Abstract
Tryptophan catabolism, including kynurenine pathway, is known to be involved in immunoregulation and its metabolites play a role in immune modulation. In the context of allergic diseases, it has been proposed that tryptophan degradation pathway, rather than tryptophan deprivation itself, plays an important role in tolerance induction during allergen-specific immunotherapy, which is, by far, the only causal treatment of allergic diseases. In addition, it has recently been demonstrated that kynurenine and, perhaps, its metabolites can act through the aryl hydrocarbon receptor, a unique cellular chemical sensor, and regulate immune functions. The present review intends to highlight the recent development on the involvement of kynurenine pathway in the regulation of allergic diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Kynurenine
- Allergy
- Allergic diseases
- Mast cells
- Allergen-specific immunotherapy
- Immune tolerance
- Aryl hydrocarbon receptor
Introduction
Tryptophan (TRP), an essential amino acid, plays an important role in the protein synthesis and serves as a precursor of many biologically active substances, such as kynurenine (KYN). The degradation of TRP takes place in the liver, kidney, brain, and peripheral tissues via three biochemical pathways: KYN pathway, tryptamine pathway, and serotonin pathway (Fig. 8.1). The KYN pathway is the major route for TRP metabolism [1]. Of the dietary TRP that is not used in protein synthesis, approximately 99 % is metabolized by the KYN pathway, where TRP is catabolized by rate-limiting enzymes, TRP 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO), to N-formyl-KYN, which is then converted to KYN [2]. Under normal physiological conditions, the level of KYN is low but is upregulated in response to infection and Th1-mediated inflammation [3] through IFN-γ-induced expression of IDO. Increase in KYN mediated by IDO/TDO activation is also found in various disease states, such as autoimmune disorders and malignant diseases [4].
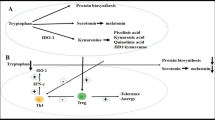
A schematic diagram depicting the tryptophan metabolic pathway and its metabolites that are suggested to be involved in the regulation of allergic diseases. Pathophysiological states of related enzymes and metabolites in allergic diseases and their functional impacts are summarized. Abbreviations: AR allergic rhinitis; AD atopic dermatitis; ICS inhaled corticosteroids; SIT allergen-specific immunotherapy; DCs dendritic cells; AhR aryl hydrocarbon receptor; 1-MT 1-methyl-l-tryptophan; TPH-1 tryptophan hydroxylase-1; 5-HT 5-hydroxytryptamine
While the role of TRP and its metabolites in immune regulation has been extensively investigated, their role in allergy has been less well defined. In recent years, a clear association has been made between TRP metabolism and immune or inflammatory responses in a vast array of disease states. These studies have focused mainly on the KYN pathway of TRP degradation occurring in the immune system, rather than in the serotonin pathway, and the importance of this pathway in allergic inflammation has recently been suggested; but, despite recent increasing interest in TRP metabolism in the field of allergy, particularly as it pertains to the tolerogenic properties of IDO or KYN metabolites in controlling allergic reactions, the biological significance of immune-related TRP breakdown remains still unclear. This review considers the current understanding of the role of the TRP metabolites, particularly those in the KYN pathway, in orchestrating and regulating the expression of allergic diseases.
Metabolites and Enzymes Along the KYN Pathwayin Allergic Diseases
There are limited numbers of studies examining the level of KYN and its metabolites or activity of IDO in patients with allergic diseases. In seasonal allergic rhinitis (AR), higher plasma KYN/TRP ratio in asymptomatic atopic individuals was found during seasonal allergen exposure compared to those in symptomatic patients with AR and normal subjects [5]. In addition, serum KYN concentration was significantly higher in patients with AR than in normal subjects and was also higher out of the pollen season than during the season [6]. These results suggest that enhanced IDO activity may be involved in the maintenance of clinical unresponsiveness. Furthermore, it has been reported that patients with AR seem to have constitutively elevated serum TRP concentrations [7], suggesting the activity of IDO is lower in symptomatic patients than in nonatopics or asymptomatic atopics. As a corollary, studies have shown that the expression of IDO was not increased in the nasal mucosa of patients with AR [8]. Therefore, the potential relationship between modified tryptophan metabolism and clinical responsiveness in AR need to be confirmed in further studies. Nonetheless, IDO seems to associate with chronic rhinosinusitis (CRS) [9].
CRS is known to be a refractory, versatile, and multifactorial immunological disease of nose and paranasal sinuses. CRS is characterized by increased local eosinophilia and a Th2 polarization with high levels of IL-5 and IgE or a Th1 polarization with high levels of IFN-γ and TGF-β, depending on patients with (CRSwNP) or without nasal polyps (CRSsNP), respectively. Luukkainen et al. found that the maxillary sinus mucosa from patients with CRSwNP, but not CRSsNP, showed a higher level of IDO expression in leukocytes but not in the epithelium when compared with normal subjects [10]. The findings of IDO expression in sinonasal biopsies were independent of AR, aspirin intolerance, asthma, smoking, use of intranasal or oral corticosteroids or antihistamines, previous operations, recurrence of polyps, sex, and age [10]. Thus, in the upper airways, IDO expression seems to associate with CRSwNP, but not with AR.
Regarding asthma, only limited studies have been performed in humans. It has been suggested that KYN/TRP ratio is decreased in sputum of asthmatics [11] and in cultured dendritic cells (DCs) from dust mite-sensitive patients with asthma compared to normal subjects [12]. It has also recently been reported in a prospective study that KYN concentration in exhaled breath condensates in stable mild asthmatics was lower at baseline level than that in normal subjects [13]. On the other hand, systemic levels of TRP and its metabolites, KYN, anthranilic acid (AA), and quinolinic acid (QA) were markedly higher in patients with allergic asthma and were associated with eosinophilic inflammation and symptom scores during experimental rhinovirus infection [13]. Thus, IDO expression appears to be associated with asthma.
But, in murine models of asthma, there have been inconsistent results with regard to the KYN’s function in either promoting or suppressing asthmatic responses. One earlier study showed that KYN elevation in the lung induced by a TLR9 ligand, synthetic immunostimulatory sequence-containing oligodeoxynucleotide (CpG-ODN) was able to inhibit Th2-driven pulmonary inflammation, which could be reversed by the addition of 1-methyl-tryptophan (1-MT), a pharmacological inhibitor of IDO [14], suggesting an immunosuppressive or tolerogenic effect of the KYN pathway. In contrast, utilizing IDO-deficient mouse models, it has been reported that IDO deficiency did not impair the induction of immune tolerance in Th2-driven pulmonary inflammation and that IDO-deficient mice displayed blunted Th2-driven airway inflammation and airway hyperresponsiveness [15], indicating a pro-inflammatory effect of IDO. The reasons for these discrepant results are currently unclear. Different models and stimuli used could be the reasons for observing differential outcomes. It has been cautioned that genetically modified mice might have developmental defects associated with the IDO deficiency from birth. Further investigations into these issues are needed to clarify these issues. In the case of atopic dermatitis (AD), the expression of IDO and kynureninase has been shown to be upregulated in the skin lesions as compared to the uninvolved skin of patients with AD [16]; also, their upregulated expression was noted in human-cultured epidermal keratinocytes and in the skin lesion of NC/Nga mice, which are considered to be an AD-like animal model [16].
Kynurenine Pathway in Monocytes, Eosinophils, and Mast Cells
Functional IDO has been detected in multiple cell types involved in allergic inflammation, including DCs [17], monocytes/macrophages [18], endothelial cells [19], fibroblasts [20], epithelial cells [21], and granulocytes [22]; but, IDO expression in mast cells or basophils has not been reported. In allergic individuals, the first contact with allergen is suggested to lead to IL-4- and IL-13-dependent production of allergen-specific IgE, with subsequent binding of these antibodies to the high-affinity receptor for IgE (FcεRI) on the surface of mast cells and basophils. Allergen reexposure results in cross-linkage of membrane-bound IgE and subsequent mediator release that induces typical immediate-type hypersensitivity reactions. FcεRI is constitutively expressed on mast cells and basophils but can also be detected on additional immune regulatory cell types, including antigen-presenting cells, of atopic individuals. In human monocytes, it has been reported that the enzymes along the KYN pathway, including IDO, were highly expressed in FcεRI-activated monocytes derived from atopics than those from nonatopics, and its expression is increased after cross-linkage of the receptors by IgE and anti-IgE [18]. These enhanced expression of IDO in monocytes from atopics lead to production of higher amount of KYN than those from nonatopics.
Eosinophil accumulation is a prominent feature of allergic inflammatory responses, such as those occurring in the lung of the allergic asthmatics [23]. Human eosinophils derived from atopic donors constitutively express IDO [22], and when treated with IL-5 in the presence of IFN-γ, they produce considerable amount of KYN. CD28 cross-linking also results in measurable KYN in culture supernatants and is inhibited by a neutralizing anti-IFN-γ antibody. Moreover, extensive infiltration of IDO-positive cells has been observed in the tissues from patients with asthma, where eosinophils are the prominent cell type expressing IDO.
Mast cells are known to be a critical cell type in the regulation of allergic responses and can be activated by a multitude of stimuli resulting in the release of inflammatory mediators and cytokines, contributing to various pathophysiological events in acute and chronic inflammation [24]. Considering the strategic location of mast cells at the site of tissue mucosa where exposure of TRP and its metabolites may occur, it is likely that KYN pathway metabolites, derived endogenously or from other cell types in the inflammatory microenvironment, may exert there in controlling the mast cell responses. Furthermore, it is still remain unsolved as to how KYN metabolites act as an immune-regulating mediator. One plausible mechanism may involve KYN serving as a ligand for aryl hydrocarbon receptor (AhR) [25]. AhR is constitutively expressed in mouse and human mast cells [26–28]. The initial report showed that KYN and kynurenic acid (KA) could affect degranulation and intracellular calcium signaling in murine mast cell lines, although the dependence of AhR and the underlying mechanisms were not investigated. We have recently demonstrated that KYN enhanced IgE-mediated mast cell responses, including degranulation, leukotriene C4 (LTC4) release, and IL-13 production in mouse bone marrow-derived cultured mast cells (BMMCs) through the activation of PLCγ, Akt, and MAPK p38 and the increase of intracellular calcium [29]. KYN also enhanced cutaneous anaphylaxis in vivo in a mouse model of passive cutaneous anaphylaxis (PCA). In addition, KYN had similar enhancing effects on human peripheral blood-derived cultured mat cells. It was noted that the effective concentration of KYN showing the enhancing effect on mast cells was around 50 μM, which could be a physiologically relevant concentration in the microenvironment at the site where inflammation occurs [25].
Metabolites in KYN Pathway Act via Aryl Hydrocarbon Receptor (AhR)
KYN’s effects on mast cells are dependent on AhR [29]. In our recent studies, the enhancing effects of KYN on IgE-mediated mast cell responses were not observed in AhR-deficient BMMCs and could be inhibited by an AhR antagonist. KYN’s enhancing effects on human-cultured mast cells could be inhibited by an AhR antagonist as well. On the other hand, KA has been proposed as a potential endogenous AhR ligand in mouse and human hepatocytes [30], although its binding activity for human AhR is 100-fold higher than that for mouse AhR. However, both KA and QA did not show any additive and synergistic effects directly in mast cells when treated with KYN [29]. Thus, KA seems not to be associated with allergic responses in mast cells. Interestingly, in the study by Maaetoft-Udsen et al., KA was suggested to be an AhR ligand and affected mast cell responses [26], but its stimulatory effect appeared to be AhR independent or through a secondary activating mechanism. Indeed, KA has been reported to be a ligand for GPR35 [31], a G-protein-coupled receptor expressed in a variety of tissues, including mast cells, which was significantly upregulated when mast cells were exposed to IgE antibodies [32]. Further, cromolyn disodium and the second-generation nedocromil sodium, known as mast cell stabilizers, have been recently reported as ligands for GPR35 [33]. But, the underlying mechanism through which mast cell degranulation is inhibited remains unclear. This suggests the possible existence of a sequential event originating from the AhR-KYN axis and its subsequent activation of the GPR35-KA axis in regulating mast cell functions. Further detailed studies are clearly needed.
Role of KYN Pathway on Allergen-Specific Immunotherapy and Induction of Immune Tolerance in Allergic Diseases
The concept of allergen-specific immunotherapy (SIT) was first introduced 100 years ago [34]. SIT is the only available causative treatment of allergic diseases that induces a number of allergen-specific immunological changes. IDO seems to play a role in the induction of SIT. It has been reported that tolerance induction against allergens is partially mediated by activation of the KYN pathway during allergen-specific sublingual immunotherapy [35, 36]. TRP administered during SIT does not inhibit the effect of SIT, and pharmacological inhibition of IDO with 1-MT does not impair allergic inflammation during the challenge [37], suggesting the likely involvement of the KYN pathway metabolites rather than TRP deprivation itself in the induction of tolerance.
There is increasing evidence that IDO could be an important modulator of immune tolerance in a variety of immune responses. At the cellular level, IDO expression in DCs is important in inducing T-cell tolerance, as well as through direct effects on T cells or through effects of IDO on the DCs themselves. The mechanism by which IDO expression in DCs induces T-cell tolerance is hypothesized to be due to either the induction of T-cell anergy [38], the induction of T-cell apoptosis [39], the deviation of the immune response (Th1/Th2) [3], or the induction of regulatory T cells/suppression of Th17 cells [40].
A recent report, in the setting of allergen-specific immunotherapy, suggested that KYN pathway metabolites were able to downregulate allergic responses by potentiating tolerance induction in a mouse model of asthma [37]. KYN, 3-hydroxy-KYN and xanthurenic acid (XA), but not KA, QA and 3-hydroxy-AA, enhanced the efficacy of suboptimal immunotherapy with allergen. Thus, certain KYN pathway metabolites particularly KYN might potentiate immune tolerance during allergic responses, with anti-allergic properties. Therefore, it is likely that KYN might contribute to potentiate both pro-allergic (enhancing mast cell functions) and anti-allergic responses (promoting immune tolerance) depending on the timing of exposure to KYN and the types of immune cells during the progression of allergic diseases.
Serotonin Pathway in Allergy
Only 1 % of ingested TRP is converted into serotonin as mentioned above [2], whereas the majority of TRP is subject to degradation via the KYN pathway. However, it has been suggested that the KYN- and serotonin-metabolic pathway compete for their mutual precursor, TRP [41]. It has recently been reported that serotonin pathway in platelets also contribute to allergic inflammation [42]. It has long been recognized that serotonin may play an important role in the pathogenesis of allergic asthma. Elevated plasma serotonin level was found in symptomatic patients with asthma, correlating with clinical severity and pulmonary function [43]. Furthermore, pharmacologic blockade of its receptors was shown to attenuate the development of allergic airway inflammation and remodeling in mice [44]. TRP hydroxylase (THP)-1 is a critical enzyme for the biosynthesis of serotonin outside of the central nervous system. Dürk et al. have reported that platelets, rather than mast cells, were the main source of serotonin released during an allergic inflammation by utilizing THP-1-deficient and mast cell-deficient mice and pharmacological approaches [42]. These results are consistent with several earlier clinical observations reporting platelet activation accompanying allergic asthmatic responses [45, 46].
Conclusion
Recent studies have made a significant progress in our understanding of the immunoregulatory properties of TRP metabolites, particularly in the context of allergic diseases. Recent advancement in elucidating the AhR-KYN and GPR35-KA axis in immune regulation provides a novel and promising regulatory mechanism supporting the importance of KYN’s metabolic pathway in the pathophysiology of allergy and other diseases as well. However, the knowledge accumulated thus far has revealed a more diverse and complex network of regulation and function than we had previously recognized. Future research might benefit greatly from increasing attention on the metabolic regulations of these two receptor-ligand axis and their functional consequences, as well as of the functional link between KYN and serotonin pathways.
Abbreviations
- 1-MT:
-
1-Methyl-l-tryptophan
- AA:
-
Anthranilic acid
- AD:
-
Atopic dermatitis
- AhR:
-
Aryl hydrocarbon receptor
- AR:
-
Allergic rhinitis
- BMMCs:
-
Bone marrow-derived mast cells
- CRSsNP:
-
Chronic rhinosinusitis without nasal polyps
- CRSwNP:
-
Chronic rhinosinusitis with nasal polyps
- DCs:
-
Dendritic cells
- FcεRI:
-
High-affinity receptor for IgE
- ICS:
-
Inhaled corticosteroids
- IDO:
-
Indoleamine 2,3-dioxygenase
- KA:
-
Kynurenic acid
- KYN:
-
Kynurenine
- QA:
-
Quinolinic acid
- SIT:
-
Allergen-specific immunotherapy
- TDO:
-
Tryptophan 2,3-dioxygenase
- TPH-1:
-
Tryptophan hydroxylase-1
- TRP:
-
Tryptophan
- XA:
-
Xanthurenic acid
References
Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. 1991;294:345–58.
Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21(12):1239–49. doi:10.1111/j.1365-2982.2009.01370.x.
Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121(1):1–6. doi:10.1016/j.imlet.2008.08.008.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–43. doi:10.1016/j.it.2012.10.001.
von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin Exp Allergy. 2004;34(7):1056–63. doi:10.1111/j.1365-2222.2004.01984.x.
Ciprandi G, De Amici M, Tosca M, Fuchs D. Tryptophan metabolism in allergic rhinitis: the effect of pollen allergen exposure. Hum Immunol. 2010;71(9):911–15. doi:10.1016/j.humimm.2010.05.017.
Kositz C, Schroecksnadel K, Grander G, Schennach H, Kofler H, Fuchs D. High serum tryptophan concentration in pollinosis patients is associated with unresponsiveness to pollen extract therapy. Int Arch Allergy Immunol. 2008;147(1):35–40. doi:10.1159/000128584.
Luukkainen A, Karjalainen J, Honkanen T, Lehtonen M, Paavonen T, Toppila-Salmi S. Indoleamine 2,3-dioxygenase expression in patients with allergic rhinitis: a case-control study. Clin Transl Allergy. 2011;1(1):17. doi:10.1186/2045-7022-1-17.
Luukkainen A, Toppila-Salmi S. Indoleamine 2,3-dioxygenase expression is associated with chronic rhinosinusitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2013;13(1):37–44. doi:10.1097/ACI.0b013e32835b350e.
Honkanen T, Luukkainen A, Lehtonen M, Paavonen T, Karjalainen J, Hurme M, et al. Indoleamine 2,3-dioxygenase expression is associated with chronic rhinosinusitis with nasal polyps and antrochoanal polyps. Rhinology. 2011;49(3):356–63. doi:10.4193/Rhino10.191.
Maneechotesuwan K, Supawita S, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Sputum indoleamine-2,3-dioxygenase activity is increased in asthmatic airways by using inhaled corticosteroids. J Allergy Clin Immunol. 2008;121(1):43–50. doi:10.1016/j.jaci.2007.10.011.
Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S, Yaikwawong M, Barnes PJ, et al. Der p 1 suppresses indoleamine 2,3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma. J Allergy Clin Immunol. 2009;123(1):239–48. doi:10.1016/j.jaci.2008.10.018.
van der Sluijs KF, van de Pol MA, Kulik W, Dijkhuis A, Smids BS, van Eijk HW, et al. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus-induced asthma exacerbation: a prospective study with a parallel-group design. Thorax. 2013;68(12):1122–30. doi:10.1136/thoraxjnl-2013-203728.
Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114(2):270–9. doi:10.1172/jci21275.
Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105(18):6690–5. doi:10.1073/pnas.0708809105.
Ito M, Ogawa K, Takeuchi K, Nakada A, Heishi M, Suto H, et al. Gene expression of enzymes for tryptophan degradation pathway is upregulated in the skin lesions of patients with atopic dermatitis or psoriasis. J Dermatol Sci. 2004;36(3):157–64. doi:10.1016/j.jdermsci.2004.08.012.
Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14(1):65–8.
von Bubnoff D, Matz H, Frahnert C, Rao ML, Hanau D, de la Salle H, et al. FcepsilonRI induces the tryptophan degradation pathway involved in regulating T cell responses. J Immunol. 2002;169(4):1810–16.
Daubener W, Spors B, Hucke C, Adam R, Stins M, Kim KS, et al. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect Immun. 2001;69(10):6527–31. doi:10.1128/iai.69.10.6527-6531.2001.
Dai W, Gupta SL. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J Biol Chem. 1990;265(32):19871–7.
Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162(2):957–64.
Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173(10):5909–13.
Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113(1):3–8. doi:10.1016/j.anai.2014.04.002.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi:10.1038/nm.2755.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi:10.1038/nature10491.
Maaetoft-Udsen K, Shimoda LM, Frokiaer H, Turner H. Aryl hydrocarbon receptor ligand effects in RBL2H3 cells. J Immunotoxicol. 2012;9(3):327–37. doi:10.3109/1547691x.2012.661802.
Sibilano R, Frossi B, Calvaruso M, Danelli L, Betto E, Dall’Agnese A, et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol. 2012;189(1):120–7. doi:10.4049/jimmunol.1200009.
Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121(16):3195–204. doi:10.1182/blood-2012-08-453597.
Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 2014;69(4):445–52. doi:10.1111/all.12346.
DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. doi:10.1093/toxsci/kfq024.
Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281(31):22021–8. doi:10.1074/jbc.M603503200.
Yang Y, Lu JY, Wu X, Summer S, Whoriskey J, Saris C, et al. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology. 2010;86(1):1–5. doi:10.1159/000314164.
MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, Hudson BD, et al. The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35. Mol Pharmacol. 2014;85(1):91–104. doi:10.1124/mol.113.089482.
Noon L. Prophylactic inoculation against hay fever. The Lancet. 1911;177(4580):1572–3. doi:10.1016/S0140-6736(00)78276-6.
Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61(2):151–65. doi:10.1111/j.1398-9995.2006.01002.x.
Kofler H, Kurz K, Grander G, Fuchs D. Specific immunotherapy normalizes tryptophan concentrations in patients with allergic rhinitis. Int Arch Allergy Immunol. 2012;159(4):416–21. doi:10.1159/000338937.
Taher YA, Piavaux BJ, Gras R, van Esch BC, Hofman GA, Bloksma N et al. Indoleamine 2,3-dioxygenase-dependent tryptophan metabolites contribute to tolerance induction during allergen immunotherapy in a mouse model. J Allergy Clin Immunol. 2008;121(4):983–91.e2. doi:10.1016/j.jaci.2007.11.021.
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–42. doi:10.1016/j.immuni.2005.03.013.
Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–77. doi:10.1038/sj.cdd.4401073.
Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra6. doi:10.1126/scitranslmed.3000632.
Keszthelyi D, Troost FJ, Jonkers DM, van Donkelaar EL, Dekker J, Buurman WA, et al. Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine? Am J Clin Nutr. 2012;95(3):603–8. doi:10.3945/ajcn.111.028589.
Durk T, Duerschmied D, Muller T, Grimm M, Reuter S, Vieira RP, et al. Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am J Respir Crit Care Med. 2013;187(5):476–85. doi:10.1164/rccm.201208-1440OC.
Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol. 1996;77(3):245–53. doi:10.1016/s1081-1206(10)63263-2.
Lima C, Souza VM, Soares AL, Macedo MS, Tavares-de-Lima W, Vargaftig BB. Interference of methysergide, a specific 5-hydroxytryptamine receptor antagonist, with airway chronic allergic inflammation and remodelling in a murine model of asthma. Clin Exp Allergy. 2007;37(5):723–34. doi:10.1111/j.1365-2222.2007.02700.x.
Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Rose MJ, et al. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol. 2003;112(1):109–18.
Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Lukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy. 2006;36(4):426–32. doi:10.1111/j.1365-2222.2006.02446.x.
Acknowledgments
This work was supported, in part, by grants from the National Health Research Institutes, Taiwan (NHRI-100A1-PDCO-03000001, NHRI-101A1-PDCO-03010201, NHRI-102A1-PDCO-03010201) and Ministry of Health, Taiwan (EODOH01).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kawasaki, H., Huang, SK. (2015). Role of Kynurenine Pathway in Allergy. In: Mittal, S. (eds) Targeting the Broadly Pathogenic Kynurenine Pathway. Springer, Cham. https://doi.org/10.1007/978-3-319-11870-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-11870-3_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11869-7
Online ISBN: 978-3-319-11870-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)