Abstract
In order to evaluate the resistance to salinity as a factor enhancing freshwater invasiveness, we assessed the tolerance of the mussel Limnoperna fortunei to salinity conditions mimicking changes in an estuary. We tested mussel mortality in 30-day exposures to constant and fluctuating salinities at different temperatures in the laboratory. Test conditions simulated different seasons of the year and locations with increasing influence of marine waters in Río de la Plata, Argentina. Significant mortality (31 % after 30 days) was observed at a constant salinity of 2 ‰, increasing to 45 and 57 % at 5 and 10 ‰, respectively. In contrast, considerably greater tolerances were observed when conditions in the experimental chamber fluctuated between salt water and fresh water. No significant mortality was observed in mussels exposed to a salinity cycle with abrupt salinity changes ranging 1–23 ‰ (mean 2.68 ‰) over a month. Tolerance to this type of regime was unaffected by different temperatures within ambient ranges. Tests at constant salinity underestimate the tolerance of this and probably other freshwater nonindigenous species (NIS) to short-term saltwater exposures. Estuarine ports account for ca. 2/3 of non-marine ports globally, thus constituting donor and recipient hotspots for the spread of NIS propagules into continental aquatic ecosystems via shipping vectors. The tolerance of L. fortunei to estuarine conditions likely contributes to the species’ remarkable invasive success. These results highlight the need to determine causes of invasiveness and to study NIS traits not alone but in combination with transport network properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the aim of building capacity to forecast and prevent new invasions, considerable effort has been devoted to elucidating the physiological and life history traits that confer invasiveness to organisms (e.g., Devin and Beisel 2007; Karatayev et al. 2009; Van Kleunen et al. 2010; Hui et al. 2011; Zalewski et al. 2011). While these surveys provided valuable insight into traits related to invasiveness, the interpretation of results is often complicated by lack of adequate information on physiology and life history, differences between terrestrial and aquatic habitats, and the confounding effect of variation in propagule pressure (Colautti et al. 2006; Gordon and Gantz 2011).
The golden mussel, Limnoperna fortunei (Dunker 1857), is a highly invasive mussel native to rivers and lakes in Southeast Asia (Ricciardi 1998). Within a few decades, this species has been unintentionally introduced to over half a dozen countries across Asia and South America (Ricciardi 1998; Boltovskoy et al. 2006). L. fortunei is a fouling species for which a considerable number of economic and ecological impacts, comparable to those described for the zebra mussel Dreissena polymorpha (Pallas 1771) in Europe and North America, have been reported, such as fouling of industrial facilities and boat hulls, competition with other filter-feeding organisms, overgrowth of native bivalves, promotion of Microcystis blooms, changes in benthic communities, and nutrient cycling (Boltovskoy et al. 2009a; Karatayev et al. 2010; Cataldo et al. 2012). Given its tolerance to a wide range of environmental conditions (e.g., high temperature, pollution levels, low pH, calcium, and dissolved oxygen), further spread of L. fortunei into other continents is expected (Boltovskoy et al. 2006; Oliveira et al. 2010). Environmental tolerance has frequently been linked to invasiveness, and many studies have focused on the environmental and community interactions that come into play after a species has been released in a new habitat. Surprisingly, the implications of environmental tolerance for transport, an earlier stage in the invasion process, have been largely ignored (Colautti and MacIsaac 2004; but see Bailey et al. 2004; Ellis and MacIsaac 2009; Briski et al. 2011). Determining the causes favouring frequent transport of propagules into new habitats is needed for understanding the drivers of invasive success and designing more efficient prevention protocols, particularly for high profile invaders such as L. fortunei.
A large number of works have examined the salinity tolerance of nuisance NIS seeking to predict their potential introduced distributions (e.g., Strayer and Smith 1993; Wilcox and Dietz 1998). Some authors have further looked into the tolerance of NIS to salinity conditions in ballast water tanks vectoring the immense majority of aquatic introductions, including L. fortunei (Ellis and MacIsaac 2009). Managing salinity in tanks by means of mandatory ballast water mid-ocean exchange and brine addition in destination ports currently represents the most effective way to prevent ballast water-mediated introductions (Bradie et al. 2010; Bailey et al. 2011). In contrast, few studies have investigated NIS tolerance to ambient conditions in propagule pickup and delivery hotspot locations such as estuaries (Ruiz et al. 1997). Because a large number of potential donor ports are located in transition areas from freshwater to marine environments where salinity values vary widely over short periods of time, we contend that survival in variable salinity conditions, rather than tolerance to high constant salinity levels per se, may confer an invasion advantage to many aquatic species. However, because investigations aimed at forecasting NIS potential distribution ranges usually focus on environmental match, rather than on introduction and transport-related mechanisms, studies of tolerance to variable salinity levels (e.g., Wilcox and Dietz 1998) strongly lag behind those of survival in constant salinity conditions (e.g., Deaton et al. 1989; Berezina 2003; Byrne and Dietz 2006).
Here we examine the tolerance of L. fortunei to a salinity cycle typical of an estuarine regime, a trait that may significantly enhance the invader’s area of introduction and, consequently, its propagule pressure from transportation hubs. The primary objectives of this work are to: (1) assess the tolerance of L. fortunei to constant and fluctuating salinity conditions; (2) determine the effect of the thermal regime on salinity tolerance; (3) interpret the mechanisms responsible for the animal’s salinity tolerance; and (4) evaluate the role of this tolerance in increasing invasiveness.
Methods
We assessed the effects of salinity on mortality of L. fortunei mussels collected from Río de la Plata near the city of Buenos Aires (Fig. 1). The site where animals were collected has permanently freshwater conditions, and hence they had no prior history of exposure to saline waters. Within 24 h of collection, mussels were transported to the laboratory and stored in tanks containing aerated, dechlorinated tap water at room temperature for a week to acclimate specimens. Acclimation to experimental temperatures (see below) was performed at 1 °C day−1.
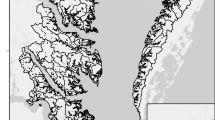
Mean November–March salinity fields (‰) at 0 and 10 m depth during normal Río de la Plata discharge conditions (between 17,370 and 28,000 m3 s−1; the estuary is dominated by higher salinities at lower discharge volumes). No isohalines below 22.5 ‰ are indicated in bottom panel because farther into the estuary bottom depths are below 10 m. Average values for the area where L. fortunei was recorded are, 0 m: 4–7 ‰, 10 m: 19–23 ‰ (bottom depths of the actual sites where L. fortunei was recorded are between 3 and 4 m, for which reason their average salinities are most probably somewhere between the above values). Salinity figures are based on historical 1911–2003 data from Guerrero et al. (2010). Presence data for L. fortunei after Giberto and Sardiña (2009)
We conducted 30-day exposure experiments to both fixed and fluctuating salinity at two temperatures in the laboratory. In all the experiments (see below), we used 90 adult (valve length 5–27 mm) mussels equally divided among three containers for each salinity and temperature tested. Experiments were conducted under controlled temperature conditions (Fig. 2). Mussels were checked for mortality every 5–59 h (below) and dead individuals (as indicated by opened valves and lack of response to tactile stimuli) were recorded and removed. The endpoint was either 100 % mortality or termination of the experiment (30 days). Saltwater was produced from commercially available sea salts (Tetra Marine salt Pro) dissolved in dechlorinated tap water. Dechlorinated tap water was used for freshwater controls. Salinity changes in the saltwater tests were accomplished by replacing the water in the experimental containers with water at the new salinity. Fresh water was renewed in the controls simultaneously with each saltwater change. Temperature was monitored throughout the experiments at 30-min intervals using autonomous programmable temperature data loggers in each experimental vessel.
Scheme of experimental setup employed for 30-day mortality experiments conducted on L. fortunei mussels at three fluctuating salinities (salinity cycles), and freshwater controls under controlled laboratory conditions. See “Methods”, Fig. 3, and Online Resource 1 for a detailed explanation of the salinity conditions in the fluctuating salinity experiments. Each container had 30 mussels (overall N = 360). Containers were distributed randomly in the bath, the spatial distribution shown being merely illustrative
We conducted three different mortality experiments. The first experiment was carried out between 4 June and 3 July 2010 at constant temperature (17 ± 1 °C), three fixed salinity concentrations: 2, 5, and 10 ‰, and a freshwater control. The objective of this trial was to explore L. fortunei’s tolerance to constant salinity at average ambient temperature (the water temperature in the lower Paraná-inner Río de la Plata estuary varies seasonally between ca. 11 and 28 °C).
With the aim of exploring the species’ tolerance to conditions mimicking those in the estuary, we conducted a second experiment (1 August to 1 September 2010) at changing salinity concentrations. For this purpose, we built a fluctuating salinity cycle using actual in situ salinity profiles obtained from data collected between 1 December 2009 and 31 May 2010 by the FREPLATA project buoy moored in the Río de la Plata estuary off Montevideo (35.193°S, 56.397°W), slightly eastward from the most seaward records of L. fortunei (Giberto and Sardiña 2009; Figs. 1, 2). The buoy’s sensors provide an hourly record of several parameters, including temperature and salinity (in Practical Salinity Units, or PSU, which in practice is identical to parts per thousand, or ‰; thus, for the sake of clarity throughout this work we use the latter unit). Data were recorded at a depth of 2 m (the bottom depth at the site of the mooring is 7 m). From this series, we extracted the values and frequencies of salinity peaks and troughs, and the length and frequency of the intervals between salinity shifts. These values were randomized and an experimental 30-day schedule was established where the frequency and duration of the different salinity levels followed those of the actual record (Fig. 3). Thus, we defined a salinity regime, herein referred to as “salinity cycle 0”, with a range of 1–23 ‰, mean time-weighted salinity throughout the 30-day experiment of 2.7 ‰, and a mean interval between salinity changes of 13.3 h (maximum: 54 h, minimum: 3 h; see details in Online Resource 1) that mimicked both the values and the timing of salinity changes in the estuary. On the basis of this salinity schedule, we established two other experimental regimes simulating sites progressively more influenced by marine waters. The first, “salinity cycle +3”, had all salinities 3 ‰ higher than salinity cycle 0, with a range of 4–26 ‰ and a time-weighted mean of 5.7 ‰. The second, “salinity cycle +6”, had all salinities 6 ‰ higher than salinity cycle 0, with a range of 7–29 ‰ and a time-weighted mean of 8.7 ‰. To simulate a spring-to-summer transition, when reproductive activity of the mussel peaks (Boltovskoy et al. 2009b), temperature in the experimental containers was raised gradually from 17 ± 1 to 21 ± 1 °C over the course of this experiment.
The third experiment (19 October to 19 November 2010) was aimed at examining the influence of winter and summer temperature extremes in the Río de la Plata estuary on mortality due to salt water. Because low mortality in the previous test was limited to salinities above those of the low salinity cycle (salinity cycle 0), but below those of the mid salinity cycle (salinity cycle +3; see “Results”), this experiment was conducted using salinity cycle 0 + 1.5 ‰ (“salinity cycle +1.5”; range: 2.5–24.5 ‰, time-weighted mean: 4.18 ‰) at 11 and 28 °C. Mortality in saltwater was compared with that in freshwater controls for the two temperatures tested.
Data analysis
In all experiments, mortality differences were examined using an Analysis of Variance (ANOVA) and post hoc Tukey’s tests on the proportion of mussels that remained alive in each container at the end of the experiment. Where necessary, we used arcsin(x1/2) transformation of the variable to meet the statistical assumptions. Descriptive survival curves (Fig. 4) were constructed using individual mussel data. A significance level of α = 0.05 was used for all statistical analyses.
Survival of L. fortunei in constant salinity (a), and fluctuating salinity at 17–21 °C (b), 11 °C, and 28 °C (c). See “Methods”, Fig. 3, and Online Resource 1 for a detailed explanation of the salinity conditions in the fluctuating salinity experiments. Curves with different letters denote significantly different survival rates
Results
Mortality in freshwater controls was always <10 % (Fig. 4). In all the experiments, L. fortunei’s mortality increased with increasing average salinity (ANOVA, P < 0.05; Fig. 4).
All constant salinity concentrations tested yielded significantly higher mussel mortalities than fresh water (Tukey’s test, P < 0.05). At the end of the 30-day exposures, between 31 % (at 2 ‰) and 57 % (at 10 ‰) of the animals died. In all cases there was no indication of a decreasing mortality rate with time, suggesting that maintenance of a viable population under these conditions was not feasible (Fig. 4a). At 10 ‰ mortalities were higher, albeit not significantly so, than at 5 ‰ (Tukey’s test, P = 0.417).
Mortality rates in exposures to fluctuating salinities at 17–21 °C were dependent on the range of values used. At the lowest salinities tested (salinity cycle 0) mortalities were only slightly higher than in the control, and did not differ significantly from the latter (Tukey’s test, P = 0.196; Fig. 4b). The overall mortality trend was similar to that in fresh water, suggesting that a sizable proportion of the animals could tolerate these conditions for extended periods. On the other hand, raising all salinity values by 3 and 6 ‰ yielded mortalities significantly higher than those in the control (Tukey’s test, P < 0.01; Fig. 4b). The continued increase in mortality was sustained until termination of the experiment.
At 11 and 28 °C mortality of mussels exposed for 30 day to the salinity cycle +1.5 was significantly higher than in fresh water (ANOVA, P < 0.01), with gradual but sustained declines especially after day 20 (Fig. 4c). Water temperature did not affect mortality rates (ANOVA, P = 0.111; Fig. 4c).
Discussion
This is the first experimental assessment of the long-term survival of a freshwater invasive bivalve in fluctuating salinity conditions. Our results indicate that tolerance to salinity extremes by L. fortunei is considerably higher than anticipated based on earlier studies of this and other closely related mussels (Table 1). Ours is likely a conservative estimate of the actual tolerance of L. fortunei to saline waters, since in our experiments salinity changes were instantaneous, while in the wild mussels benefit from short-term (minutes to hours) acclimation periods between salinity shifts (Fig. 3).
Tolerance to constant versus variable salinity conditions
Although L. fortunei is considered a freshwater animal (Brandt 1974; Morton 1977), it is common in estuarine habitats owing to its considerable tolerance to high salinity concentrations (Huang et al. 1981; Table 1). Our results indicate that ability of L. fortunei to survive in the middle section of the Río de la Plata estuary, periodically influenced by salinities in excess of 20 ‰ (Figs. 1, 3), is not due to its tolerance to high, constant salinities, but to its capacity to endure saline pulses of up to 23 ‰ for periods of up to several hours. The physiological tolerance and distribution potentials of invasive molluscs are underestimated when taking chronic tolerance values as indicators of their ability to withstand saline waters. Indeed, judging from previous studies (Angonesi et al. 2008; Barbosa and Melo 2009) and our own results at constant salinity concentrations (Fig. 4a), L. fortunei should be unable to maintain a viable population in mixohaline habitats where it actually occurs (Fig. 1). Another likely source of misjudgement on the distribution of freshwater species is the assumption that isohalines based on synoptic surveys can be used for defining their geographic spread in mixohaline environments. Our results show that although L. fortunei cannot maintain a viable population at constant salinities above 2–3 ‰ (Fig. 4a), in the Río de la Plata estuary it lives in areas beyond the 5 ‰ isohaline (Fig. 1).
The tolerance of L. fortunei to constant salinity (<2 ‰) is substantially lower than that reported for invasive dreissenid mussels, known to inhabit marshes, coastal lagoons, canals, and inland lakes at salinities of up to 6 ‰ (D. polymorpha), 14 ‰ (D. polymorpha andrusovi), 17.6 ‰ (D. polymorpha aralensis), and 18.4 ‰ (D. polymorpha obtusecarinata, probably extinct) (Table 1). Higher tolerance ranges have been obtained for mussels in laboratory studies when salinity changes take place gradually (Kilgour et al. 1994; Dietz et al. 1996). Deaton et al. (1989) reported low mortality of L. fortunei at constant salinities of up to 7 ‰ preceded by periods of acclimation at intermediate salinities. This result likely represents an exception where unusually high tolerances might be the result of acclimation and the use of mussels that had survived successive stepwise salinity increases thus reflecting the behaviour of a selection of the most resistant individuals (Deaton et al. 1989; Table 1). We anticipate that, unlike some other freshwater invasive mussels, L. fortunei will most probably be excluded from habitats dominated by permanently high salinities (Table 1; Fig. 4a).
Tolerance to sudden salinity fluctuations is a trait likely shared by other invasive mussels, such as Mytella charruana, a South American brackish water mussel introduced to coastal and estuarine habitats of the southern US (Yuan et al. 2010; Table 1). Surprisingly, the abundant literature on the zebra and quagga (D. rostriformis bugensis) mussels has rarely focused on their tolerance to intermittent salinity (but see Casper 2007). Yet evidence for it is provided by recent laboratory experiments reporting unexpectedly low mortality rates of zebra and quagga mussels exposed to a salinity peak of 30 ‰ for 5 h (Ellis and MacIsaac 2009). Field and laboratory studies have found excellent survival of zebra mussels exposed to diurnal cycles of salinity between 0.45 and 5 ‰ (Walton 1996; Wilcox and Dietz 1998). Unfortunately, tolerance to wider fluctuations has not been tested. These results suggest that very significant differences in the tolerance to constant versus variable salinity are not exclusive to L. fortunei (Table 1).
Salinity tolerance and temperature
Osmotic balance in mussels is maintained using free amino acids and inorganic ions (Deaton et al. 1989; Dietz et al. 1996). Similar to other physiological processes, L. fortunei’s resistance to high salinities was expected to be influenced by temperature. Surprisingly, we did not find any differences between salinity tolerances at quite different temperatures (11 and 28 °C). A possible explanation for this might be that our observations lie at the two tails of a Gaussian tolerance curve. Salinity exposure experiments have shown that mortality of D. polymorpha is lowest at 10–12 °C, increasing below 4 °C and above 18 °C (Kilgour et al. 1994). The salinity tolerance of L. fortunei may change with temperature, with a maximum tolerance somewhere between 11 and 28 °C. Alternatively, our data might simply indicate the lack of any influence of temperature on salinity resistance of L. fortunei over that temperature range (which represents the yearly range of environmental conditions in the Río de la Plata estuary).
Tolerance mechanisms
We hypothesize that valve gaping is the main mechanism by which L. fortunei can withstand swift salinity changes. The use of valve closure to prevent exposure to harmful environmental conditions is well known for bivalves (Jørgensen 1990). Toxicity experiments have shown that, by closing its valves, L. fortunei can survive several weeks of continuous exposure to concentrations of up to 100 mg L−1 of chlorine (Cataldo et al. 2003). Upon cessation of the adverse conditions, mussels open and reassume their normal activity (Jørgensen 1990; Cataldo et al. 2003). Valve closure is so effective in preventing exposure to the environment that, in long-term experiments, mussels exposed to toxicants are affected by starvation and debilitation of byssal attachment as much as by the toxicant (Cataldo et al. 2003; Rajagopal et al. 2003).
Tolerance to variable salinity conditions and invasiveness
The ability to survive in estuaries has very important implications for the invasion ecology of aquatic ecosystems. In the first place, it involves an increase in the geographical range where tolerant species can be delivered and picked up by transport vectors. Most importantly, the habitats involved in this areal range increase are particularly significant in terms of human-mediated species dispersion, because they host a large number of ports. Several workers have recently modelled the spread of aquatic NIS in terms of a network of ports functioning as transport hubs connected by shipping vectors (vessel ballast water and hull fouling) (e.g., Drake and Lodge 2004; Floerl et al. 2009). Transport hubs play a key stepping-stone role in the spread of biological invasions providing species that can make use of a greater number of hubs with increased transport opportunities (Muirhead and MacIsaac 2005; Floerl et al. 2009). According to a broad classification of ports based on their salinity and distance to the ocean, estuarine areas include about 20 % of the ports worldwide (Keller et al. 2011). Considering that freshwater ports barely amount to about 10 % of the total, tolerance to estuarine conditions potentially triples the number of transport hubs for the spread of L. fortunei.
In the second place, the capability of withstanding salinity fluctuations can substantially increase effective colonization rates. Estuarine and freshwater ports serviced by oceanic vessels, usually located relatively close to the ocean, are invariably subjected to seaward water flow. Thus, a major challenge for the NIS released in these ports is to avoid being flushed out into the sea before having established a self-sustaining population capable of withstanding the high expatriation rates typical of these environments. The ability to survive in mixohaline waters significantly enlarges the area suitable for colonisation, which facilitates initial introduction and subsequent secondary spread. The saltwater tolerance of embryos and larvae, which likely differs from that of adult mussels, need to be studied before we can confidently determine settling boundaries of L. fortunei in the estuary (Wright et al. 1996; Barnard et al. 2003). Yet, the ability of L. fortunei larvae to settle and survive in saline regions is evidenced by the presence of colonies (unlikely formed by accumulation of drifting adults) near the Río de la Plata salinity front (Fig. 1).
It might seem surprising that in the case of Ponto-Caspian dreissenid mussels, it was D. polymorpha, the species with the lowest tolerance to high constant salinity levels, that resulted in widespread invasions (Karatayev et al. 1998; Table 1). As previously observed, the tolerance of L. fortunei to constant salinity is also quite modest, yet this was not an obstacle to the species’ invasive success. This suggests that mere resistance to high constant salinity values is not necessarily translated into invasive success. Rather, resistance to abrupt salinity changes seems to be more advantageous in some situations. In the case of L. fortunei, the advantage provided by this resistance to salinity changes is not restricted to the environmental tolerance during establishment, but perhaps more importantly, also at an earlier stage in the invasion process during the transport of propagules (Colautti and MacIsaac 2004). We thus contend that tolerance to a wide range of salinities, and likely many other ‘invasive traits’, are not only (and perhaps not primarily) advantageous features per se but advantageous in association with transport network characteristics.
Implications for vessel-mediated NIS dispersal
Ballast water is the most likely vector of introduction of L. fortunei to over half a dozen countries across South America and Asia (Ricciardi 1998; Boltovskoy et al. 2006). Current international ballast water regulations require mid-ocean exchange (MOE), at least 200 miles (370 km) off-shore, of all ballast originated from freshwater to coastal sources (IMO—International Maritime Organization 2004). Although MOE is effective in reducing the number of larvae transported in ballast water (Bailey et al. 2011), adult freshwater mussels may survive attached to the walls of exchanged tanks (Carlton and Geller 1993). While for some mussels the window for adult survival after MOE seems to be small (<5 h for zebra and quagga mussels: Ellis and MacIsaac 2009), we have found 90 % survival of L. fortunei exposed to a salinity peak of 23 ‰ for 9 h. In terms of the distance covered by a commercial vessel typically sailing at 13–24 knots (24–44 km h−1), 9 h represents anywhere between 217 and 400 km, which in many areas exceeds the distance between the destination port and the site of MOE. International and national regulations in most countries, including Argentina, require salinities of at least 30 ‰ of all ballast to be discharged—e.g., IMO and regulations for Canada and the US (IMO 2004; Saint Lawrence Seaway Development Corporation 2008). Further research is needed to determine L. fortunei’s ability to withstand these salinity levels, and whether vessels in strict compliance with these regulations may still pose an invasion threat.
Hull fouling, on the contrary, does not seem to be a viable option for intercontinental introduction of freshwater species with a salinity tolerance similar to that observed for L. fortunei. This is consistent with the absence of freshwater bivalves from extensive hull fouling inventories for marine and freshwater environments (Sylvester and MacIsaac 2010; Sylvester et al. 2011).
Conclusions
Physiological tolerance to chronic exposures in adverse settings and distribution studies may underestimate NIS capabilities to colonize habitats characterised by fluctuating conditions, in particular salinity. In estuaries, the freshwater mussel L. fortunei appears to be able to tolerate very high salinity concentrations provided saltwater pulses are interrupted by periods of freshwater conditions. The ability of L. fortunei to live in estuaries not only expands the potential distribution range of the species, but also greatly increases the likelihood of its vessel-mediated transport and effective introduction to new habitats. This invasive trait may be shared by other successful aquatic NIS around the world. Given the prevalence of human-mediated introductions, with a prime influence of propagule pressure on NIS invasive success, research on species invasiveness should examine species characteristics in the light of vector and transport network properties.
References
Angonesi LG, Da Rosa NG, Bemvenuti CE (2008) Tolerance to salinities shocks of the invasive mussel Limnoperna fortunei under experimental conditions. Iheringia Sér Zool 98:66–69
Bailey SA, Duggan IC, Van Overdijk CDA, Johengen TH, Reid DF, MacIsaac HJ (2004) Salinity tolerance of diapausing eggs of freshwater zooplankton. Freshw Biol 49:286–295
Bailey SA, Deneau MG, Jean L, Wiley CJ, Leung B, MacIsaac HJ (2011) Evaluating efficacy of an environmental policy to prevent biological invasions. Environ Sci Technol 45:2554–2561
Barbosa FG, Melo AS (2009) Predictive model of survival of the Golden Mussel (Limnoperna fortunei) in relation to variations of salinity in the Laguna dos Patos, RS, Brazil. Biota Neotropica 9:407–412
Barnard C, Frenette JJ, Vincent WF (2003) Planktonic invaders of the St. Lawrence estuarine transition zone: environmental factors controlling the distribution of zebra mussel veligers. Can J Fish Aquat Sci 60:1245–1257
Berezina NA (2003) Tolerance of freshwater invertebrates to changes in water salinity. Russ J Ecol 34:261–266
Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and impact of invasive freshwater bivalves: Limnoperna fortunei in the Río de la Plata watershed and beyond. Biol Invasions 8:947–963
Boltovskoy D, Karatayev AY, Burlakova L, Cataldo D, Karatayev VA, Sylvester F, Mariñelarena A (2009a) Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636:271–284
Boltovskoy D, Sylvester F, Otaegui AY, Leytes V, Cataldo D (2009b) Environmental modulation of the reproductive activity of the invasive mussel Limnoperna fortunei in South America. Austral Ecol 37:719–730
Bradie JN, Bailey SA, van der Velde G, MacIsaac HJ (2010) Brine-induced mortality of non-indigenous invertebrates in residual ballast water. Mar Environ Res 70:395–401
Brandt RAM (1974) The non-marine aquatic Mollusca of Thailand. Archiv Molluskenkd 105:1–423
Briski E, Ghabooli S, Bailey SA, MacIsaac HJ (2011) Assessing invasion risk across taxa and habitats: life stage as a determinant of invasion success. Diversity Distrib 17:593–602
Byrne RA, Dietz TH (2006) Ionic and acid–base consequences of exposure to increased salinity in the zebra mussel, Dreissena polymorpha. Biol Bull 211:66–75
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 61:78–82
Casper AF (2007) Chapitre III. Life at the edge: physiological constraints on freshwater mussels (Dreissena polymorpha Pallas and D. bugensis Andrusov) in a fluvial estuary. In: Contraintes écophysiologiques de la distribution d’une espèce: divergence parmi les populations sympatriques de Dreissena polymorpha (Pallas) et de D. bugensis (Andrusov) dans l’estuaire et du fleuve Saint-Laurent. PhD theses available from http://archimede.bibl.ulaval.ca/archimede/fichiers/24295/24295.html. Accessed 31 Oct 2012
Cataldo D, Boltovskoy D, Pose M (2003) Toxicity of chlorine and three nonoxidizing molluscicides to the pest mussel Limnoperna fortunei. J Am Water Works Assoc 95:66–78
Cataldo D, Vinocur A, O′Farrell I, Paolucci E, Leites V, Boltovskoy D (2012) The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680:25–38
Colautti RI, MacIsaac HJ (2004) A neutral terminology to define invasive species. Divers Distrib 10:135–141
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Deaton LE, Derby JGS, Subhedar N, Greenberg MJ (1989) Osmoregulation and salinity tolerance in two species of bivalve mollusc: Limnoperna fortunei and Mytilopsis leucophaeta. J Exp Mar Biol Ecol 133:67–79
Devin S, Beisel JN (2007) Biological and ecological characteristics of invasive species: a gammarid study. Biol Invasions 9:13–24
Dietz TH, Wilcox SJ, Byrne RA, Lynn JW, Silverman H (1996) Osmotic and ionic regulation of north american zebra mussels (Dreissena polymorpha). Am Zool 36:364–372
Drake JM, Lodge DM (2004) Global hot spots of biological invasions: evaluating options for ballast-water management. Proc R Soc B 271:575–580
Ellis S, MacIsaac HJ (2009) Salinity tolerance of Great Lakes invaders. Freshw Biol 54:77–89
Floerl O, Inglis GJ, Dey K, Smith A (2009) The importance of transport hubs in stepping-stone invasions. J Appl Ecol 46:37–45
Giberto DA, Sardiña P (2009) Mytella charruana D’Orbigny 1842 y Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) en la zona mixohalina del Río de la Plata: ¿bancos residuales o futuras poblaciones locales? VII Jornadas Nacionales de Ciencias del Mar, 30 November–4 December 2009, Bahía Blanca, Argentina
Gordon DR, Gantz CA (2011) Risk Assessment for invasiveness differs for aquatic and terrestrial plant species. Biol Invasions 13:1829–1842
Guerrero RA, Piola AR, Molinari GN, Osiroff AP, Jáuregui SI (2010) Climatología de temperatura y salinidad en el Río de la Plata y su frente marítimo. Argentina-Uruguay. Instituto Nacional de Investigación y Desarrollo Pesquero, Secretaría de Agricultura, Ganadería y Pesca, Mar del Plata, Argentina
Huang Z, Li C, Zhang L, Li F, Zheng C (1981) The distribution of fouling organisms in Changjiang river estuary. Oceanol Limnol Sin 12:531–537
Hui C, Richardson DM, Robertson MP, Wilson JRU, Yates CJ (2011) Macroecology meets invasion ecology: linking the native distributions of Australian acacias to invasiveness. Divers Distrib 17:872–883
IMO—International Maritime Organization (2004) International convention for the control and management of ships’ ballast water and sediments. Adopted 13 Feb 2004
Jørgensen CB (1990) Bivalve filter feeding: Hydrodynamics, bioenergetics, physiology and ecology. Olsen and Olsen, Fredensborg
Karatayev AY, Burlakova LE, Padilla DK (1998) Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). J Shellfish Res 17:1219–1235
Karatayev AY, Burlakova LE, Padilla DK, Mastitsky SE, Olenin S (2009) Invaders are not a random selection of species. Biol Invasions 11:2009–2019
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves. J Shellfish Res 29:975–984
Keller RP, Drake JM, Drew MB, Lodge DM (2011) Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network. Divers Distrib 17:93–102
Kilgour BW, Mackie GL, Baker MA, Keppel R (1994) Effects of salinity on the condition and survival of zebra mussels (Dreissena polymorpha). Estuaries 17:385–393
Morton B (1977) The population dynamics of Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) in Plover Cove Reservoir, Hong Kong. Malacologia 16:165–182
Muirhead JR, MacIsaac HJ (2005) Development of inland lakes as hubs in an invasion network. J Appl Ecol 42:80–90
Oliveira MD, Hamilton SK, Jacobi CM (2010) Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Aquat Inv 5:59–73
Orlova MI, Panov VE (2004) Establishment of the zebra mussel, Dreissena polymorpha (Pallas), in the Neva Estuary (Gulf of Finland, Baltic Sea): distribution, population structure and possible impact on local unionid bivalves. Hydrobiologia 514:207–217
Rajagopal S, Van Der Velde G, Van Der Gaag M, Jenner HA (2003) How effective is intermittent chlorination to control adult mussel fouling in cooling water systems? Water Res 37:329–338
Ricciardi A (1998) Global range expansion of the Asian mussel Limnoperna fortunei (Mytilidae): another fouling threat to freshwater systems. Biofouling 13:97–106
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Saint Lawrence Seaway Development Corporation (2008) Seaway regulations and rules. Code of federal regulations 33-CFR Part 401, 2008
Strayer DL (2006) The benthic animal communities of the tidal-freshwater Hudson River estuary. In: Levinton JS, Waldman JR (eds) The Hudson River estuary. Cambridge University Press, New York, pp 266–278
Strayer DL, Smith LC (1993) Distribution of the zebra mussel (Dreissena polymorpha) in estuaries and brackish waters. In: Nalepa TF, Schloesser D (eds) Zebra mussels biology, impacts, and control. Lewis Publishers, Boca Raton, pp 715–728
Sylvester F, MacIsaac HJ (2010) Is vessel hull fouling an invasion threat to the Great Lakes? Divers Distrib 16:132–143
Sylvester F, Kalaci O, Leung B, Lacoursière-Roussel A, Murray CC, Choi FM, Bravo MA, Therriault TW, MacIsaac HJ (2011) Hull fouling as an invasion vector: can simple models explain a complex problem? J Appl Ecol 48:415–423
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245
Walton WC (1996) Occurrence of zebra mussel (Dreissena polymorpha) in the oligohaline Hudson River, New York. Estuaries 19:612–618
Wilcox SJ, Dietz TH (1998) Salinity tolerance of the freshwater bivalve Dreissena polymorpha (Pallas, 1771) (Bivalvia, Dreissenidae). Nautilus 111:143–148
Wright DA, Setzler-Hamilton EM, Magee JA, Kennedy VS, Mcininch SP (1996) Effect of salinity and temperature on survival and development of young zebra (Dreissena polymorpha) and quagga (Dreissena bugensis) mussels. Estuaries 19:19–628
Yuan W, Walters LJ, Schneider KR, Hoffman EA (2010) Exploring the survival threshold: a study of salinity tolerance of the nonnative mussel Mytella charruana. J Shellfish Res 29:15–422
Zalewski A, Michalska-Parda A, Ratkiewicz M, Kozakiewicz M, Bartoszewicz M, Brzeziński M (2011) High mitochondrial DNA diversity of an introduced alien carnivore: comparison of feral and ranch American mink Neovison vison in Poland. Divers Distrib 17:57–768
Acknowledgments
We are grateful to Raúl Guerrero for putting at our disposal the salinity record produced by the FREPLATA project, and to Diego Giberto for information and samples of L. fortunei collected in the Río de la Plata mixohaline area. Gerardo Cueto helped with the statistical analysis. Erik Thuesen, Ladd Johnson, and two anonymous reviewers provided very helpful comments on this work. This work was financed by grants from the University of Buenos Aires (UBA X-020 and 20020100100035) and from the Argentine Agencia Nacional de Promoción Científica y Tecnológica (PICT 2007 1968) to DB.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sylvester, F., Cataldo, D.H., Notaro, C. et al. Fluctuating salinity improves survival of the invasive freshwater golden mussel at high salinity: implications for the introduction of aquatic species through estuarine ports. Biol Invasions 15, 1355–1366 (2013). https://doi.org/10.1007/s10530-012-0373-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0373-z