Abstract
The automation of the detection of fractured bone tissue would allow to save time in medicine. In many cases, specialists need to manually revise 2D and 3D CT images and detect bone fragments and fracture regions in order to check a fracture. The identification of bone fragments from CT images allows to remove image noise and undesirable parts and thus improves image visualization. In addition, the utilization of models reconstructed from CT images of patients allows to customize the simulation, since the result of the identification can be used to perform a reconstruction that provides a 3D model of the patient anatomy. The detection of fracture zones increases the information provided to specialists and enables the simulation of some medical procedures, such as fracture reduction. In this paper, the main issues to be considered in order to identify bone tissue and the additional problems that arise if the bone is fractured are described. The identification of fractured bone includes not only bone tissue segmentation, but also bone fragments labelling and fracture region detection. Moreover, some fragments can appear together after the segmentation process, hence additional processing can be required to separate them. After that, currently proposed approaches to identify fractured bone are analysed and classified. The most recently proposed methods to segment healthy bone are also reviewed in order to justify that the techniques used for this type of bone are not always suitable for fractured bone. Finally, the aspects to be improved in the described methods are outlined and future work is identified.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The automatic identification of bone tissue from computed tomographies (CT images) is a helpful procedure in medical visualization and simulation. Nowadays, the specialist has to manually revise 2D and 3D CT images to detect bone fragments and fracture regions to check a fracture in many cases. The segmentation of bone fragments removes image noise and undesirable parts and therefore improves image visualization. Advances in the visualization of medical images are rewarding because they prevent the specialists reviewing 2D and 3D images manually and thus they enable time saving. In medical simulation, the result of the segmentation can be used to perform a reconstruction that provides a 3D model of the patient anatomy which can be utilized to customize the simulation. These generated models are also useful to provide additional information during the intervention. On the other hand, the detection of fracture zones increases the information provided to specialists and enables the simulation of some medical procedures, such as bone fracture reduction.
In the literature, many methods have been proposed to segment healthy bone. Most of these methods are focused on a specific bone or require previous learning. These constraints do not allow to apply them to the segmentation of fractured bone, since the shape of the bone fragments is often unpredictable, especially in fractures caused by trauma. On the other hand, the identification of fractured bone adds some additional tasks. Specifically, it requires to label fragments and, in some cases, to separate wrongly joined fragments. Moreover, some applications also require to detect bone regions. Thus, specific methods are needed in order to identify fractured bones from CT images. In addition, each type of fracture has different features, hence there are necessary different methods in order to identify bone fragments in all type of fractures. In this paper, the main aspects to be considered to identify healthy and fractured bone are described. This allows to check what techniques applied in healthy bone segmentation may or may not be used to identify fractured bone. Moreover, the identification of fractured bone includes not only bone tissue segmentation, but also bone fragment labelling and fracture region detection, hence these processes are also analysed. After the segmentation process, several bone fragments can appear together as only one. Therefore, some additional processing can be required. Once all these issues are analysed, currently proposed approaches to segment healthy bone, identify fractured bone, separate bone fragments and detect fracture zones are revised and classified. This enables the outline of the aspects to be improved and the identification of future work.
In the next section, the main issues for both healthy and fractured bone detection are discussed. This includes the special aspects to be considered in each type of bone fracture. Then, we describe and classify previous work related to the segmentation of healthy and fractured bone. In the case of fractured bone, the approaches used to label fragments, to separate wrongly joined fragments and to detect fracture regions are also classified. Finally, this review allows to know the strengths and weakness of each approach and thus the issues that remain unsolved.
2 Issues for Bone Detection
2.1 Healthy Bone
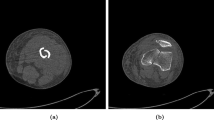
The segmentation of bone tissue from CT images is a complex process. It is difficult to find a solution that works in all cases. In a bone, there are two very distinct zones: cortical and trabecular tissue. Cortical tissue is very dense and it can be found in the outer part of the bone. Trabecular tissue is mainly in the inner part of the bone. This type of tissue is more heterogeneous and it has less intensity in a CT image. In addition, the intensity value for the same tissue differs between slices. This happens with both cortical and trabecular tissues. For instance, intensity values on the diaphysis and the epiphysis are different in a long bone (Fig. 1). Near the joints, the cortical zone is very thin. This zone even disappears in the area closest to the join. Therefore, the transition of the intensity values near the joints generally appears to be fuzzy and some areas within the bone may have similar intensity than the soft tissue surrounding the bone. This may cause incomplete segmentation or overgrowing [14].
2.2 Fractured Bone
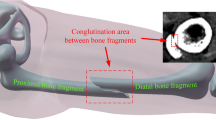
Fractured bone tissue is more difficult to identify because it has some additional features to be considered. Due to the fact that bone fragments may have arbitrary shape and can belong to any bone in a nearby area, it is necessary to label all the fragments during the segmentation process. In some cases, this labelling requires expert knowledge. In addition, a priori knowledge can not be easily used because it is uncommon to find two identical fractures and therefore it is difficult to predict the shape of the bone fragments, specially in comminuted fractures. On the other hand, bone fragments are not completely surrounded by cortical tissue, since they have areas on the edges without cortical tissue due to the fracture. Finally, proximity between fragments and the resolution of the CT image may cause that different fragments appear together as one in the image. For this reason, smoothing filters should be used with caution. This type of filters can deform the shape of bone fragments and fracture zones or even remove small bone fragments. In some cases, it is necessary to detect the fracture zone of each fragment after its segmentation. The fracture zone is the area of the bone where the fracture occurs and is composed of trabecular tissue (Fig. 2). In situations in which bone fragments appear connected, it is difficult to accurately identify the fractured zone of each fragment. Therefore, post-processing can be necessary to delimit fracture zones in these situations.
The method applied in fractured bone identification depends on the fracture type. Based on the fracture line, a fracture can be classified as (Fig. 3): greenstick, transverse, oblique, spiral, avulsed, segmental and comminuted [7]. In a greenstick fracture (Fig. 4a) there are no fragments because the bone is not completely broken. Thus, labelling is not necessary. Since the fracture barely changes the shape of the bone, segmentation methods that are based on previous knowledge are available. Nevertheless, the edges of the fracture zone, composed of trabecular tissue, may require special processing. The detection of the fracture zone is specially complicated since the bone is not completely broken and trabecular tissue is very heterogeneous. Therefore, the fracture zone can be fuzzy in the CT image.
Transverse, oblique and spiral fractures (Fig. 4b, c,d,e, and f) can be similarly treated during the segmentation. Despite of having different fracture lines, these types of fracture generate two fragments with similar shape. Labelling is necessary, but expert knowledge is not required. Segmentation methods that can be applied depend on whether or not there is displacement. If there is no displacement (Fig. 4c, d, e, and f), they can be processed as a greenstick fracture but considering that there are two fragments. These two fragments can be completely joined, hence an additional processing to separate them may be required. In order to detect fracture zones, the same issues applicable to greenstick fractures should be considered. In the case that there is displacement (Fig. 4b), the probability that both fragments are jointly segmented decreases and methods based on prior knowledge are almost discarded. In return, the fracture zone is easier to be identified. Avulsed fractures normally occur near a join thus the fracture zone is composed almost exclusively by trabecular tissue and the boundaries of the fragments are weak. This complicates the identification of the fracture zone because practically the entire fragment is surrounded by trabecular tissue. Segmental fractures are simple fractures that generate three bone fragments. Therefore, they can be treated as transverse or oblique fractures but considering that there are two distinct fracture regions. Comminuted fractures (Fig. 5) add some additional constraints, hence this is the type of fracture that is more complicated to be segmented. Comminuted fractures usually generate small fragments and bone may be deformed due to the fracture. This is because comminuted fractures are usually associated with crush injuries. In most cases, some fragments overlap in the CT image and require additional processing to be separated. Labelling is necessary and expert knowledge is strongly required to identify fragments. The detection of fracture zones is complicated in this case. Due to the complexity of the fracture, several fracture zones are generated. Since the relationship between fragments in this type of fractures is many-to-many, it can be necessary not only to identify fracture zones, but also to delimit which part of the fracture zone corresponds to each fragment. As mentioned before, some fragments can overlap due to the fracture and therefore post-processing and expert knowledge can be required to accurately identify fracture zones.
3 Currently Proposed Approaches
3.1 Healthy Bone
In recent years, many approaches have been proposed in order to segment bone tissue from CT images. Most of these methods are focused on the segmentation of a specific area. In [25] authors combine region growing, active contours and region competition to segment carpal bones. An expectation maximization algorithm has been utilized to segment phalanx bones [23]. The method requires a previously generated CT atlas. In [18], 3D region growing is used to segment the inferior maxillary bone from CT images. In order to fill holes in the segmented surface, a morphological operation of closing is used. Then, 3D ray casting is applied to segment the internal region of the bone by determining which points are inside of the outer shell. The segmented voxels are classified as cortical or trabecular bone using a fuzzy c-means algorithm. To improve the result, an adapted median filter allows to remove outliers. A 3D region growing method has also been used to segment bone tissue in [32]. Both the seeds and the threshold are calculated automatically. Since they use an unique threshold, some areas of bone are not segmented and they propose a method to fill them. This segmentation approach has been tested to segment skull and spine bones. A novel active contour model is utilized to segment bone tissue in [28]. The statistical texture method has also been proposed to segment mandible bones from CT images [19]. In [17] authors use a 3D deformable balloon model to segment the vertebral bodies semi-automatically. Graph cuts have also been used to segment vertebrae [2]. Previously, seeds are automatically placed using the matched filter and vertebrae are identified with a statistical method based on an adaptive threshold. Cortical and trabecular bone are then separated by using a local adaptive region growing method. In [15], Willmore flow is integrated into the level set method to segment the spinal vertebrae. Graph cuts have also been employed to segment the hip bone [16]. Most of these approaches can not be applied to the segmentation of fractured bone tissue because they take advantage of the prior knowledge of the shape of the bones.
Statistical methods are frequently used to segment bone tissue [3]. In this case, they use a generative model to classify pixels into cortical bone or another tissue. A learned model is constructed by modeling probability functions using Gaussian mixture models. Then, the learned model allows to assign a probability to each pixel and a maximum a-posteriori probability rule enables a crisp classification. In [12], a genetic algorithm is used to search the better procedure to segment bone tissue and to separate cortical and trabecular tissue. For that, the genetic algorithm requires previous expert information. Despite the results obtained, learning based methods can not be easily used to segment fractured bones because previous learning is not available in most cases.
Several methods are based on the fact that the shape and the anatomy of the bone are known [31]. In this work, an adaptive threshold method is utilized to segment bone tissue. However, the method can not be applied to segment bone fractures because it is based on the supposition that bone fragments are completely surrounded by cortical tissue, and this is not always true in the case of a fracture. All the revised works for segmenting healthy bone from CT images are summarized in Table 1.
3.2 Fractured Bone
The methods applied to the segmentation of healthy bone could not be suitable for segmenting fractured bone. This is because, as seen in the previous section, fractured bone has different features. Moreover, the identification of fracture bone requires to carry out additional steps, such as labelling the fragments or splitting wrongly joined fragments. Currently proposed methods to perform these steps are described below.
3.2.1 Fragment Segmentation and Labelling
There are several papers that are focused on the identification of fractured bone. With this aim, threshold-based methods are used in most cases. The most basic threshold-based method consist in defining an intensity interval that corresponds to bone tissue and calculating the pixels in the image that belong to this interval [24]. The intensity interval can be defined manually or can be calculated from the information provided by the image. On the other hand, the interval can be used in the hole stack or can be defined for each slice. The second option is usually the most successful because, as seen in Sect. 2, intensity values differ between slices. Several works propose to use thresholding to segment fractured bone. In [20], ulna, radius and carpus are segmented to simulate a virtual corrective osteotomy. Therefore, the segmentation is performed on non-fractured bones and then the segmented bones are virtually cut. In order to separate bone from other tissues, an user-defined threshold is used. In [27], the area where the bones are located is detected using a threshold-based method. Then, they present manual and semi-automatic tools for interactively segmenting bone fragments. This toolkit includes separation, merge and hole filling tools to generate individually segmented fragments from the result of the threshold-based segmentation. Thus, the method achieves accuracy at the expense of requiring a lot of user intervention. A global fixed threshold method has been utilized in [26] to detect the trabecular bone fracture zone. Due to the difference of intensity values between slices, it is difficult to set a threshold that fits all the slices.
Region growing is a threshold-based method that allows to limit the segmentation to a specific area [8]. To that end, the algorithm requires to place seeds before starting the segmentation. The selection of the seed points can be performed manually or automatically. The manual placement of the seeds enables the labelling of the different bone fragments. Moreover, the algorithm also needs to define an intensity interval. As in the previous case, the interval can be defined globally or for each slice. Once the seeds have been placed and the interval has been defined, the algorithm check all their neighbouring pixels. If the intensity of a neighbouring pixel is outside of the defined interval, it is discarded. Otherwise, the pixel is included in the segmented area and its adjacent pixels are studied. The algorithm stops when there are no pixels to study. The result of the algorithm can differ depending on the criteria used to accept or discard pixels. The basic algorithm accepts a pixel if its intensity is inside the interval. This approach allows to detect small bone features but image noise can also be segmented. However, noise can be mostly reduced using smoothing filters. Therefore, this approach can be suitable for segmenting fractured bone. Other approaches decide to accept or discard a pixel based on the intensity value of its neighbours. The simplest option is to accept a pixel if all its neighbours have intensity values inside the interval. Another option is to use a criteria based on statistical values calculated from the neighbouring pixels. In this case, small features could be discarded. Thus, this variation could not be suitable for segmenting fractured bone.
Region growing based methods are the best used for segmenting fractured bone. A semi-automatic threshold-based method and region growing have been utilized to extract bone contours from CT scans in [10]. Before that, thresholding is applied to obtain the area where bone tissue is located. Then, redundant contours are removed using an absolute and a relative spatial criterion. To improve the result, smoothing algorithms are applied and close contours are joined. In [11], authors use an interactive method to segment complex humeral bone fractures. In a first step, the method calculates a sheetness measure in order to extract the cortical layer of the fragments. Then, a semi-automatic region growing is performed on the obtained 3D sheetness data. Voxels with a sheetness measure less than a threshold are labeled as belonging to cortical bone fragments. Region growing is performed using a wave propagation strategy in order to reduce memory consumption and increase computation speed. Seed points and the sheetness threshold are interactively selected by the user. The placement of the seed is used to label the bone fragments, hence this process is repeated until all the fragments have been labelled. In [9], authors also use a sheetness-based method to segment fractured pelvic bones. In order to identify cortical tissue, a local adaptive thresholding method, based on the sheetness measure and a weight factor, is utilized. In order to segment trabecular tissue, a region growing method, based on the previous cortical bone segmentation, is applied using an adaptive threshold. In [14], authors present a multi-region segmentation approach to identify pelvic fractures. The seed points are automatically established by searching in the image pixels that have an intensity value higher than a threshold. Once a seed is found, its region is propagated to avoid finding another seed inside it. After that, a region growing algorithm propagates all regions in turns. In each cycle of propagation, the gray values of the fronts are set to be equal and reduced by the threshold iteratively. To that end, the threshold value is determined in an iterative process.
Other proposed method to segment fractured bone is based on registration [22]. In order to automatically segment fractured hip bones, they use an extension of the non-rigid Morphon registration [13]. The proposed method registers each bone fragment with a prototype. The method is limited to simple fractures, since it requires a prototype for registering each bone fragment. The main disadvantage of this method is that it requires prototypes of the fractured parts, hence it is limited to the specific fractures defined by the prototypes. Other segmentation methods [1] could be tested in order to segment fractured bone tissue. Table 2 summarizes all the revised methods for identifying fractured bones.
3.2.2 Fragment Separation
The proximity between fragments and the resolution of the medical images can cause that several bone fragments appear together after the segmentation procedure. In that case, these bone fragments must be separated. Current works usually propose methods not only to identify bone fragments, but also to separate wrongly joined fragments.
Some proposed methods allow to separate bone fragments manually. These methods achieve accuracy at the expense of requiring a lot of user intervention. In [11], authors use a manual procedure to separate erroneously connected fragments. To that end, the user can draw a cut line onto the surface of the bone fragments to define a set of separation voxels. Then, these set is grown parallel to the screen and extruded along the viewing vector. After that, the segmentation process is repeated to determine if the connection still exists. This manual procedure takes about five minutes. In [27], authors present a tool to separate bone fragments in a 3D model. For this purpose, the user must position seed points on different fracture locations and the tool calculates the fracture line in between. If there is no fragment line visible, a cut tool can be used.
Manual tasks take a long time, hence other methods try to split bone fragments as automatically as possible. A semi-automatic watershed-based method has been used to separate erroneously joined bone fragments resulted from a threshold-based segmentation [20]. The proposed method needs that the user selects a voxel located on the boundary between the two fragments. Then, a watershed based segmentation algorithm performs the separation. This method achieves good results, but manual corrections need to be performed in case of inaccuracies. In [9], authors propose to apply a 3D connected component algorithm to separate bone fragments in simple cases. Moreover, the algorithm also allows to reject small fragments and remove false positive labelled structures. In order to deal with fractures in which the boundary of the bone is weak, they propose to use graph cuts. For that, seeds have to be added by the user to each bone fragment. They also introduce an optimized Ransac algorithm to detect fracture gap planes and thus to identify incorrect bone fragment separation. With the aim of refining the segmentation in zones with low bone density, they use another graph cut based approach. Another proposed solution consists in performing a re-segmentation [14]. If the proposed multi-region segmentation fails, authors provide a manual region combination algorithm that allows to blend the wrongly-segmented regions, and a region re-segmentation that enables the separation of the incompletely-segmented objects. Region combination allows to combine several fragments into one interactively. The user needs to select the fragments one by one and the algorithm combines them into one. The region re-segmentation consists in applying the multi-region segmentation algorithm to a specific region defined by the user. The initial threshold is set higher than usual in order to ensure that the two regions are detected. The target threshold does not change during the growing process. These two algorithms, region combination and region re-segmentation, can be executed repeatedly until all the bone fragments are accurately separated. All the revised works to separate wrongly joined bone fragments are summarized in Table 3.
3.2.3 Fracture Zone Identification
Sometimes, it is useful to perform the identification of the fractured area. For instance, the simulation of a fracture reduction and the virtual analysis of the fracture can require to previously calculate this area. Therefore, some approaches have been proposed to calculate the fractured area after the segmentation of bone fragments.
Statistical based approaches have been proposed to identify fractured zones [29]. In this work, authors semi-automatically reconstruct highly fragmented bone fractures. Before performing the fracture reduction, they need to separate intact and fractured zones of each bone fragment. For that purpose, they propose to use a mixture model consisting of two Gaussian probability distributions to perform a binary classification. They choose a threshold that enables the classification of intact-surface intensities and minimizes the type I classification errors. Thus, this threshold allows to separate fractured and intact surfaces. After classifying all points, the fractured surface is the largest continuous region of fractured surface points. In [33], an extension of the previous method that improves fragment alignment in highly fragmented bone fractures has been presented. In order to separate fractured and intact surfaces, they use a two-class Bayesian classifier based on the intensity values previously mapped on the surface vertices.
Other proposals take advantage of the specific shape of a particular type of bone. In [30], authors present an approach to semi-automatically perform the reduction of cylindrical bones. In order to identify vertices of the fractured area, they check the normal orientation of each vertex and compare it with the bone axis. This method does not work when fracture lines are almost parallel to the bone axis.
Curvature analysis has also been used to identify fractured surfaces [21]. In this work, authors present a procedure to virtually reduce proximal femoral fractures. In order to obtain fracture lines in each slice, they use curvature analysis. For that purpose, a 3D curvature image is generated. To begin with, 0 or 1 values are assigned to each voxel depending on the voxel position: 1 is assigned if the voxel is inside the fragment region and 0 is assigned if it is outside. After that, the surface voxels are defined as 1-value voxels adjacent to 0-value voxels. The 3D curvature image is generated by setting K abs to each voxel belonging to the fracture surface and 0 to the rest of voxels, where \(K_{abs} = |k_{1}| + |k_{2}|\). k 1 and k 2 are the maximum and the minimum curvature respectively, and are obtained from K and H
where \(h(x,y)\) is a quadratic function fitted to 3D points generated from the surface voxels. Once the 3D curvature image is generated, an interactive line-tracking software allows to extract the fracture zone from the generated 3D curvature image.
In [26], authors perform a comparison with healthy models in order to identify trabecular tissue in fractured zones. To that end, authors compare the fractured region of interest in both pre-failure and post-failure slices. These regions are identified as disconnected trabecular tissue in the slice. If the regions of interest of both slices overlap less that a predefined threshold, the region is classified as broken. The threshold is determined by minimizing the root mean square error (RMSE) between resulted values and values manually calculated
where \(a_{i(x)}\) and v i are the calculated and the visually obtained values respectively and n is the number of analysed cases. Finally, they apply a median filter to remove the generated noise.
Interactive methods have also been proposed to identify fracture surfaces in order to be used in virtual craniofacial reconstruction [4, 6, 5]. In these works, fracture contours are extracted interactively from segmented bone fragments. With that aim, user has to select points belonging to the fractured area and then a contour tracing algorithm generates the rest of the points. Once the fracture contours are calculated, the 3D surface is generated by collating the contours extracted from each slice. Table 4 summarizes all the analysed works to detect fracture zones.
4 Discussion
The previous revision allows us to made a classification of the methods used to identify both healthy and fractured bone (Fig. 6). In order to identify fractured bones, it is necessary not only to segment, but also to label the bone fragments. Considering the previous revision, threshold-based methods have been used in most cases. Currently proposed threshold-based methods obtain good results, but they can be improved in some aspects. The selection of threshold intensity values is one of the most challenging procedures. Threshold values are difficult to be determined even manually and each slice may require a different threshold value. In addition, it is particularly difficult to set the threshold to segment bone tissue near the joints. The ideal would be that the threshold values were selected automatically from the information available in the set of slices in all cases. Because of the complexity of the fractures, it is difficult to label bone fragments automatically. This procedure may require expert knowledge, but it must be reduced as possible. Thresholding-based approaches do not label bone fragments, hence fragments have to be labelled after the segmentation process. Other approaches try to solve it by using seeded-based methods. By the time they place the seeds, they identify the bone fragments. Thus, seeds should be placed by an expert in some cases. Ideally, all the bone fragments should be segmented automatically and simple bone fragments should be identified without user intervention. Then, the expert could decide the bone to which each fragment belongs in the most complex cases.
Due to the fracture, two different fragments can be completely joined. This is specially common in fractures caused by crashes. In addition, the image resolution can cause that very close fragments appear joined. These joined fragments are difficult to be separated during the segmentation process, hence current fractured bone identification approaches propose to separate them after the segmentation. New methods that solve this problem in a more automatic way are required. One solution would be to improve the segmentation method, hence no joined fragments are generated. This would be the faster solution, because no additional methods are required. However, the usual resolution of the CT scans makes it very difficult. The alternative is to implement a method that automatically separates wrongly joined fragments resulted from the segmentation. Manual and semi-automatic fragment separation takes a lot time, hence these new methods would be important to enable time saving. On the other hand, the use of higher resolution images, such as μCT, could avoid that fragments appear together in most cases. Nevertheless, this type of images is not always available.
Once all the bone fragments have been identified, some applications, such as fracture reduction or fracture analysis, require to detect fracture zones. Different interactive methods have been proposed to delimit the fracture area. Some of these methods propose to calculate fracture lines in each slice and then join them to generate the fracture area. Following this approach, it is easier to detect and fix anomalies in each slice. In contrast, this type of methods usually requires more time since fracture line detection is performed in each slice and user interaction is needed. Other methods use 3D interactive techniques to identify the fracture zone. These methods are usually faster but the interaction is usually much more complex. Methods based on prior knowledge have also been proposed to identify the fracture zone. These methods are usually faster but are restricted to specific bones and fracture types. In summary, currently proposed methods to detect fracture zones are based on previous knowledge or need user interaction (Fig. 6). Therefore, new methods that calculate fracture zones using the information available in the slice would be useful. In addition, these new methods should be as automatic as possible.
All these shortcomings are summarized in the following points:
-
Separate wrongly joined bone fragments after or during the segmentation process without user intervention.
-
Select the threshold for each slice automatically from the information available in the CT stack.
-
Label the bone fragments with minimal user interaction.
-
Detect fracture zones using information from the CT stack as automatically as possible.
5 Conclusion
In this paper, the main issues to be considered when identifying both healthy and fractured bone tissues have been described. Moreover, currently proposed methods for healthy and fractured bone identification have been discussed and classified. This revision has shown that most of the methods applied to the segmentation of healthy bone can not be utilized to identify fractured bone. Moreover, it has allowed to know which algorithms have been applied in order to identify each type of bone and fracture as well as the results obtained. In the case of the identification of fractured bones, emphasis has also been placed in the proposed methods to label bone fragments, separate fragments that have been segmented together incorrectly and detect fracture zones. Finally, the shortcomings of the currently available methods have been revised and identified.
References
Allili MS, Ziou D (2007) Automatic colour–texture image segmentation using active contours. Int J Comput Math 84(9):1325–1338
Aslan MS, Ali A, Rara H, Farag AA (2010) An automated vertebra identification and segmentation in CT images. In: 2010 IEEE International conference on image processing, IEEE, 233–236
Battiato S, Farinella GM, Impoco G, Garretto O, Privitera C (2007) Cortical bone classification by local context analysis. In: Gagalowicz A, Philips W (eds) Computer vision/Computer graphics collaboration techniques, vol. 4418. Springer, Berlin pp 567–578
Bhandarkar SM, Chowdhury AS, Tang Y, Yu JC, Tollner EW (2007) Computer vision guided virtual craniofacial reconstruction. Comput Med Imaging Graph J Comput Med Imaging Soc 31(6):418–427
Chowdhury AS, Bhandarkar SM, Robinson RW, Yu JC (2009) Virtual craniofacial reconstruction using computer vision, graph theory and geometric constraints. Pattern Recognit Lett 30(10):931–938
Chowdhury AS, Bhandarkar SM, Robinson RW, Yu JC (2009) Virtual multi-fracture craniofacial reconstruction using computer vision and graph matching. Comput Med Imaging Graph J Comput Med Imaging Soc 33(5):333–342
Egol K, Koval KJ, Zuckerman JD (2010) Handbook of fractures. Lippincott Williams & Wilkins (LWW), Philadelphia
Fan J, Zeng G, Body M, Hacid MS (2005) Seeded region growing: an extensive and comparative study. Pattern Recognit Lett 26(8):1139–1156
Fornaro J, Székely G, Harders M (2010) Semi-automatic segmentation of fractured pelvic bones for surgical planning. In: Bello F, Cotin S (eds) Biomedical simulation, vol. 5958. Springer, Berlin pp 82–89
Gelaude F, Vander Sloten J, Lauwers B (2006) Semi-automated segmentation and visualisation of outer bone cortex from medical images. Comput Meth Biomech Biomed Eng 9(1):65–77
Harders M, Barlit A, Gerber C, Hodler J, Székely G (2007) An optimized surgical planning environment for complex proximal humerus fractures. In: MICCAI Workshop on interaction in medical image analysis and visualization
Janc K, Tarasiuk J, Bonnet AS, Lipinski P (2011) Semi-automated algorithm for cortical and trabecular bone separation from CT scans. Comput Meth Biomech Biomed Eng 14(1):217–218
Knutsson H., Andersson M. (2005) Morphons: segmentation using elastic canvas and paint on priors. In: IEEE International conference on image processing 2005, IEEE, II–1226
Lee PY, Lai JY, Hu YS, Huang CY, Tsai YC, Ueng WD (2012) Virtual 3D planning of pelvic fracture reduction and implant placement. Biomed Eng Appl Basis Commun 24(3):245–262
Lim PH, Bagci U, Bai L (2013) Introducing Willmore flow into level set segmentation of spinal vertebrae. IEEE Transac Biomed Eng 60(1):115–122
Malan DF, Botha CP, Valstar ER (2013) Voxel classification and graph cuts for automated segmentation of pathological periprosthetic hip anatomy. Int J Comput Assist Radiol Surg 8(1):63–74
Mastmeyer A, Engelke K, Fuchs C, Kalender WA (2006) A hierarchical 3D segmentation method and the definition of vertebral body coordinate systems for QCT of the lumbar spine. Med Image Anal 10(4):560–577
Moreno S, Caicedo SL, Strulovic T, Briceño JC, Briceño F, Gómez S, Hernández M (2010) Inferior maxillary bone tissue classification in 3D CT images. In: Bolc L, Tadeusiewicz R, Chmielewski LJ, Wojciechowski K (eds) Computer vision and graphics, vol. 6375. Springer, Berlin, pp 142–149
Nassef TM, Solouma NH, Alkhodary M, Marei MK, Kadah YM (2011) Extraction of human mandible bones from multi-slice computed tomographic data. In: 2011 1st Middle East conference on biomedical engineering, IEEE, 260–263
Neubauer A, Bühler K, Wegenkittl R, Rauchberger A, Rieger M (2005) Advanced virtual corrective osteotomy. Int Congr Ser 1281:684–689
Okada T, Iwasaki Y, Koyama T, Sugano N, Chen Y, Yonenobu K, Sato Y (2009) Computer-assisted preoperative planning for reduction of proximal femoral fracture using 3-D-CT data. IEEE Transac Biomed Eng 56(3):749–759
Pettersson J, Knutsson H, Borga M (2006) Non-rigid registration for automatic fracture segmentation. In: IEEE International conference on image processing, 1185–1188
Ramme AJ, DeVries N, Kallemyn NA, Magnotta VA, Grosland NM (2009) Semi-automated phalanx bone segmentation using the expectation maximization algorithm. J Digital Imaging 22(5):483–491
Sahoo P, Soltani S, Wong A (1988) A survey of thresholding techniques. Comput Vision Graph Image Process 41(2):233–260
Sebastian TB, Tek H, Crisco JJ, Kimia BB (2003) Segmentation of carpal bones from CT images using skeletally coupled deformable models. Med Image Anal 7(1):21–45
Tassani S, Matsopoulos GK, Baruffaldi F (2012) 3D identification of trabecular bone fracture zone using an automatic image registration scheme: A validation study. J Biomech 45(11):2035–2040
Tomazevic M, Kreuh D, Kristan A, Puketa V, Cimerman M (2010) Preoperative planning program tool in treatment of articular fractures: process of segmentation procedure. In: XII Mediterranean conference on medical and biological engineering and computing 2010, 29, 430–433
Truc PTH, Kim TS, Lee S, Lee YK (2011) Homogeneity and density distance-driven activecontours for medical image segmentation. Comput Biol Med 41(5):292–301
Willis A, Anderson D, Thomas T, Brown T, Marsh JL (2007) 3D reconstruction of highly fragmented bone fractures. Medical Imaging 2007: Image processing. Proceedings of the SPIE, 6512
Winkelbach S, Westphal R, Goesling T (2003) Pose estimation of cylindrical fragments for semi-automatic bone fracture reduction. In: Pattern recognition, Springer, Berlin, pp 566–573
Zhang J, Yan CH, Chui CK, Ong SH (2010) Fast segmentation of bone in CT images using 3D adaptive thresholding. Comput Biol Med 40(2):231–236
Zhao K, Kang B, Kang Y, Zhao H (2010) Auto-threshold bone segmentation based on CT image and its application on CTA bone-subtraction. In: 2010 Symposium on photonics and optoelectronics, 1–5
Zhou B, Willis A, Sui Y, Anderson D, Thomas T, Brown, T (2009) Improving inter-fragmentary alignment for virtual 3D reconstruction of highly fragmented bone fractures. SPIE Medical Imaging, 7259
Acknowledgements
This work has been partially supported by the Ministerio de Economía y Competitividad and the European Union (via ERDF funds) through the research project TIN2011-25259.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Paulano, F., Jiménez, J., Pulido, R. (2015). Fractured Bone Identification from CT Images, Fragment Separation and Fracture Zone Detection. In: Tavares, J., Natal Jorge, R. (eds) Developments in Medical Image Processing and Computational Vision. Lecture Notes in Computational Vision and Biomechanics, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-13407-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-13407-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13406-2
Online ISBN: 978-3-319-13407-9
eBook Packages: EngineeringEngineering (R0)