of IDO, by binding to the catalytic domain (Samelson-Jones and Yeh 2006). In turn, IDO inhibition then leads to higher tryptophan levels.
As described above, BH4 is required for a proper function of NO. synthesis. However, in human macrophages, there is a lack of PTPS to produce BH4, and instead, neopterin accumulates. When BH4 levels become deficient, the oxygenase domain of NOS enzymes produces O2 .– instead of NO.. The produced O2 .– can promote further reactions to form other ROS/RNS such as ONOO– or H2O2, which can disturb the redox balance and in the end lead to cellular injury and inflammation. Toxic ROS products like H2O2, O2 .–, or ONOO– can suppress the growth of target cells and pathogens (Schroecksnadel et al. 2010; Wink et al. 2011) but also lead to the dysfunction of protective cellular antioxidant mechanisms in inflamed tissue, which can result in a high oxidative stress milieu (Hesslinger et al. 2009; Bowler and Crapo 2002). In turn, a high degree of oxidative stress can activate signaling cascades such as mitogen-activated protein kinase (MAPK), transcription factor nuclear factor-κB (NF-κB), and activator protein (AP) pathways and initiate the expression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1, chemokines, and adhesion molecules (Aggarwal 2004).
The increased ROS production can further limit BH4 availability through the oxidation of the oxidation-sensitive molecule BH4 itself (Lindeman et al. 1995). Oxidative stress is probably involved in a wide range of clinical pathologies like cardiovascular or neurodegenerative disorders (Halliwell 1996, 2006). To counteract ROS effects, different strategies have evolved. For example, some small molecules function as antioxidants or enzymes, which can neutralize ROS, like catalase, glutathione peroxidase (EC 1.11.1.9), and superoxide dismutase (Sies 1997; Halliwell 1999). Antioxidants may be synthesized in the body or can be obtained from the diet. An intake of dietary antioxidants can counteract oxidation processes by scavenging ROS and other redox-sensitive molecules and therefore protect against cellular damage (Schroecksnadel et al. 2007; Jenny et al. 2011). However, if antioxidants are present in a normal milieu without inflammation, an excess can shift the Th1-type immune response to Th2-type immunity, which can promote or accelerate allergic reactions when an allergen is met (Zaknun et al. 2012).
3.5 Food Antioxidants and Tryptophan Metabolism
In the last decades, antioxidant exposure and uptake have extremely increased. Food and beverages are supplemented with vitamins such as A, C, or E, and this is done because of the conviction that this should be healthy. However, it is still unclear whether supplemented antioxidant vitamins and other compounds have a benefit comparable to that of their natural counterparts. Moreover, meta-analyses demonstrated that supplemented antioxidant vitamins like vitamin A, C, and E may even increase mortality rather than reduce it (Bjelakovic et al. 2007); especially, vitamins E and A and ß-carotene seem to exert also adverse effects.
Today, when antioxidants are supplemented to almost every food or beverage, it is not easy to avoid overexposure. Thereby, extra vitamins are usually advertised. This is not the case for food preservatives and colorants. These are usually declared only as fine print. Food preservatives like sodium sulfite or benzoate but also colorants like curcumin or betalain are widely known for their antioxidant activity (Zaknun et al. 2012). However, a well-functioning human organism does not need extra antioxidant supplementation; the content in the normal Western diet is sufficient.
An excess of food preservatives or antioxidants may increase allergy risks. They can suppress Th1-type immune responses and cytokine expression, and consequently, due to the cross-regulation of Th1- and Th2-type immune responses, Th2-type cytokines are upregulated, and the development of asthma and allergic diseases might be favored (Murr et al. 2005). If an allergen is presented, Th2-type cytokines are produced in a high concentration, and this condition can strengthen the Th2-type immune response. Under antioxidant-loaded conditions, allergic response already initially becomes stronger when an allergen is met, than in conditions with normal antioxidant levels (Zaknun et al. 2012).
It is well documented that antioxidants can stabilize BH4 and promote NO. production, which leads in the end to the inhibition of IDO and an increase of tryptophan and higher serotonin levels. In parallel, high serotonin levels could even precipitate the serotonin syndrome, which is a life-threatening disease, characterized by a clinical triad of mental-status changes, autonomic hyperactivity, and neuromuscular abnormalities. It is known to be induced either by adverse drug reaction; from therapeutic drug use, intentional self-poisoning, or inadvertent interaction between drugs; or by an excess of antioxidants (Boyer and Shannon 2005).
Physical exercise is the most convenient way to escape from reductive caused by excess intake of antioxidative compounds. Sports help not only in burning fat; it especially oxidizes the even stronger antioxidants like vitamins, spices, and food preservatives. This interaction may help to understand the findings that supplementation with antioxidant vitamins was found to slow down the antioxidant defense response induced by physical exercise and sports (Ristow et al. 2009; Peternelj and Coombes 2011). Thus, moderate sports and physical exercise can be recommended to combat allergic responses.
Unfortunately, in the current generation, every negative effect of food is first denominated by the public as an allergy. However, food allergy is often not an allergy in its sense. In its strict sense, food allergy is an adverse reaction to the food itself, and the classical immune mechanism is specific, for it being indicated by the presence of IgE antibodies is typical. The diagnosis will be taken after a case history, the demonstration of IgE sensitization by a skin-prick test on an in vitro test, and will be confirmed by a positive oral provocation (Wüthrich 2009). By contrast, food intolerance is considered as a “nonimmune”-mediated adverse reaction to the food. There are enzymatic (e.g., lactose intolerance, lactase deficiency), pharmacological (reactions against biogenic amines, histamine intolerance), or undefined food intolerances (against food additives). Interestingly, under such conditions, huge amounts of H2 are produced and exhaled, H2 under certain circumstances being a strong antioxidative compound. Still, it has to be kept in mind that not every sign of sickness after food intake has to be an allergy.
Recently, performed studies reported an association between fast-food consumption and the prevalence of asthma, rhinoconjunctivitis, and eczema in children and adolescents (Ellwood et al. 2013). In addition, antioxidants or additives may disturb the endogenous appetite and satiation regulatory circuits. On the one hand, antioxidants may suppress tryptophan breakdown by IDO (Jenny et al. 2011) and thus increase the availability of tryptophan for serotonin production and as a consequence contribute to mood improvement. Tryptophan metabolic changes may also contribute to the weight gain after a calorie-restricted diet (Berger et al. 2013), when under starvation conditions tryptophan levels decline, which increases carbohydrate craving as a substitute for brain serotonin (Wurtman and Wurtman 1995) followed by weight gain. This sequence of events can explain the often observed yo-yo effect, also known as weight cycling when people rapidly gain weight after a diet.
Also, the histamine content of beverages and food has to be taken in consideration. As mentioned above, histamine is known to trigger acute symptoms like acute rhinitis, bronchoconstriction, diarrhea, or cutaneous wheal. It has a strong activity on endothelium and bronchial or smooth muscle cells and modulates also chronic inflammatory events (Jutel et al. 2002). Histamine is important in the early and late phase response to soluble antigens. It increases the vascular permeability and is involved in the recruitment, adherence, and activation of inflammatory cells (Andersson et al. 1994). Histamine content is increased in preserved food and thus could play an important role in the precipitation of allergic symptoms, if too high histamine uptake can trigger allergy development. Moreover in vitro, an inhibitory effect of histamine on neopterin formation in myelomonocytic cells has been described (Gruber et al. 2000).
Also air pollution can be responsible for increasing allergy appearance. An important compound is carbon monoxide (CO), which accumulates in the blood or is inhaled during cigarette smoke. CO can downregulate Th1-type immune responses via inhibition of IFN-γ and inhibition of IDO activity and thus activate Th2-type immunity (Naito et al. 2012). An excess of antioxidants can explain the connection of obesity, smoking or pollution, and their association with the increase of allergies (Hosick and Stec 2012), when CO, a gas with well-known antioxidant properties, exerts its effect to counteract Th1-type immune activation. As another consequence, tryptophan availability will increase, when IDO is suppressed. In turn, the higher tryptophan and thus serotonin availability may enhance mood and thus support addiction to tobacco smoking.
3.6 Conclusion
Significant alterations of tryptophan metabolism have been described in patients suffering from allergy. Allergy development is characterized by Th2-type immune activation that is related to Th2-type cytokine expression like IL-4, IL-5, and IL-10 by Th2-type cells. The immoderate increase of allergies in the past decades posed the question of the underlying trigger. Various approaches were taken into consideration, like the hygiene hypothesis or the pollution of the air. Still the aspect of antioxidants and allergy development has to be investigated in more detail. The enormous presence of antioxidants as food additives, preservatives, or colorants is indispensable in our lifestyle. Antioxidants can inhibit Th1-type immune response and thus can result in an insufficient clearance of infectious pathogens. The inhibition can be mediated by downregulation of IFN-γ and/or by the inhibition of IDO leading to higher tryptophan levels. The radical scavenging property of antioxidants can stabilize BH4, the cofactor for iNOS, and thus promote high NO. output. The high NO. level inhibits IDO activity by binding to the catalytic domain. For the allergy diagnosis, high levels of NO. and tryptophan can be good biomarkers that may be of value for the judgment of treatment response. The excess of antioxidants can represent the missing link between the constant growing number of allergy and obesity patients. The downregulation of Th1-type immune response and expression of Th2-type cytokines can be attributed to the inhibiting nature of anti-inflammatory agents like antioxidants. The impression is emerging that in otherwise healthy people, stress due to overwhelming exposure to antioxidants is more relevant than oxidative stress, which is critical in clinical conditions related with Th1-type immunity and excess IFN-γ and thus ROS production. A right balance between cellular produced ROS and antioxidant uptake via nutrition is essential to support human health.
References
Aggarwal BB (2004) Nuclear factor-kappa B: the enemy within. Cancer Cell 6:203–208
Allen BW, Stamler JS, Piantadosi CA (2009) Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15:452–460
Andersson M, Greiff L, Svensson C (1994) Allergic rhinoconjunctivitis: the role of histamine. Mediators Inflamm 3:171–175
André C, Vatrinet C, Galvain S, Carat F, Sicard H (2000) Safety of sublingual-swallow immunotherapy in children and adults. Int Arch Allergy Immunol 121:229–234
Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43:521–531
Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172:2522–2529
Badawy AA (2013) Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol 27:878–893
Balakrishnan K, Adams LE (1995) The role of the lymphocyte in an immune response. Immunol Invest 24:233–244
Barth H, Berg PA, Klein R (2003) Methods for the in vitro determination of an individual disposition towards TH1- or TH2-reactivity by the application of appropriate stimulatory antigens. Clin Exp Immunol 134:78–85
Berger K, Strasser B, Fuschelberger R (2013) Effect of hypocaloric nutrition on inflammation markers and insulin sensitivity. Pteridines 24:128–129
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J Am Med Assoc 297:842–857
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A et al (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 63(Suppl 86):8–160
Bowler RP, Crapo JD (2002) Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol 110:349–356
Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352:1112–1120
Brown JM, Wilson TM, Metcalfe DD (2008) The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy 38:4–18
Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG (1990) Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345:346–347
Chen Y, Guillemin GJ (2009) Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res 2:1–19
Ciprandi G, Fuchs D (2013) Tryptophan, neopterin, and nitrite in allergy. Allergy 67:1083
Ciprandi G, Amici M, Tosca M, Fuchs D (2010) Tryptophan metabolism in allergic rhinitis: the effect of pollen allergen exposure. Hum Immunol 71:911–915
Ciprandi G, Tosca M, Fuchs D (2011a) Nitric oxide metabolites in allergic rhinitis: the effect of pollen allergen exposure. Allergol Immunopathol 39:326–329
Ciprandi G, De Amici M, Tosca M, Fuchs D, Marseglia G (2011b) Serotonin in allergic rhinitis: a possible role for behavioural symptoms. Iran J Allergy Asthma Immunol 10:183–188
De Rosa S, Cirillo P, Pacileo M, Petrillo G, D’Ascoli GL, Maresca F, Ziviello F, Chiariello M (2011) Neopterin: from forgotten biomarker to leading actor in cardiovascular pathophysiology. Curr Vasc Pharmacol 9:188–199
Ellwood P, Asher MI, García-Marcos L, Williams H, Keil U, Robertson C, Nagel G, ISAAC Phase III Study Group (2013) Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax 68:351–360
Fuchs D (2012) Antioxidant intake and allergic disease. Clin Exp Allergy 42:1420–1422
Fuchs D, Hausen A, Reibnegger G, Werner ER, Dierich MP, Wachter H (1988) Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today 9:150–155
Fuchs D, Möller AA, Reibnegger G, Stöckle E, Werner ER, Wachter H (1990) Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr 3:873–876
Fuchs D, Möller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, Wachter H (1991) Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 28:207–212
Fuchs D, Weiss G, Reibnegger G, Wachter H (1992) The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious and malignant diseases. Crit Rev Clin Lab Sci 29:307–341
Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra Sanchez L, Kaski JC (2009) The role of neopterin in atherogenesis and cardiovascular risk stratification. Curr Med Chem 16:4644–4653
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27:2783–2802
Gostner J, Ciardi C, Becker K, Fuchs D, Sucher R (2014) Immunoregulatory impact of food antioxidants. Curr Pharm Des 20(6):840–849
Gruber A, Murr C, Wirleitner B, Werner-Felmayer G, Fuchs D (2000) Histamine suppresses neopterin production in the human myelomonoctoma cell line THP-1. Immunol Lett 72:133–136
Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ (2007) Characterization of the kynurenine pathway in human neurons. J Neurosci 27:12884–12892
Halliwell B (1996) Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res 25:57–74
Halliwell B (1999) Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res 31:261–272
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C (2009) Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans 37:886–891
Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A (1992) Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115:1249–1273
Holt PG, Macaubas C, Stumbles PA, Sly PD (1999) The role of allergy in the development of asthma. Nature 402(6760 Suppl):B12–B17
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Hosick PA, Stec DE (2012) Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am J Physiol Regul Integr Comp Physiol 302:R207–R214
Isolauri E, Huurre A, Salminen S, Impivaara O (2004) The allergy epidemic extends beyond the past few decades. Clin Exp Allergy 34:1007–1010
Jenny M, Klieber M, Zaknun D, Schroecksnadel S, Kurz K, Ledochowski M, Schennach H, Fuchs D (2011) In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm Res 60:127–135
Juniper EF, Guyatt GH (1991) Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 21:77–83
Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA (2002) Immune regulation by histamine. Curr Opin Immunol 14:735–740
Klein C, Patte-Mensah C, Taleb O, Bourguignon JJ, Schmitt M, Bihel F, Maitre M, Mensah-Nyagan AG (2013) The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 70:254–260
Kositz C, Schroecksnadel K, Grander G, Schennach H, Kofler H, Fuchs D (2008) Serum tryptophan concentration in patients predicts outcome of specific immunotherapy with pollen extracts. Int Arch Allergy Immunol 147:35–40
Krämer U, Heinrich J, Wjst M, Wichmann HE (1999) Age of entry to day nursery and allergy in later childhood. Lancet 353:450–454
Kuo CH, Kuo HF, Huang CH, Yang SN, Lee MS, Hung CH (2013) Early life exposure to antibiotics and the risk of childhood allergic diseases: an update from the perspective of the hygiene hypothesis. J Microbiol Immunol Infect 46:320–329
Lindeman KS, Aryana A, Hirshman CA (1995) Direct effects of inhaled nitric oxide on canine peripheral airways. J Appl Physiol 78:1898–1903
Liu AH, Murphy JR (2003) Hygiene hypothesis: fact or fiction? J Allergy Clin Immunol 111:471–478
Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS (1988) Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27:8706–8711
McNeill E, Channon KM (2012) The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb Haemost 108:832–839
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774
Moncada S (1999) Nitric oxide: discovery and impact on clinical medicine. J R Soc Med 92:164–169
Mungan D, Misirligil Z, Gürbüz L (1999) Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite-sensitive patients with rhinitis and asthma–a placebo controlled study. Ann Allergy Asthma Immunol 82:485–490
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191–1193
Murr C, Fuith LC, Widner B, Wirleitner B, Baier-Bitterlich G, Fuchs D (1999) Increased neopterin concentrations in patients with cancer: indicator of oxidative stress? Anticancer Res 19:1721–1728
Murr C, Widner B, Wirleitner B, Fuchs D (2002) Neopterin as a marker for immune system activation. Curr Drug Metab 3:175–187
Murr C, Schroecksnadel K, Winkler C, Ledochowski M, Fuchs D (2005) Antioxidants may increase the probability of developing allergic diseases and asthma. Med Hypotheses 64:973–977
Murr C, Schroecksnadel K, Winklhofer-Roob BM, Mangge H, Böhm BO, Winkelmann BR, Maerz W, Fuchs D (2009) Inverse association between serum concentrations of neopterin and antioxidants in patients with and without angiographic coronary artery disease. Atherosclerosis 202:543–549
Naito Y, Uchiyama K, Takagi T, Yoshikawa T (2012) Therapeutic potential of carbon monoxide (CO) for intestinal inflammation. Curr Med Chem 19:70–76
Nathan CF (1986) Peroxide and pteridine: a hypothesis on the regulation of macrophage antimicrobial activity by interferon gamma. Interferon 7:125–143
Nathan CF, Murray HW, Wiebe ME, Rubin BY (1983) Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158:670–689
Pedersen ER, Midttun Ø, Ueland PM, Schartum-Hansen H, Seifert R, Igland J, Nordrehaug JE, Ebbing M, Svingen G, Bleie Ø, Berge R, Nygård O (2011) Systemic markers of interferon-?-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 31:698–704
Peternelj TT, Coombes JS (2011) Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med 41:1043–1069
Poljsak B, Milisav I (2012) The neglected significance of “antioxidative stress”. Oxid Med Cell Longev 2012:480895
Raitala A, Karjalainen J, Oja SS, Kosunen TU, Hurme M (2006) Indoleamine 2,3-dioxygenase (IDO) activity is lower in atopic than in non-atopic individuals and is enhanced by environmental factors protecting from atopy. Mol Immunol 43:1054–1056
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106:8665–8670
Romagnani S (2004) Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol 113:395–400
Romagnani S (2006) Regulation of the T cell response. Clin Exp Allergy 36:1357–1366
Samelson-Jones BJ, Yeh SR (2006) Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry 45:8527–8538
Saporta D (2012) Efficacy of sublingual immunotherapy versus subcutaneous injection immunotherapy in allergic patients. J Environ Public Health 2012:492405
Sas K, Robotka H, Toldi J, Vécsei L (2007) Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci 257:221–239
Schröcksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D (1996) Decreased plasma tryptophan in pregnancy. Obstet Gynecol 88:47–50
Schroecksnadel K, Winkler C, Fuith LC, Fuchs D (2005a) Tryptophan degradation in patients with gynecological cancer correlates with immune activation. Cancer Lett 223:323–329
Schroecksnadel K, Frick B, Winkler C, Wirleitner B, Schennach H, Fuchs D (2005b) Aspirin downregulates homocysteine formation in stimulated human peripheral blood mononuclear cells. Scand J Immunol 62:155–160
Schroecksnadel K, Wirleitner B, Winkler C, Fuchs D (2006) Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 364:82–90
Schroecksnadel K, Fischer B, Schennach H, Weiss G, Fuchs D (2007) Antioxidants down-regulate Th1-type immune response in vitro. Drug Metab Lett 1:166–171
Schroecksnadel S, Jenny M, Kurz K, Klein A, Ledochowski M, Ueberall F, Fuchs D (2010) LPS-induced NF-kappaB expression in THP-1Blue cells correlates with neopterin production and activity of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 399:642–646
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Stewart L, Katial RK (2012) Exhaled nitric oxide. Immunol Allergy Clin North Am 32:347–362
Strachan DP (1989) Hay fever, hygiene, and household size. Br Med J 299:1259–1260
Thomas SR, Mohr D, Stocker R (1994) Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem 269:14457–14464
Tsutsui M, Shimokawa H, Otsuji Y, Ueta Y, Sasaguri Y, Yanagihara N (2009) Nitric oxide synthases and cardiovascular diseases: insights from genetically modified mice. Circ J 73:986–993
Van Voorhis M, Fechner JH, Zhang X, Mezrich JD (2013) The aryl hydrocarbon receptor: a novel target for immunomodulation in organ transplantation. Transplantation 95:983–990
Villanueva C, Giulivi C (2010) Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med 49:307–316
von Bubnoff D, Bieber T (2012) The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy 67:718–725
Weiss G, Murr C, Zoller H, Haun M, Widner B, Ludescher C, Fuchs D (1999) Modulation of neopterin formation and tryptophan degradation by Th1- and Th2-derived cytokines in human monocytic cells. Clin Exp Immunol 116:435–440
Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, Pfleiderer W, Wachter H (1990) Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1 and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 265:3189–3192
Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger R, Yim JJ, Wachter H (1991) Biochemistry and function of pteridine synthesis in human and murine macrophages. Pathobiology 59:276–279
Widner B, Ledochowski M, Fuchs D (2000a) Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr Drug Metab 1:193–204
Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D (2000b) Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm 107:343–353
Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D (2002) Neopterin production tryptophan degradation and mental depression: what is the link? Brain Behav Immun 16:590–595
Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA (2011) Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol 89:873–891
Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, Trusinskis K, Fuchs D (2003) Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 33:550–554
Wurtman RJ, Wurtman JJ (1995) Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res 3(Suppl 4):477S–480S
Wüthrich B (2009) Food allergy, food intolerance or functional disorder? Prax (Bern 1994) 98:375–387
Yazdanbakhsh M, Kremsner PG, van Ree R (2002) Allergy, parasites, and the hygiene hypothesis. Science 296:490–494
Yu JJ, Gaffen SL (2008) Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 13:170–177
Zaknun D, Schroecksnadel S, Kurz K, Fuchs D (2012) Potential role of antioxidant food supplements, preservatives and colorants in the pathogenesis of allergy and asthma. Int Arch Allergy Immunol 157:113–124
Zhou L, Chong MM, Littman DR (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30:646–655
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Becker, K., Ciprandi, G., Gostner, J., Kofler, H., Fuchs, D. (2015). Tryptophan and Nitric Oxide in Allergy. In: Engin, A., Engin, A. (eds) Tryptophan Metabolism: Implications for Biological Processes, Health and Disease. Molecular and Integrative Toxicology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-15630-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-15630-9_3
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-15629-3
Online ISBN: 978-3-319-15630-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)