Abstract
Sensorimotor behaviors are by definition “closed-loop” processes in which sensory feedback modulates behavioral output. Sensory feedback can be provided by visual, auditory, and vestibular inputs or direct proprioceptive inputs from muscle contraction. Although sensory feedback is not necessary for oscillation underlying locomotion to occur, there is evidence in the cat that sensory feedback can initiate locomotion [128] or reset the rhythm [183]. The contribution of sensory feedback to active locomotion is however difficult to estimate for technical reasons. Indeed, most physiological studies of genetically identified cells in spinal circuits involved in sensorimotor integration rely on preparations where mechano-muscles are paralyzed or dissected out, and are therefore deprived of sensory feedback.
In this chapter, we will first explain closed-loop processes, and we will review the precious information obtained using “open-loop” experimental paradigms on how spinal neurons generate the neural rhythms that are at the basis of locomotion [82]. Optical and genetics techniques offer today alternatives to electrophysiology for monitoring neuronal activity from genetically defined populations of spinal neurons. We will then discuss how innovative tools for monitoring and manipulating neural activity, together with conducting sophisticated behavioral analysis, have provided exciting opportunities for “closing the loop” in genetically accessible model organisms with a special emphasis on zebrafish.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 A Closed-Loop Approach to Sensorimotor Behaviors
8.1.1 Defining Sensorimotor Behaviors
8.1.1.1 Eliciting Sensory Input
A fly approaching a fruit odor is a rich example of sensorimotor integration [188]: the fly must first detect the odor [32], extract information regarding its environmental relevance, and adapt its course to approach the fruit. All those steps have to be achieved while the animal is moving, thus adjusting its locomotor output to changing visual, olfactory, and mechanosensory feedback [67]. Combining multiple sensory modalities and their closed-loop feedbacks is critical to adapt to a noisy sensory environment and enhances the robustness of the behavioral output [67]. Multisensory processing relies on interdependent sensory signals, allowing for increased efficiency during sensorimotor tasks compared to unimodal sensory stimuli [127].
In mammals, it has long been clear that “high-level” cortical areas, such as parietal and prefrontal cortices, are able to integrate multiple sensory modalities. However, increasing evidence suggests that multisensory integration also occurs in “low-level” cortices that were previously thought to be unisensory [71, 185]. Studying sensorimotor integration, even at a relatively low level, thus requires one to reproduce a behaviorally relevant multisensory environment. However, practical considerations often make this difficult.
One solution proposed by the field of neuroethology [49] is to consider that neural circuits can be experimentally understood in the context of the animal’s natural behavior. By focusing on innate behaviors in which the animal extracts critical sensory inputs to produce a behaviorally meaningful locomotor output, neuroethology has provided important models for sensorimotor integration. For instance, escape behaviors, by which an animal escapes from its predator, are a perfect example of a sensorimotor task that is crucial for the animal’s survival. Escape responses can be found in many species, including Drosophila [36], C. elegans [160], and several fish species [186], allowing for comparative studies of sensorimotor integration across taxa.
Determining which sensory stimulus to control experimentally is a critical step of sensorimotor studies. We cannot reproduce the highly variable and multidimensional sensory inputs from the animal’s natural environment, but we should at least choose a stimulus that replicates the minimum set of sensory cues necessary to elicit a behaviorally relevant and consistent motor output [39]. We also need to reliably record and quantify the locomotor output elicited by this sensory input.
8.1.1.2 Measuring Motor Output
The behavioral output of a sensorimotor transformation can be measured at different spatial and temporal scales, from the migration of an entire population of animals over several days to the analysis of single muscle fibers at millisecond timescale [39]. Choosing the right scale for addressing the sensorimotor process of interest is not trivial.
At one extreme of this scale, “taxis” behaviors, such as chemotaxis in Drosophila [69] or rheotaxis in zebrafish [195], examine the cumulative change in spatial position of a group of animals over a relatively long period of time. It is also possible to look at the level of the individual in order to identify sequences of stereotyped behaviors such as mating in C. elegans [125]. Sequential analyses of canonical behaviors can allow the description of the complete locomotor repertoire for a given species, such as zebrafish [31]. Lastly, a more detailed kinematics analysis could measure the movements of individual joints and couple this analysis with muscle activity recordings, as has been done in rodents [41].
With the refinement of locomotor analysis, and the increasing set of kinematic parameters that can be measured simultaneously, automated tracking programs have become crucial to reliably quantify behavior. Such programs have been successfully applied to track individuals and classify behaviors in C. elegans [12], Drosophila [66], and zebrafish [141]. Automated tracking programs have also been used to identify interactions between populations of multiple animals [26, 141], characterize mutant behaviors and build behavioral phenotypes databases [210], and might be applied to high-throughput drug screening [141].
Analyzing complex datasets with multiple kinematic parameters per animal and several animals interacting simultaneously raises important technical challenges. Reducing the dimensionality of the behavioral dataset can be achieved either by arbitrarily focusing on a restricted number of kinematic parameters or though statistical dimensionality reduction as in principal component analysis (PCA) [145]. The main issue with dataset reduction is to determine and preserve the behavioral output related to the sensory stimulus of interest. This can be achieved by computing the level of prediction or correlation between the sensory input and motor output [28].
Although sensory input and motor output are the two ends and most accessible parts of a sensorimotor circuit, they are not sufficient to infer sensorimotor neural computation. Modulating inputs from “top-down” afferents or “bottom-up” feedback also heavily influence sensorimotor processing.
8.1.2 Modulating Sensorimotor Behaviors
8.1.2.1 Sensory Feedback
In the real world, sensorimotor integration is a dynamic process where the animal constantly updates its sensory inputs according to its behavioral output: as the fly approaches the fruit, olfactory and visual stimuli change continuously. By tracking these changes, the fly can adjust its flight to reach the target [67]. In an experimental setting, the animal must often be restrained or paralyzed to allow recording of neuronal activity. Such preparations are called “open-loop” because the motor output does not influence subsequent sensory input. But one might hypothesize that neuronal activity is not the same in the absence of sensory feedback.
“Closed loop” experiments, where new sensory information is acquired as the motor output is produced, can be obtained mainly through two complementary approaches: by attaching a miniaturized device onto a free moving animal interacting with a controlled environment or by providing simulated sensory inputs to a restrained animal. The developing field of brain-machine interfaces has provided key examples of how to go about this, for example in studies where cortical activity is recorded through chronically implanted electrode arrays and decoded in real time to control a motor effector, such as prosthetic limb [37]. It has also been possible to restore tactile sensation using a “brain-machine-brain interface,” by providing a way to produce a virtual motor output and to generate the corresponding sensory feedback [157, 197].
Such tools make it possible to monitor neuronal activity while the animal is freely behaving, but they do not provide precise control over its sensory inputs. Virtual reality environments [50] reproduce a simulated sensory environment that is continuously updated based on the animal’s behavior. Besides providing a better-controlled sensory input, virtual environments most importantly enable simultaneous neural recording by allowing the animal to perform a closed loop sensorimotor task while being physically restrained.
Combined with electrophysiology or genetically encoded calcium imaging, virtual environments have been applied in mice [87], Drosophila [190], and zebrafish [2, 161]. Notably, the zebrafish studies have shown that larvae were able to quickly modify their motor output in response to unexpected visual feedback (Fig. 8.1a) [161] and that this adaptive behavior correlated with state-dependent neural activity in a subset of brain areas identified using brain-wide calcium imaging [2].
Open loop and closed loop paradigms illustrated in zebrafish. A. A visual closed-loop virtual reality paradigm in the zebrafish larva. A moving visual stimulus is showed to a head-embedded larva (aged 6-7 days post-fertilization) while its behavior is monitored and its speed (red arrow) is modified by the swimming speed of the larva (A1). In this virtual visual closed-loop environment, a “gain„ is used as a constant factor to adjust the grating speed to the larval swimming speed (A2). For three different gains, several kinematics parameters of the larvae locomotor output are modified consistently: bout duration (A3), interbout interval (A4), number of bouts (A5) and latency (A6). Adapted from Portugues et al. 2011. B. An open-loop experimental fictive preparation for investigating with electrophysiology the role of spinal cells in the absence of mechanosensory feedback. To record from spinal neurons in a juvenile zebrafish (aged 8-15 weeks), the skin and muscles are dissected out to expose the isolated spinal cord (B1), and a stimulating electrode (1s, 40Hz) is placed at the junction with brainstem to elicit episodes of “fictive„ swimming, while the motor output can be recorded from the ventral nerve root or from patched-clamp spinal neurons (B2). Bath application of pharmacological substances, such as the glycinergic antagonist strychnine, is used to modify the fictive motor output on the ventral nerve root recordings (B3). Short (10 minutes) application of strychnine results in increased swimming burst frequency, while longer application (20 minutes) leads to a decreased duration of the swimming episode as well as disruption of the left-right alternation (B4). Adapted from Kyriakatos et al. 2011.
8.1.2.2 Neuromodulation
State-dependent sensorimotor processing, in which the activity of a given population of neurons differs according to the behavioral state of the animal, is investigated within the larger framework of neuromodulation.
The core hypothesis underlying the concept of “multifunctional circuits” is that a given neural circuit should not be considered as a hard-wired diagram, activated during discrete states, but rather as a distributed network that is able to switch continuously between a variety of dynamical states to produce different patterns of activity, and eventually different behaviors [27]. In a multifunctional sensorimotor circuit, a given neuron can be active during multiple locomotor behaviors [179], producing different patterns of activity based on its modulatory inputs [27]. External parameters, such as modulatory neurotransmitters [129] or synaptic input, for example from sensory afferents [116], can control the transitions between these different phases.
The neuromodulatory functions of monoaminergic substances have been extensively studied in invertebrate sensorimotor models such as the crustacean somato-gastric ganglion (STG) [130]. These central pattern generator (CPG) circuits can generate fictive locomotor patterns and are modulated by numerous substances, from neurotransmitters released locally by projecting sensory neurons to diffuse hormones released at distance by secretory structures [20]. In rats with induced spinal cord injuries, the role of monoaminergic (in particular serotoninergic and dopaminergic) substances in modulating spinal locomotor circuits has been well documented [145]. Pharmacological manipulation, together with electrical spinal cord stimulation, could restore some locomotion independently of supraspinal input regeneration [42]. Such neuromodulatory-mediated functional recovery is also phase specific, that is, the observed recovery depends on the phase of the locomotor cycle during which it occurs, suggesting that different interventions facilitate distinct phases of the locomotor pattern [59]. This observation is in line with a multifunctional framework for the spinal sensorimotor circuits driving locomotion in spinal cord injured rats.
Intrinsic sensory states, that is neural dynamics that are not directly affected by an external physical stimulus, can also modulate multifunctional sensorimotor networks. One interesting example is the dual role of the gravimetric organ of the mollusk Clione limacina, which can switch between two very different rhythmic patterns, and associated behavioral outputs, depending on whether the animal is under control of a “hunting neuron” [116]. Another example of intrinsic sensory modulation is the feeding behavior of the Aplysia californica, where the same neurons drive both ingestion and rejection of food, but are differentially modulated by the coupling between the mouth muscles [209].
8.1.3 Modeling Sensorimotor Behaviors
8.1.3.1 Behavioral Computations
Analyzing sensorimotor transformations is more complicated than just correlating an observed motor output with an experimentally elicited sensory input. Computational models for sensorimotor integration have proven more and more helpful as the number of measured variables increased with the improvement of experimental techniques.
For any sensorimotor task, the underlying computation is complex and can be modeled on a coarse behavioral scale, or on a more refined neuronal scale. These approaches are complementary but have so far mostly been developed independently. The long-term objective is to map one onto the other.
One major issue when dealing with sensorimotor computation is that our motor system is highly nonlinear [65]. In a linear system, one can easily predict the behavioral response to a multisensory stimulus by calculating the sum of the motor outputs for each individual sensory stimulus. However, the force developed by a muscle in response to its nervous input largely depends on other variables such as muscle length, velocity, tendons, and joint positions, among others [211]. Similarly, multiple sensory inputs create combined representations that are more than merely the sum of the individual modalities [78].
Besides nonlinearity, many other issues increase the complexity of sensorimotor computations. For instance, noise limits our ability to perceive sensory inputs (e.g., for our approaching fly, estimating the location of the fruit on the table) and to produce motor outputs precisely (e.g., adjusting speed by modifying wing movements to reach the target) [172]. Other issues include redundancy, that is the fact that multiple combinations of motor sequences can achieve the same behavioral task; nonstationarity, that is the fact that sensory and motor systems are modified throughout development and aging; sensory ambiguity, partial information; and even multiple and variable delays, whether due to sensory or motor processing [65].
One approach to resolve such complex sensorimotor computations is Bayesian decision theory [207]. Bayesian decision theory aims to produce, using a probabilistic reasoning, optimal inferences based on uncertain inputs by combining prior beliefs and multiple sensory modalities. Based on these inferences, decision theory is subsequently used to decide which action is more likely to achieve the task objectives [65]. In a Bayesian system, the probability of a sensory state being true (called the “posterior”) is produced by combining the probability of receiving a set of sensory information if that state were true (the “likelihood”) with the prior probability of that state (the “prior”) [114].
Such Bayesian sensorimotor computation can be easily tested using a simple task where a subject is asked to reach a cursor in a virtual-reality environment. A discrepancy is introduced between the subject’s actual and displayed hand positions [113]. The “prior” distribution can be experimentally set by varying the discrepancy, while the sensory feedback “likelihood” is adjusted by varying the degree of visual blur controls. Using this approach, the authors showed that subjects combined prior statistical distribution with sensory feedback likelihood in a Bayesian manner to optimize their performance during sensorimotor learning.
8.1.3.2 Circuit Computations
Mapping behavioral sensorimotor computations onto identified neural circuits requires knowing how those circuits process sensory inputs to produce a motor output at a cellular scale.
One important challenge for computing sensorimotor transformations, whether on a behavioral or cellular scale, is that they are mostly nonlinear. Geometrically, this means that modeling any neural network underlying a sensorimotor process requires at least a three-layered transformation, with an intermediate layer (referred to as the “hidden layer”) used to recode sensory inputs before they are transformed into motor output. Such nonlinear transformations can be approximated using a linear combination of “basis functions” (such as sine and cosine functions in a Fourier transform) as the intermediate layer: this is a called the “basis function approach” [164].This basis function approach is particularly relevant in the context of sensorimotor transformations. For instance, if a subject wants to reach toward a visual target as in the previously described experiment, the motor command can be approximated by the weighted sum of several nonlinear basis functions of the visual and postural inputs [163]. On a cellular scale, this “intermediate layer” would be constituted by neurons whose firing properties, or “tuning curve,” can be described as a basis function for both visual and postural sensory inputs. Such neurons whose gain is modulated by visual and postural inputs can actually be found in the parietal [6], occipital [202], and prefrontal [23] cortices.
Besides nonlinearity, another major concern when looking at sensorimotor transformations is variability. Most experiments, whether looking at sensorimotor processes or not, rely on mean statistics calculated from populations. However, it has been repeatedly shown that multiple physiological solutions can produce similar circuit outputs [129]. Even the most stereotyped motor behaviors such as rhythms generated by CPGs can be highly variable across animals [131]. The variability of the behavioral outputs evoked by similar sensory inputs is well known, although not always documented. Most studies describe the “typical” behavior of the system by a single model. One attempt to take into account variability in sensorimotor circuits models would be to construct of population of models reproducing the actual behavioral data rather than trying to use a single model to reproduce the generic behavior [131].
8.2 An Open-Loop Access to Sensorimotor Circuits in the Spinal Cord Across Vertebrates
In the particular case of spinal sensorimotor circuits, a great wealth of anatomical and electrophysiological data has been accumulated over the years. However, being able to elaborate broader models in order to fit those data onto observed behaviors still remains a challenge, largely due to the fact that available techniques have prevented us from monitoring sensory inputs concomitantly with motor outputs until recently.
8.2.1 Extrinsic Inputs to Spinal Sensorimotor Circuits
8.2.1.1 Descending Motor Control
Located in the periphery of the spinal cord, white matter tracts comprise both ascending fibers, mainly located dorsally and laterally, carrying sensory information, and descending axons, mainly located ventrolaterally and laterally, carrying motor information (Fig. 8.2a).
Descending and ascending inputs to spinal circuits involved in sensorimotor reflexes. A. Motor and sensory inputs to spinal neurons and sites for sensorimotor integration. Descending motor control from the corticospinal and rubrospinal tracts in the dorsolateral funiculus) and reticulospinal and vestibulospinal tracts (in the the ventrolateral funiculus are integrated with ascending sensory inputs from proprioceptive afferents Ia and II from muscle spindles and Ib from Golgi tendon organs at various premotor locations. Adapted from Rossignol et al. 2006. B. Some spinal sensorimotor reflexes and underlying interneuronal networks. Presynaptic inhibition of sensory afferents by GABAergic premotor interneurons in the intermediate laminae of the spinal cord is a common control mechanism for filtering sensory inputs (B1). Reciprocal Ia inhibition by glycinergic interneurons allows for antagonist muscles inhibition during a flexion movement (B2). Non-reciprocal Ib inhibition facilitates synergist muscle contraction though polysynaptic pathways (B3).
Descending motor tracts mainly include corticospinal tracts, which forms monosynaptic connections between motoneurons located in the primary motor cortex and spinal motoneurons located in the anterior horn of the grey matter at each segment. Eighty to ninety percent of the corticospinal axons decussate to the contralateral side at the pyramid level in the medulla oblongata (hence the name “pyramidal tracts”) and travel in the dorsolateral funiculus [83]. Corticospinal tracts are mostly involved in voluntary skilled movements.
Other descending motor tracts originate mainly in subcortical nuclei in the brainstem, and particularly in the reticular formation, and are called “extra-pyramidal tracts.” Extra-pyramidal tracts are composed of the rubrospinal (located along the corticospinal tract in the dorsolateral funiculus), vestibulospinal, tectospinal, and reticulospinal tracts (all three located in the ventrolateral funiculus) [19, 174].
Those descending inputs are mainly involved in autonomic functions, postural control, and locomotion. More specifically, they facilitate contralateral upper limb flexion (rubrospinal tract), neck and head motor control (tectospinal tract), autonomic functions (reticulospinal tract), and regulate ipsilateral extensors and antigravity muscles to control tone and posture (vestibulospinal tract) [83]. Extra-pyramidal tracts project mainly on premotor lamina (lamina VI–VIII) of the spinal cord grey matter at each segment [19].
The role of reticulospinal pathways originating from the brainstem in the initiation and control of locomotion has been extensively studied, leading to the concept that, while the spinal CPG produces the basic locomotor rhythm (see Sect. 8.2.2.1), brainstem structures are necessary to activate and regulate this structure [98, 205].
Numerous studies, mainly using decerebrate cat preparations, have identified several areas within the brainstem that can lead to the production of locomotion when activated, whether chemically or electrically. The mesencephalic locomotor region (MLR), first identified by Shik et al. [191], receives inputs from both the basal ganglia, the limbic system and the frontal cortex, and projects to neurons of the medial medullary reticular formation (MRF), and then on to interneurons in the spinal cord [205]. When stimulated electrically in decerebrate cats, the MLR can generate different gait patterns (walking, trotting, galloping) depending on the strength of the electrical stimulus [176]. Interestingly, after its initial description in cats, areas homologous to the MLR have been described in many vertebrate species, including the rat [70], lamprey [136], and monkey [60].
Other areas in the midbrain, such as the medial MLR, the pontomedullary locomotor strip (PLS) or areas in the subthalamic nucleus (subthalamic locomotor region), have been shown to be involved in the control of locomotion by projecting onto spinal circuits through reticulospinal pathways in rodents [205]. More recently, isolated spinal cord preparations from neonatal rats and mice have allowed the identification of various neurotransmitters (N-methyl-D-aspartate, 5-hydroxytryptamine, dopamine, noradrenaline) that can elicit locomotor rhythmic activity by stimulating the spinal CPG through descending reticulospinal pathways [99].
In nonmammalian vertebrates, the descending control of locomotion has been particularly well documented in the lamprey [52]. Trigeminal relay cells activate reticulospinal neurons in a “all-or-nothing” fashion to elicit escape responses in response to a mechanical cutaneous stimulus [203]. In contrast, MLR inputs to reticulospinal neurons initiate locomotion in a graded fashion through monosynaptic cholinergic and glutamatergic inputs, with the middle rhombencephalic reticular nucleus (RRN) being activated for low intensity stimulation, and the posterior RRN being activated as the stimulation strength increases [204] (Fig. 8.3a). Lastly, recent investigations in zebrafish larvae have demonstrated the role of descending reticulospinal neurons in the nucleus of the medial longitudinal fasciculus (nMLF) in the modulation of swimming speed [189] and swim posture [200].
Neural substrates of spinal sensorimotor integration across vertebrates. A. Descending motor control. In the lamprey, a mechanical stimulation to the head activates reticulospinal neurons through the trigeminal nerve, eliciting escapes reponses in an all-or-nothing fashion (A1 left). Swimming episodes can also be elicited by stimulating the Mesencephalic Locomotor Region (MLR), which projects onto reticulospinal neurons in the middle and posterior rhombencephalic reticular nuclei with a graded synaptic input (A1, right) Adapted from Dubuc et al. 2008. In mammalian vertebrates, forebrain regions such as the primary motor cortex can initiate locomotion by projection on the MLR, which in turn activate descending motor pathways that modulate the spinal circuitry (A2). Adapted from Goulding, 2009. B. Intraspinal circuitry. Based on this molecular homology, similar neuronal cell types can be identified in the zebrafish (B1) and mouse (B2) spinal cords, as indicated by the same color in the schematic. Zebrafish homologs of the mouse interneurons are: CoSA/MCoD (V0), CiA (V1), CiD (V2a), VeLD (V2b), UCoD/VeMe (V3). Adapted from Goulding, 2009. C. Ascending sensory feedback. In the lamprey, intraspinal stretch receptors called the “edge cells” are activated upon mechanical bending of the spinal cord and could serve as mechanoreceptor during swimming (C1, top. Adapted from Grillner et al. 1984 and Di Prisco et al. 1990). In the zebrafish, the lateral line can be used to sense the water flow and provide feedback for rheotaxis behavior. Ablation of the lateral line neuromasts results in the inability for the fish to successfully escape a suction source (percentage of larvae holding against the water flow in black) (C1, bottom. Adapted from Olszewski et al. 2012). In mammalian vertebrates, cutaneous and proprioceptive muscle receptors provide sensory feedback to the spinal circuitry and can modulate the motor output in a phase and state-dependent manner (C2. Adapted from Rossignol et al. 2006).
8.2.1.2 Ascending Sensory Feedback
While descending inputs schematically provide the motor command to spinal sensorimotor circuits, ascending afferents to the spinal cord mainly provide sensory information. In mammals, ascending sensory afferents include proprioceptive inputs (group Ia and II afferents from, respectively, primary and secondary endings of muscles spindles, and Ib afferents from Golgi tendon organs) and cutaneous inputs (chemosensitive group III/Aδ and group IV/C fibers from nociceptive receptors). These have been extensively studied in the context of local spinal reflex pathways [108, 176] (Fig. 8.2b).
The simplest, and fastest, somatic reflex is mediated by the monosynaptic pathway between primary sensory afferents from primary muscle spindles (Ia) and homonymous alpha motoneurons in the ventral horn of the corresponding segment grey matter. This drives the basic myotatic reflex that is elicited by a muscle stretch due to a tendon tap, but it is also involved in tonus and postural adjustments [83]. The experimental analog of the Ia reflex, the Hoffman reflex (H-reflex), where the mechanical stretch is replaced by a subthreshold electrical stimulation of the afferent nerve, has been extensively used to investigate spinal sensorimotor circuits, and in particular presynaptic and reciprocal inhibition [95, 108], see (Sect. 8.2.2.1).
Golgi tendon organs are force-sensitive receptors located at the muscle-tendon junction, which are activated by passive and active muscle force. The Ib reflex arc, also known as the “inverse myotatic reflex,” is a disynaptic pathway by which group Ib sensory afferents from Golgi tendon organs inhibit alpha-motoneurons. This is the reflex arc responsible for the abrupt termination of the myotatic reflex, the well-known “clasp-knife” phenomenon [93]. Although stimulating the Golgi tendon organs at rest cannot induce any movement, the Ib reflex has been suggested to be important for regulating muscle stiffness [108].
While group Ib afferents from Golgi tendon organs provide information about the tension developed during muscle contraction, and group Ia afferents from primary muscle spindles inform spinal circuits about the dynamic of changes in muscle length, group II afferents from muscle spindle secondary endings provide information of muscle length itself [96]. Group Ia, Ib, and II muscle afferents taken together constitute what is generally termed the “proprioception” input. Together with cutaneous afferents from nociceptors (Aδ and C fibers) and other muscle afferents (thinly myelinated group III and unmyelinated group IV fibers), group II muscle afferents constitute the flexion reflex afferents (FRA) involved in the withdrawal reflex, by which a painful stimulus leads to withdrawal of the limb through ipsilateral flexion and contralateral extension [55]. This sensorimotor reflex, more sophisticated than the “myotatic” and “inverse myotatic” reflexes, involves at least to two interneurons that either activate or inhibit the ipsilateral flexor or extensor alpha-motoneurons over several spinal segments [83].
Sensory feedback pathways in nonmammalian vertebrates still remain unclear. Indeed, there is no clear equivalent to mammalian peripheral proprioceptive receptors in swimming vertebrates. However, in the lamprey, intraspinal mechanosensitive receptors called the “edge cells” [81] might provide movement-related sensory feedback [48]. Interestingly, it has recently been proposed that edge cells could be modulated by GABAergic cerebrospinal fluid contacting neurons (CSF-cNs) [94]. Similar CSF-cNs, called “Kolmer-Agduhr” cells, have been described in the zebrafish and were able to modulate slow swimming upon optical activation [208]. Another sensory feedback pathway in larvae and adult zebrafish is the lateral line system [72]. Mechanosensory hair cells in the lateral line neuromasts provide information about the water flow, contributing to orientating the fish against the water, a behavior called “rheotaxis” [154] (Fig. 8.3c).
8.2.2 Intrinsic Spinal Sensorimotor Circuitry
8.2.2.1 Sensorimotor Interneuronal Networks
Presynaptic Inhibition
As we have seen, spinal circuits are continuously provided with multiple ascending sensory inputs from various sources. This sensory feedback needs to be controlled to allow for the proper execution of a motor task [108]. One way to control this sensory input is through presynaptic inhibition of muscle afferents on alpha-motoneurons through GABAergic axo-axonal synapses [178] (Fig. 8.2b). A similar control can be achieved through primary afferent depolarization (PAD), and the two phenomena are now actually considered to be mediated by the same interneurons [95].
Initially described in relation to group Ia afferents from primary endings of muscle spindles [64], presynaptic inhibition through GABAergic interneurons has more recently also been described for group Ib and group II muscle afferents, as well as cutaneous and articular afferents [177]. Although it has traditionally been thought that different subgroups of interneurons mediate PAD of distinct muscle sensory afferents [95], it has also been demonstrated that the same interneurons, located within Rexed’s laminae VI–VII of the spinal cord grey matter (intermediate zone), could be coexcited by group Ia and group Ib afferents [62]. More surprisingly, even group Ib and group II inputs can be integrated by a common pool of interneurons, located within laminae V–VII [14]. These results led some authors to consider that all those subpopulations of interneurons (groups Ia, Ib and II) may actually operate as a single functional population with multisensory inputs from both several types of afferents and several muscles [96]. (Fig. 8.2b)
Reciprocal Ia Inhibition
Considering that the same Ia muscle afferents innervate motoneurons belonging to many different motor pools, it has long been postulated that a neural pathway involving Ia afferents allowed for inhibition of alpha-motoneurons controlling antagonist muscles. The reciprocal Ia inhibition is mediated by a single glycinergic inhibitory interneuron activated by Ia afferents from a given flexor muscle, which in turn inhibits alpha-motoneurons controlling the antagonistic extensor muscle [58, 95]. As for PAD interneurons, it has later been showed that these reciprocal Ia inhibitory interneurons, located dorsomedially to the motor nuclei in the ventral horn, actually also receive convergent inputs, both excitatory and inhibitory, from multiple descending and ascending sources, including Renshaw cells (see below) [92] (Fig. 8.2b).
Nonreciprocal Ib Inhibition
Group Ib sensory afferents from Golgi tendon organs inhibit motoneurons projecting to synergist muscles and facilitate motoneurons projecting to antagonist muscles through di- or tri-synaptic pathways involving respectively one or two inhibitory glycinergic interneurons [57, 95]. As for Ia interneurons mediating reciprocal inhibition, Ib inhibitory interneurons exhibit a wide convergence of inputs from both descending inputs (excitatory corticospinal, rubrospinal, and inhibitory reticulospinal afferents) and ascending inputs (excitatory group Ia and Ib muscle afferents, as well as cutaneous and joint afferents) [92] (Fig. 8.2b).
Recurrent Inhibition
Lastly, another sensorimotor interneuronal pathway involving an inhibitory interneuron is the one formed by Renshaw cells, located in the ventral horn (next to Ia reciprocal inhibitory interneurons) [166]. Renshaw cells are excited by cholinergic axonal collaterals from alpha-motoneurons and provide glycinergic recurrent inhibition to the same or synergistic muscles [56]. Again, as for other sensorimotor interneurons, Renshaw cells also receive inputs from other afferents, including ipsilateral group II and III muscle afferents, cutaneous afferents, and descending motor afferents, and project themselves not only to alpha-motoneurons but also to gamma-motoneurons, Ia reciprocal inhibitory interneurons, and other Renshaw cells within the same spinal segment [206].
8.2.2.2 Spinal Central Pattern Generator (CPG) Across Vertebrates
Along with this complex interplay between sensory afferents and sensorimotor interneuronal networks, a large amount of work has converged toward the identification of a spinal network able to generate the elementary patterns and rhythms of locomotion: the spinal CPG. First postulated from studies of decerebrated cats more than a century ago [29], extensive research in nonmammalian vertebrate species such as the lamprey [80] and the Xenopus tadpole [169] has provided many insights into the swimming CPG and its cellular mechanisms, leading to rapid advances in the understanding of the mammalian walking CPG [102].
Homology Across Vertebrates
Interestingly, new insights into the genetic profiles of spinal interneurons have allowed direct comparison between different classes of interneurons across all vertebrates [76]. Based on the dynamic expression pattern of transcription factors, five major subclasses of spinal ventral interneurons have been described, called “V0, V1, V2, V3, and Hb9 interneurons” (Fig. 8.3). Each class being characterized by a specific transcription factor, such as “molecular code” opens the way for functional investigation of genetically targeted, rather than morphologically or electrophysiologically identified, spinal interneurons within the CPG (Fig. 8.3b).
Excitatory Rhythm-Generating Circuits
Several lines of evidence suggest that the rhythmogenic neurons of the CPG are glutamatergic excitatory neurons projecting ipsilaterally onto inhibitory left–right and flexor–extensor coordinating cells at each spinal segment [104]. Indeed, blocking inhibitory commissural or ipsilateral interneurons does not prevent rhythm generation, whether in the lamprey [35], rodent [22] or cat [100], therefore discarding the “half-center model” for CPG rhythm generation [102]. Various putative candidates for the role of “pacemakers” neurons have been recently investigated [103]. Among them, Hb9 [199] and V2a-Chx10 expressing interneurons [88] have been shown to have rhythmogenic properties in neonatal mouse models. Morphological homologs in the lamprey [80] and tadpole [123], and molecular homologs in zebrafish [139] support the hypothesis of a glutamatergic ipsilateral drive to the spinal CPG.
Flexor–Extensor Coordination
Ipsilateral-projecting glycinergic inhibitory interneurons are known to be involved in alternation of extensor and flexor muscles activation, since flexor–extensor coordination is suppressed when glycinergic transmission is blocked but can persist in hemisected spinal preparations [22]. Putative candidate interneurons include Ia inhibitory interneurons and Renshaw cells (see Sect. 2.2.1), as both have been shown to fire rhythmically during locomotion and in opposing phases with respect to their flexor/extensor afferents [138].
However, a recent study challenged this assumption [74]. V1-derived interneurons expressing the transcription factor Engrailed-1 (En1) are inhibitory ipsilaterally projecting interneurons that give rise to Renshaw cells and Ia inhibitory interneurons. Genetic knockout of En1-expressing neurons induced slower locomotor activity and increased step cycle, but did not suppress flexor–extensor coordination. This suggests the existence of other ipsilateral inhibitory interneurons, which might be specific to mammalian locomotor CPG [102].
Left–Right Coordination
Coordination of left–right activity during locomotion is mainly achieved through commissural interneurons that cross the midline via the ventral commissure [102]. Experiments in mice have revealed a dual system for left/right coordination: (1) during alternative walking, inhibition of contralateral motoneurons is achieved either through mixed glycinergic and GABAergic inhibitory commissural interneurons that project monosynaptically to contralateral motoneurons, or excitatory commissural interneurons that project onto contralateral inhibitory premotor interneurons; (2) during synchronous “hopping,” contralateral motoneurons are excited by glutamatergic commissural interneurons [165].
Candidate commissural interneurons for this dual model are derived from Dbx1 positive cells from the V0 transcription domain [115], in which about one-third of commissural interneurons are glutamatergic (Evx-1-positive, V0V interneurons) and two-thirds are inhibitory (Evx1-negative, V0D interneurons) [143]. A recent study [198] confirmed and further refined this hypothesis by showing that V0-ablated mice exhibited a hopping gait at all frequencies. Selective ablation of inhibitory V0 interneurons (V0D) led to a lack of left–right alternation only at low frequencies, whereas selective ablation of excitatory V0 interneurons (V0V) led to similar hopping gait but only at medium and high frequencies.
Neurons participating in the left–right alternation spinal network have also been identified in nonmammalian vertebrates. In the Xenopus tadpole, inhibitory glycinergic commissural interneurons are responsible for mid-cycle reciprocal inhibition and are driven by descending glutamatergic interneurons [169]. In the lamprey, both inhibitory and excitatory commissural interneurons have been described with a left–right alternating pattern of activity [80]. Lastly, similar glycinergic inhibitory and glutamatergic excitatory commissural interneurons have been identified in the zebrafish, sharing molecular markers with the mouse V0 neurons, although the details of their network functions have not yet been worked out [61].
8.2.3 Dynamic Spinal Sensorimotor Interactions
8.2.3.1 Modulation of Spinal Circuitry from Extrinsic Inputs
Both descending motor inputs and ascending sensory feedback can modulate the activity of the spinal CPG. Dynamic sensorimotor interactions with both supraspinal and peripheral inputs continuously modulate CPG-generated activity patterns to achieve a flexible adaptation to the environment. Such interactions take place in a phase-dependent (swing/stance) and state-dependent (forward/backward) manner, such that extrinsic inputs will result in different modulations depending on the ongoing phase of the locomotor cycle [176].
As discussed in Sect. 2.1.1, supraspinal pathways, such as the MLR and its projections through the reticulospinal tract, can induce locomotion in “fictive preparations” such as isolated spinal cord or decerebrated adult cat. However, descending pathways, whether carrying sensory or motor information, can also modulate ongoing locomotion. This can be achieved either though modulation of brainstem command circuitry or through the direct modulation of spinal circuitry [137].
Vestibular inputs (relaying information about balance and posture) phasically modulate the activity of reticulospinal neurons during fictive locomotion in lampreys, thereby avoiding a counteractive drive from reticulospinal neurons during ongoing locomotion [34]. A recent study in zebrafish larvae suggested that vestibular inputs are able to differentially recruit dorsal and ventral premotor spinal microcircuits during postural correction, possibly prefiguring the mammalian modular organization of spinal flexor/extensor microcircuits [13]. The influence of visual feedback on the control of locomotion can be experienced on a daily basis when one needs to anticipate and adjust his gait to avoid an obstacle [173]. New experimental paradigms, such as the optomotor response in zebrafish [155], have started to shed light on the neural circuitry responsible for visually induced locomotion.
Besides descending inputs, ascending sensory feedback, from either proprioceptive or cutaneous inputs, can also modulate the activity of the spinal CPG. Cutaneous inputs (C and A fibers, see Sect. 2.1.2) are mainly involved in correcting the steps in response to external perturbations, such as an uneven floor, during the different phases of the step cycle [137, 176]. Interestingly, the same cutaneous stimulus can lead to responses in flexor or extensor muscles depending on the initial position of the limb, thereby exciting a given muscle group in one locomotor phase, and the antagonist muscles in the opposite phase, a phenomenon termed “reversal” [175].
Proprioceptive feedback also has an important role in modulating ongoing locomotion, in particular by adjusting the duration of, and facilitating the switch between, the different phases of the step cycle, therefore setting the frequency of locomotion [176]. In decerebrate cat preparations, stimulation of group Ib afferents from the Golgi tendon organs of extensor muscles can reset the locomotor cycle by abruptly terminating the ongoing fictive flexor activity and initiating a new burst in the extensor recording [40]. Similarly, stretch-evoked Ia inputs can increase the duration of the stance phase, but only when stimulated during flexor activity [84].
In all, patterns of fictive locomotion produced by the spinal CPG should not be considered the fixed output of a hard-wired circuit, but should be viewed rather as a dynamic multimodal process whose output is dramatically modulated by sensory experience.
8.2.3.2 Implications for Plasticity After Spinal Cord Injury
The emerging concept that intrinsic spinal circuits can produce adaptive locomotion through modulation by sensory feedback, and do so independently, at least to some extent, from supraspinal inputs, bears important consequences for new neurorehabilitative strategies after spinal cord injury.
Experimental paradigms with adult cats walking on a treadmill have demonstrated that neither bilateral lesion of the dorsolateral spinal cord (interrupting cortico- and rubrospinal tracts) [97] nor bilateral lesion of the ventrolateral spinal cord (interrupting vestibulo- and reticulospinal tracts) [30] could permanently suppress quadrupedal locomotion. However, after unilateral complete hemisection at the lower thoracic (T13) level, interrupting both dorsal and ventral descending pathways, cats showed a complete paralysis of the ipsilateral hindlimb during the first 3 days, followed by a progressive recovery over the following 3 weeks [174]. Interestingly, this recovery was accompanied by a modification of the step cycle, forelimb/hindlimb, and left/right coordination [135]. These results suggest that the intrinsic spinal circuitry is able to produce locomotion even after removal of all supraspinal inputs and that this recovery is underpinned by extensive reorganization of the spinal sensorimotor network [134]. They also suggest that treadmill-induced locomotor training, by providing sensory feedback, is crucial to drive the reorganization of spinal circuits [174].
To test this hypothesis of a plastic spinal CPG, Rossignol et al. designed a dual-lesion paradigm in which a first hemisection performed at the T10/T11 spinal level is followed, after several weeks of locomotor training and complete recovery, by a complete spinal transection at the T13 level [15, 134]. The major finding was that cats regained full locomotor performance after only 24 h, without any training of pharmacological intervention [15], therefore indicating that intrinsic changes within the spinal CPG had indeed occurred during the rehabilitation period, and could be retained after the complete removal of supraspinal inputs.
Similar results have been obtained recently in rodents [59], in which recovery of coordinated hindlimbs locomotion on a treadmill could be achieved only 1 week after complete thoracic (T7) spinal transection when combined lumbosacral electrical epidural stimulation (EES) and systemic application of serotoninergic agonists were applied [41]. Interestingly, removing peripheral sensory inputs by unilateral dorsal rhizotomy prevented EES-facilitated locomotor recovery after complete spinal transection, but only on the deafferented side, thereby confirming the hypothesis that sensory feedback drives the reorganization of intrinsic spinal circuitry [117].
However, those results only concerned treadmill-induced “automatic” locomotion. To what extent can we exploit the plasticity of spinal sensorimotor circuits to induce restoration of voluntary locomotion? This question was investigated by a recent study [25], in which the authors used a simultaneous dual hemisection paradigm in adult rats together with a so-called electrochemical neuroprosthesis (i.e., the combination of lumbosacral epidural electrical stimulation together with systematic administration of a cocktail of monoaminergic agonists). They observed that rats trained with a robotic postural interface encouraging supraspinally mediated locomotion could regain voluntary control through remodeling of corticospinal projections. A similar approach has even been used successfully in a paraplegic human subject, who could regain some voluntary control of one of his lower extremities after intensive rehabilitation and electrical epidural stimulation, although this recovery was very limited and observed in few individuals [7, 86].
These results have raised hopes that clinically significant locomotor recovery can be achieved through reorganization of intrinsic sensorimotor circuitry, facilitated by intensive training and electrical and/or chemical manipulation. However, one major issue of such studies is that they can probe changes in spinal circuitry only in a very indirect manner.
Indeed, until now, one had to choose whether to access spinal circuitry in open-loop “fictive” preparations, discarding sensory feedback in favor of identifying and recording from neurons within the spinal cord, or to preserve active locomotion and sensory feedback at the cost of having only limited and indirect access to spinal circuits. New tools and animal models might change this conundrum in a near future.
8.3 Closing the Loop? Optogenetic Manipulation of Spinal Sensorimotor Circuits in Zebrafish
8.3.1 Genetic Targeting of Spinal Sensorimotor Circuits in Zebrafish
8.3.1.1 Identified Sensorimotor Neurons in the Zebrafish Spinal Cord
“Closing the loop” in sensorimotor studies should involve the ability to easily target neuronal populations of interest and monitoring their activity in vivo while the sensorimotor integration actually occurs. Over the last 10 years, zebrafish has become an increasingly popular model organism for such studies, thanks to its genetic accessibility with numerous transgenic lines targeting specific subsets of neurons being shared among the community [180, 187], its optical transparency allowing the use of the always expanding palette of optogenetic actuators and reporters [2, 51, 90, 208], and its relatively simple and stereotyped behavioral repertoire [31, 139, 141].
As in other vertebrates, neurons in the zebrafish spinal cord can be broadly classified as motoneurons, sensory neurons, and interneurons [119]. The recent development of genetic tools allowing specific targeting of subtypes of neurons has allowed marked progress in our understanding of their functional roles, and has led to a refined classification.
Sensory neurons within the spinal cord comprised mainly mechanosensitive Rohon-Beard cells, of which homologs can be found in most anamniotic vertebrates, such as Xenopus tadpoles and lampreys [167]. Rohon-Beard neurons are derived from the same neural plate domain that generates neural crest cells, and die during development to be replaced by dorsal root ganglion cells in adult zebrafish [119]. When stimulated optically, Rohon-Beard neurons are able to trigger escape responses [51, 208], through either direct excitation of reticulospinal cells [51] or activation of CoPA interneurons [159].
In larvae, both primary and secondary motoneurons (together with oligodendrocytes) are derived from the pMN transcription domain in the ventral spinal cord, are positive for olig2 expression, and persist through adulthood [105, 119]. Primary motoneurons are located more dorsally (with subtypes according to their position from caudal to rostral: CaP, MiP, RoP), innervate fast muscles, and mediate fast swimming and the startle response, while secondary motoneurons are located more ventrally, innervate both slow and fast muscles, and are involved in slow swimming [119].
To explore the differences between slow swimming and escape spinal networks, Ritter et al. [168] used a head-embedded preparation in which they could elicit either slow swim by illuminating the head with a fiber optic, or escapes by tapping the head with a piezoelectric actuator. They simultaneously monitored the activity of morphologically identified interneurons in the embedded part of the tail using calcium imaging and recorded the movements of the caudal tail using a high-speed camera. They showed that “circumferential ipsilateral descending” (CiDs) interneurons were activated during escapes but not during slow swim movements, while excitatory glutamatergic “multipolar commissural and descending” (MCoDs) interneurons were, on the contrary, activated during swimming but not during escapes [168].
A subsequent study from the same group [18] combining calcium imaging and paired patch recording confirmed that CiDs were responsible for motoneuron excitation during escapes and showed that stronger escapes elicited by a head tap were associated with the recruitment of a larger number of CiDs than delayed escapes elicited by a tail tap, although the effect of the descending control from the hindbrain seems subject of debate [18, 126]. Interestingly, the same authors also demonstrated that reinnervation of CiDs by regenerating Mauthner axon, following injection of cAMP, was associated with improved locomotor performances [17].
Using isolated spinal cord from larval zebrafish, a “topographic map” of recruitment for motoneurons and premotor interneurons has been documented [139, 140]. MCoDs interneurons, located in the ventral spinal cord, provide a phasic drive to a subset of ventral contralateral motoneurons during slow swimming patterns. When the swimming frequency was increased, MCoDs were inhibited through glycinergic synapses, while CiDs interneurons became progressively activated along a dorsoventral gradient [140]. Of interest is the fact that CiDs interneurons are the fish homologs of the mouse V2a interneurons [5, 13, 106, 107] (see Sect. 2.2.2).
It has also been shown in adult zebrafish that different motoneuron pools exhibit different patterns of recruitment, with slow, intermediate, and fast secondary motoneurons being recruited progressively as the fictive locomotion frequency increased, while fast primary motoneurons were recruited only during presumed escapes. Moreover, the distribution of these different motoneurons pools also followed a ventro-dorsal gradient, from slow secondary motoneurons to fast primary motoneurons [5, 68].
Apart from premotor interneurons, other populations of interneurons are also rhythmically activated during fictive locomotion. Glycinergic “circumferential ascending (CiA) interneurons,” that are Engrailed-1 positive interneurons derived from the V0 transcription domain, monosynaptically inhibit “commissural primary ascending” (CoPA) interneurons during swimming [91]. Remarkably, CoPA interneurons are glutamatergic and relay excitation from Rohon-Beard sensory neurons, providing a connectivity pattern that would be consistent with a homologous sensorimotor gating pathway to that observed in the Xenopus tadpole [120, 121].
“Commissural local” (CoLo) interneurons are inhibitory glycinergic interneurons driven by gap junctional inputs from reticulospinal cells (Mauthner cells, see Sect. 3.3) that have been shown to exert monosynaptic inhibition on contralateral primary motoneurons during fast swimming, thereby enhancing the efficiency of the escape responses [180]. Lastly, Kolmer-Agduhr interneurons, which are GABAergic cells located next to the central canal and have cilia extending into the cerebrospinal fluid, have been shown to trigger slow swimming when optically stimulated [208].
Many other subtypes of spinal interneurons have been identified and classified, mainly according to their morphology and neurotransmitter phenotype [85, 181], but their involvement in sensorimotor circuits remains to be elucidated.
8.3.1.2 A Genetic Toolbox for Targeting Populations of Neurons
Considering the large number of cells involved in spinal sensorimotor circuits, even in a simple vertebrate such as the zebrafish, one crucial requirement for investigating their functional roles is to be able to specifically target the neural subpopulation of interest. Rather than relying on morphological cues, identification of specific promoters and new tools to efficiently generate and screen transgenic lines has recently allowed researchers to take full advantage of the optical and genetic accessibility of the zebrafish model.
The most straightforward approach to target a given neuronal population is to identify a specific gene with selective expression in the population of interest and generate a bacterial artificial chromosome (BAC) incorporating a reporter such as green fluorescent protein (GFP) into the gene’s genomic locus. Alternatively, if a minimal enhancer sequence can be identified, it can be subcloned into a smaller, plasmid-based expression system. The engineered construct is then microinjected into embryos at the single-cell stage to allow integration into the genome. Injected fish larvae are subsequently raised to adulthood and their offspring screened for fluorescence in order to establish the transgenic line [11]. Such an approach has been successfully used to produce transgenic lines labeling cranial motoneurons or trigeminal/Rohon-Beard sensory neurons with the Islet-1 promoter [89]. Animals that transiently expressed channelrhodopsin-2 were also used to investigate the role of Rohon-Beard and trigeminal neurons in the sensorimotor escape circuitry [51].
This approach can be combined with the bipartite Gal4/UAS system, widely used in drosophila, which relies on the specific expression of the yeast Gal4 transcriptional activator to drive the expression of a reporter gene placed under the control of repetitive Gal4-responsive upstream activator sequences (UAS) [9, 44]. Enhanced reporter expression can be obtained using Gal4-VP16 [112] or Gal4FF [9] fusion sequences and multiple (14X) repeats of the UAS. Stable zebrafish transgenic lines using the Gal4/UAS system have been created using Tol2-mediated transposition. A plasmid carrying the Tol2 transposon is injected in zebrafish embryos along with the Tol2 transposase mRNA, generating insertions throughout the zebrafish genome [10, 101]. Tol2-mediated Gal4-UAS transgenesis has been used to successfully perform enhancer-trap screens, leading to identification of a large number of stable transgenic lines selectively labeling subsets of spinal neurons [1, 9, 181, 187].
Another recent approach for genetic targeting of neurons in zebrafish is to combine viral gene delivery, using for instance rabies of sindbis viruses, together with the Tet system [214]. The Tet system works in a similar fashion to the Gal4/UAS system, with the transactivator (itTA) binding to the tTA-responder element (Ptet) to drive transcription of the downstream gene [75]. However, the Tet system has the advantage of being regulated by exogenously administered doxycycline, which binds to tTA and dramatically reduces its affinity to Ptet, turning off the expression of the gene of interest [214]. Interestingly, such silencing can also be used to generate sparse labeling in pan-neuronal HuC transgenic lines [214]. Combing the Tet and Gal4 systems provide exciting opportunities for combinatorial gene targeting of several neuronal populations of interest in zebrafish.
8.3.2 Optogenetic Tools for Monitoring and Breaking Neural Circuits
8.3.2.1 Reporters: Monitoring Neural Circuits
Monitoring neural activity can be indirectly achieved by measuring the intracellular calcium levels, since action potentials typically lead to a calcium influx through voltage dependent calcium channels [79]. This strategy has led to the elaboration of a number of chemical and genetically encoded calcium indicators (GECIs) that have been successfully used in many different animal models [149, 201] (Fig. 8.4a). GECIs consist of engineered fluorescent proteins having two key features: their emission properties are modified depending upon the intracellular level of calcium, and their pattern of expression can be restricted genetically. They include either permutated single fluorescent proteins whose fluorescence properties are modified when calcium is bound to an attached Ca2+ recognition element—as with the GCaMP family of indicators [147]—or pairs of fluorescent proteins in which conformational changes induced by calcium binding lead to FRET (Förster Resonance Energy Transfer)-mediated modulation of fluorescence [142].
Monitoring and manipulating neural circuits with genetically encoded reporters and actuators. A. Calcium indicators. Genetically encoded calcium indicators (GECIs) allows for monitoring neural activity through changes in intracellular calcium concentration. In a Förster resonance energy transfer (FRET)-based GECI (A1), such as Cameleon, a conformational change occurs after calcium ions binding between the two fluorescent proteins, leading to FRET, with a decrease in the 480 nm fluorescence and an increase in the 530 nm fluorescence. In a single-fluorophore GECI (A2), such as GCaMP, conformational modification upon calcium binding is intra-molecular, leading to an increase in the emitted fluorescence (515 nm). Bioluminescent GECIs, such as Aequorin, binding of calcium ions leads to oxidation of coelenterazine. Chemiluminescence resonance energy transfer (CRET) between aequorin and GFP is responsible for the emission of a green photon. Adapted from Grienberger et al., 2012. B. Optogenetic actuators. Following illumination with blue light (470 nm, blue pulses in B3), channelrhodopsin-2 allows the entry of cations into the cell (B1), triggering action potentials in whole-cell current-clamp (B3). Following illumination with yellow light (580 nm, yellow line in B3), halorhodopsin pumps chloride anions in the cell (B2), leading to hyperpolarisation and silencing of neuronal activity (B3). Adapted from Zhang et al. 2007.
The transparency of the zebrafish larva and its genetic accessibility make it an ideal model to use such optical tools for monitoring neural activity. In the first zebrafish study using a GECI (cameleon), expressed under the islet-1 promoter [89] (see Sect. 3.1.2), calcium transients could be observed within the spinal cord, in Rohon-Beard neurons activated by electrical cutaneous stimulation, and in motoneurons and CiD interneurons during escapes triggered by a mechanical head tap [90]. Since this first study, GECIs have been extensively used in zebrafish to monitor neural activity in various behavioral paradigms, including investigating the role of the optic tectum in prey capture [46], performing brain-wide monitoring of neural dynamics in a sensorimotor virtual environment [2] or testing neural coding of odors by the olfactory bulb [21]. Targeted mutagenesis and high-throughput screening have led to the continuous improvement of GECIs by optimizing their calcium affinity, kinetics, and dynamic range [3, 146, 149, 201]. From the first GCaMP [149] to the current GCaMP6 [38], and including the new generation of multicolor variants [4], the always improving GECI arsenal allows one to monitoring of neural activity under a wide range of conditions.
One major limitation of GECIs with particular relevance to the investigation of closed-loop sensorimotor behaviors in vivo is the need to provide focal excitation to the fluorescent proteins. This limitation implies constraining the neurons of interest (and therefore the animal itself) to a given location, either by partially embedding and/or paralyzing the animal. One alternative approach is to use the bioluminescent protein aequorin-GFP, derived from the jellyfish Aequorea victoria [192]. ApoAequorin, the naturally occurring complex of aequorin with GFP, binds to its substrate coelenterazine, which is then oxidized in the presence of calcium leading to the emission of a green photon by the GFP through chemiluminescence resonance energy transfer (CRET) [16]. Bioluminescence assays based on aequorin-GFP have been used not only for noninvasive monitoring of neural activity in vitro [170], but also in restrained flies [133] and freely behaving mice [171].
Taking advantage of this bioluminescence approach, neural activity in freely behaving zebrafish larvae has been monitored by genetically targeting the expression of aequorin-GFP to a specific subset of neurons and simultaneously counting the number of photons emitted over time while imaging locomotor activity [151]. Remarkably, the authors could monitor the activity of a small group of hypocretin-positive neurons in the hypothalamus over several days or combine a gated photomultiplier tube with stroboscopic illumination to record visually evoked behaviors [151]. While aequorin allows for noninvasive monitoring of an entire population of neurons in a moving animal, it does not provide any spatial information, thus making the specificity of genetic targeting a crucial limitation.
8.3.2.2 Actuators: Breaking Neural Circuits
Besides monitoring neural activity, the optical and genetic accessibility of the zebrafish larva also constitute an optimal playground for optogenetic actuators, making it possible to selectively activate or inhibit genetically targeted neurons [45, 162, 212] (Fig. 8.4b). Channelrhodopsin-2 (ChR2) is a light-gated channel derived from the unicellular alga Chlamydomonas reinhardtii that allows nonspecific influx of cations when illuminated with blue light [122, 148]. ChR2 can therefore be used to control a genetically targeted neuronal population with millisecond precision in a dynamic and reversible manner [24]. First tested in zebrafish to trigger escape responses by photoactivating Rohon-Beard neurons [51], ChR2 has subsequently been used to investigate diverse behaviors such as the optokinetic response [184] and modulation of odor responses [33]. Synthetic excitatory actuators that combine a chemical ligand with an ion channel, such as the light-gated ionotropic glutamate receptor (LiGluR, [73, 196]) and the light-gated metabotropic glutamate receptor (LimGluR2, [118]), have been successfully used to trigger neural activity in zebrafish. For instance, the potential role of Kolmer-Agduhr interneurons in modulating slow locomotion was investigated by combining LiGluR activation and Gal4/UAS enhancer-trap transgenics [208].
Optogenetics has also been used to selectively silence genetically targeted neurons in zebrafish, using the light-gated chloride pump halorhodopsin (NpHR), derived from the archaebacterium Natronomonas pharaonis [182, 194]. NpHR hyperpolarizes neurons by pumping chloride ions upon activation with yellow light, leading to optical silencing. Optical silencing with NpHR, and its improved variant eNpHR [77], can be combined with ChR2-mediated photoactivation to provide a versatile optogenetic toolbox to dissect circuits within the same animal [213].
Such a combined strategy has been successfully used in zebrafish to identify neurons in the hindbrain able to initiate locomotion through a rebound activity after eNpHR silencing [8], and for dissecting the mechanism of eye saccades during the optokinetic response [184]. In those two studies, light was delivered using optical fibers to achieve a high spatial selectivity in photoactivation. However, new microscopic techniques relying on light patterning with multimirror devices [21, 132] or temporal focusing of two-photon excitation [158] should allow for more complex 2D stimulation patterns. Lastly, 3D optical stimulation with a high spatiotemporal resolution could be achieved by combining digital holography and temporal focusing [156], opening the way for simultaneous imaging and neural manipulation in multiple planes in vivo [162].
8.3.3 The Escape Response as a Model for Sensorimotor Integration
8.3.3.1 The Escape Response and its Supraspinal Control
The “escape response” is a stereotyped sensorimotor behavior whereby an animal aims to avoid an approaching predator, which has been extensively described not only in many teleost fish species, including the goldfish and zebrafish [53], but also in other anamniotic vertebrates such as the lamprey [43] and the Xenopus tadpole [169]. Escape responses in zebrafish can be elicited by several types of sensory stimuli, such as touch to the head or the tail [18], a water jet to the otic vesicle [110] or an auditory-vestibular stimulus produced by a sound vibration [180]. In zebrafish larvae aged 6–9 days post-fertilization (dpf), the escape behavior typically consists of an initial, fast “C-shaped” bend, followed by a counter-bend in the opposite direction, and lastly a burst swim [31]. Typical kinematics parameters for escapes in zebrafish larvae are a mean angular velocity of 21.2°/ms, a mean duration until completion of the first bend of 10.4 ms, and a mean counter-bend angle of 125.1 [31].
The roles played by reticulospinal neurons, and in particular the “Mauthner cell” (M-cell), in the initiation of escape responses have been extensively documented, initially in the goldfish [54, 111]. The M-cell and its homologs MiD2 cm and MiD3 cm are paired reticulospinal neurons, located respectively in hindbrain rhombomeres 4 and 6, sending their descending axons to the contralateral spinal cord. The M-cells receive excitatory inputs from the auditory and vestibular branches of the VIIIth nerve, the posterior lateral line, and the optic tectum [150].
In head-embedded zebrafish larvae, monitoring of neural activity in reticulospinal cells by calcium imaging has demonstrated that, while M-cells are activated by mechanical stimulation of both the head and tail, its homologs MiD2 cm and MiD3 cm are only activated by head taps [153]. Ablation studies confirmed this result, showing that destruction of all three cell types delayed the escape responses elicited by both head and tail stimulation, while ablation of the M-cell alone specifically increased the latency of tail-induced escapes [126]. These results demonstrate significant functional overlap among the neurons that drive escape behavior.
Recent studies by the group of Oda [109, 110, 150] further refined our understanding of the descending control of this multimodal sensorimotor behavior. Using simultaneous calcium imaging of reticulospinal neurons and high-speed video recording of actual escapes elicited by a water jet to the otic vesicle, the authors demonstrated that activation of the Mauthner cell led to fast-onset (4–8 ms) escapes while activity in the MiD3 cm homolog gave rise to delayed escapes (8–12 ms), and that activation of these cells was mutually exclusive [109]. The authors subsequently showed that (1) before 75 h post-fertilization (hpf), suppression of auditory-vestibular inputs by selective ablation of the otic vesicle did not increase escape latency, whereas ablating the trigeminal ganglia responsible for relaying tactile input did; (2) after 90 hpf, eliminating auditory-vestibular inputs increased escape latency, whereas suppressing tactile input did not. These results therefore suggest a dual control of the escape behavior, switching during development from a preferentially touch-driven, long-latency, M-independent escape to a preferentially auditory-vestibular driven, short-latency, M-dependent pathway [110].
8.3.3.2 Monitoring Spinal Neurons During Active Locomotion
The ability to simultaneously record active locomotor behavior and monitor neural activity in partially restrained zebrafish has proven very valuable in dissecting the descending motor and sensory control of escape responses. Similar head-embedded experimental paradigms have also been used to investigate the recruitment of spinal interneurons during active locomotion [18, 168] (see Sect. 3.1.1). Although studies based on calcium imaging of either hindbrain or spinal neurons in partially restrained animals have been an important step forward in the study of sensorimotor behaviors such as the escape response, they cannot provide information about neural activity in the moving tail of the fish and so fail to account for sensory feedback arising from locomotion itself.
New techniques such as aequorin-based bioluminescence recording (see Sect. 8.3.2.1) will allow monitoring of specific neurons in actively moving animals, whether head-restrained or freely swimming. Using an experimental setup adapted from Naumann et al. [151] in which escape responses are elicited in head-embedded zebrafish larvae either by a water jet to the otic vesicle or by an auditory-vestibular sound stimulus, we can simultaneously record detailed kinematic parameters and count photons emitted by the aequorin-GFP. Taking advantage of the Gal4/UAS system to restrict the expression of aequorin-GFP to motoneurons, we obtain bioluminescence signals that report the recruitment of spinal motoneurons (Fig. 8.5, Knafo et al. unpublished) during behavior. This approach could prove particularly useful in investigating the recruitment of sensory spinal neurons during active locomotion and determining how sensory feedback from the moving part of the tail modulates the recruitment of motoneurons.
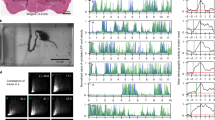
Monitoring the activity of spinal neurons during active escape responses in zebrafish larva. A. A setup for simultaneously recording active locomotion using a high-speed camera. B. A custom tracking software detects the animal body movement while a PMT is counting photons emitted by spinal motoneurons during escape responses in the transgenic line s1020t:gal4/UAS:GFP-Aequorin. C. Signals of bioluminescence and tail angle are plotted simultaneously: in blue: tail angle (in degree) between the first and last points of the tail over time, superimposed with the bioluminescent signal in green (number of photons emitted /10 ms).
8.4 Conclusion
The ability to monitor active behaviors in vivo with precise kinematics also provides a new framework in which results obtained from fictive recordings could be validated to confirm their environmental relevance. Moreover, the variability observed in real-world locomotor behaviors also questions whether “hard-wired” connectivity diagrams are actually the most suitable mean of modeling sensorimotor integration [131]. The emergence of multifunctional neuronal populations, that is. neurons that are recruited during multiple behaviors [124], as opposed to specialized neurons that are only active for a given motor output [180], will also benefit from in vivo studies involving active locomotion, in which multiple behaviors can be tested within the same animal [27].
The advances in genetic targeting and the identification of molecular markers to classify homologous populations of spinal neurons have allowed bringing together results obtained across animal models. However, the extent to which the walking CPG of mammalian vertebrates (such as rodents and cats) and the swimming CPG of nonmammalian vertebrates (such lampreys, zebrafish or tadpoles) can mutually inform each other remains unclear. In this regard, amphibian metamorphosis, during which the swimming CPG of a tadpole is transformed into a frog walking CPG, could provide an intriguing and unique model [193].
Sensorimotor behaviors are inherently a closed-loop process, where sensory feedback heavily influences the motor output. Although spinal networks do integrate this sensory information to modulate locomotion, detailed access to spinal sensorimotor circuitry has so far been only possible in open-loop preparations, where sensory feedback was not taken into account. New tools, such as optogenetic reporters and actuators, combined with genetically accessible animal models like zebrafish, should provide bright opportunities for monitoring targeted spinal sensorimotor neurons in actively moving animals and, possibly, closing the loop.
References
Abe, G., Suster, M. L., & Kawakami, K. (2011). Tol2-mediated Transgenesis, gene trapping, enhancer trapping, and the Gal4-UAS System. Methods in cell biology (3rd edn., Vol. 104, pp. 23–49). San Diego: Elsevier Inc. doi:10.1016/B978-0-12-374814-0.00002-1.
Ahrens, M. B., Li, J. M., Orger, M. B., Robson, D. N., Schier, A. F., Engert, F., et al. (2012). Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature, 485(7399), 471–477. doi:10.1038/nature11057.
Akerboom, J., Chen, T. W., Wardill, T. J., Tian, L., Marvin, J. S., Mutlu, S., et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(40), 13819–13840. doi:10.1523/JNEUROSCI.2601-12.2012.
Akerboom, J., Carreras Calderón, N., Tian, L., Wabnig, S., Prigge, M., Tolö, J., et al. (2013). Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in Molecular Neuroscience, 6, 2. doi:10.3389/fnmol.2013.00002.
Ampatzis, K., Song, J., Ausborn, J., & Manira, El. A. (2013). Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(26), 10875–10886. doi:10.1523/JNEUROSCI.0896-13.2013.
Andersen, R. A., Essick, G. K., & Siegel, R. M. (1985). Encoding of spatial location by posterior parietal neurons. Science , 230(4724), 456–458.
Angeli, C. A., Edgerton, V. R., Gerasimenko, Y. P., & Harkema, S. J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain: A Journal of Neurology, 137(Pt 5), 1394–409. doi:10.1093/brain/awu038.
Arrenberg, A. B., Del Bene, F., & Baier, H. (2009). Optical control of zebrafish behavior with halorhodopsin. Proceedings of the National Academy of Sciences, 106(42), 17968–17973. doi:10.1073/pnas.0906252106.
Asakawa, K., & Kawakami, K. (2009). The Tol2-mediated Gal4-UAS method for gene and enhancer trapping in zebrafish. Methods, 49(3), 275–281. doi:10.1016/j.ymeth.2009.01.004.
Asakawa, K., Suster, M. L., Mizusawa, K., Nagayoshi, S., Kotani, T., Urasaki, A., et al. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 105(4), 1255–1260. doi:10.1073/pnas.0704963105.
Asakawa, K., Abe, G., & Kawakami, K. (2013). Cellular dissection of the spinal cord motor column by BAC transgenesis and gene trapping in zebrafish. 1–14. doi:10.3389/fncir.2013.00100/abstract.
Baek, J.-H., Cosman, P., Feng, Z., Silver, J., & Schafer, W. R. (2002). Using machine vision to analyze and classify Caenorhabditis elegans behavioral phenotypes quantitatively. Journal of Neuroscience Methods, 118(1), 9–21. doi:10.1016/S0165-0270(02)00117-6.
Bagnall, M. W., & McLean, D. L. (2014). Modular organization of axial microcircuits in zebrafish. Science, 343(6167), 197–200. doi:10.1126/science.1245629.
Bannatyne, B. A., Liu, T. T., Hammar, I., Stecina, K., Jankowska, E., & Maxwell, D. J. (2009). Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: Differences in axonal projections and input. The Journal of Physiology, 587(Pt 2), 379–399. doi:10.1113/jphysiol.2008.159129
Barrière, G., Leblond, H., Provencher, J., & Rossignol, S. (2008). Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(15), 3976–3987. doi:10.1523/JNEUROSCI.5692-07.2008.
Baubet, V. V., Le Mouellic, H. H., Campbell, A. K. A., Lucas-Meunier, E. E., Fossier, P. P., & Brúlet, P. P. (2000). Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proceedings of the National Academy of Sciences of the United States of America, 97(13), 7260–7265.
Bhatt, D. H., Otto, S. J., Depoister, B., & Fetcho, J. R. (2004). Cyclic AMP-induced repair of zebrafish spinal circuits. Science, 305(5681), 254–258. doi:10.1126/science.1098439.
Bhatt, D. H., Mclean, D. L., Hale, M. E., & Fetcho, J. R. (2007). Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron, 53(1), 91–102. doi:10.1016/j.neuron.2006.11.011.
Bican, O., Minagar, A., & Pruitt, A. A. (2013). The spinal cord: A review of functional neuroanatomy. Neurologic Clinics, 31(1), 1–18. doi:10.1016/j.ncl.2012.09.009.
Blitz, D. M., & Nusbaum, M. P. (2011). Neural circuit flexibility in a small sensorimotor system. Current Opinion in Neurobiology, 21(4), 544–552. doi:10.1016/j.conb.2011.05.019.
Blumhagen, F., Zhu, P., Shum, J., Schärer, Y.-P. Z., Yaksi, E., Deisseroth, K., et al. (2012). Neuronal filtering of multiplexed odour representations. Nature, 479(7374), 493–498. doi:10.1038/nature10633.
Bonnot, A., Whelan, P. J., Mentis, G. Z., & O’Donovan, M. J. (2002). Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Research Reviews, 40(1–3), 141–151.
Boussaoud, D., Barth, T. M., & Wise, S. P. (1993). Effects of gaze on apparent visual responses of frontal cortex neurons. Experimental Brain Research (Experimentelle Hirnforschung. Expérimentation Cérébrale), 93(3), 423–434.
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., & Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience, 8(9), 1263–1268. doi:10.1038/nn1525.
Brand, R. V. D., Heutschi, J., Barraud, Q., DiGiovanna, J., Bartholdi, K., Huerlimann, M., et al. (2012). Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science, 336(6085), 1182–1185. doi:10.1126/science.1217416.
Branson, K., Robie, A. A., Bender, J., Perona P., & Dickinson, M. H. (2009). High-throughput ethomics in large groups of Drosophila. Nature Methods, 6(6), 451–457. doi:10.1038/nmeth.1328.
Briggman, K. L., & Kristan, W. B. (2008). Multifunctional pattern-generating circuits. Annual Review of Neuroscience, 31, 271–294. doi:10.1146/annurev.neuro.31.060407.125552.
Briggman, K. L., Abarbanel, H. D. I., & Kristan, W. B. (2006). From crawling to cognition: Analyzing the dynamical interactions among populations of neurons. Current Opinion in Neurobiology, 16(2), 135–144. doi:10.1016/j.conb.2006.03.014.
Brown, T. G. (1911). The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society B: Biological Sciences, 84(572), 308–319. doi:10.1098/rspb.1911.0077.
Brustein, E., & Rossignol, S. (1998). Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. Journal of Neurophysiology, 80(3), 1245–1267.
Budick, S. A., & O'Malley, D. M. (2000). Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. Journal of Experimental Biology, 203(17), 2565–2579.
Budick, S. A., & Dickinson, M. H. (2006). Free-flight responses of Drosophila melanogaster to attractive odors. Journal of Experimental Biology, 209(15), 3001–3017. doi:10.1242/jeb.02305.
Bundschuh, S. T., Zhu, P., Schärer, Y.-P. Z., & Friedrich, R. W. (2012). Dopaminergic modulation of mitral cells and odor responses in the zebrafish olfactory bulb. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(20), 6830–6840. doi:10.1523/JNEUROSCI.6026-11.2012.
Bussières, N., & Dubuc, R. (1992). Phasic modulation of transmission from vestibular inputs to reticulospinal neurons during fictive locomotion in lampreys. Brain Research, 582(1), 147–153.
Cangiano, L. (2005). Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: The lamprey hemicord. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(4), 923–935. doi:10.1523/JNEUROSCI.2301-04.2005.
Card, G. M. (2012). Escape behaviors in insects. Current Opinion in Neurobiology, 22(2), 180–186. doi:10.1016/j.conb.2011.12.009.
Carmena, J. M., Lebedev, M. A., Crist, R. E., O’Doherty, J. E., Santucci, D. M., Dimitrov, D. F., et al. (2003). Learning to control a brain–machine interface for reaching and grasping by primates. PLoS Biology, 1(2), e2. doi:10.1371/journal.pbio.0000042.
Chen, T.-W., Wardill, T. J., Sun, Y., Pulver, S. R., Renninger, S. L., Baohan, A., et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature, 499(7458), 295–300. doi:10.1038/nature12354.
Clark, D. A., Freifeld, L., & Clandinin, T. R. (2013). Mapping and cracking sensorimotor circuits in genetic model organisms. Neuron, 78(4), 583–595. doi:10.1016/j.neuron.2013.05.006.
Conway, B. A., Hultborn, H., & Kiehn, O. (1987). Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research (Experimentelle Hirnforschung. Expérimentation Cérébrale), 68(3), 643–656.
Courtine, G., Gerasimenko, Y., Brand, R. V. D., Yew, A., Musienko, P., Zhong, H., et al. (2009a). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nature Neuroscience, 12(10), 1333–1342. doi:10.1038/nn.2401.
Courtine, G., Gerasimenko, Y., Brand, R. V. D., Yew, A., Musienko, P., Zhong, H., et al. (2009b). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nature Neuroscience, 12(10), 1333–1342. doi:10.1038/nn.2401.
Currie, S. N. (1991). Vibration-evoked startle behavior in larval lampreys. Brain, Behavior and Evolution, 37(5), 260–271.
Davison, J. M., Akitake, C. M., Goll, M. G., Rhee, J. M., Gosse, N., Baier, H., et al. (2007). Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Developmental Biology, 304(2), 811–824. doi:10.1016/j.ydbio.2007.01.033.
Del Bene, F., & Wyart, C. (2012). Optogenetics: A new enlightenment age for zebrafish neurobiology. Developmental Neurobiology, 72(3), 404–414. doi:10.1002/dneu.20914.
Del Bene, F., Wyart, C., Robles, E., Tran, A., Looger, L., Scott, E. K., et al. (2010). Filtering of visual information in the tectum by an identified neural circuit. Science, 330(6004), 669–673. doi:10.1126/science.1192949.
Descending control of swim posture by a midbrain nucleus in zebrafish (2014). Descending control of swim posture by a midbrain nucleus in zebrafish. Neuron, 83(3), 679–691. doi:10.1016/j.neuron.2014.04.018.
Di Prisco, G. V., Wallén, P., & Grillner, S. (1990). Synaptic effects of intraspinal stretch receptor neurons mediating movement-related feedback during locomotion. Brain Research, 530(1), 161–166.
Dickinson, M., & Moss, C. F. (2012). Neuroethology. Current Opinion in Neurobiology, 22(2), 177–179. doi:10.1016/j.conb.2012.03.001.
Dombeck, D. A., & Reiser, M. B. (2012). Real neuroscience in virtual worlds. Current Opinion in Neurobiology, 22(1), 3–10. doi:10.1016/j.conb.2011.10.015.
Douglass, A. D. A., Kraves, S. S., Deisseroth, K. K., Schier, A. F. A., & Engert, F. F. (2008). Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Current Biology: CB, 18(15), 5–5. doi:10.1016/j.cub.2008.06.077.
Dubuc, R., Brocard, F., Antri, M., Fénelon, K., Gariépy, J.-F., Smetana, R., et al. (2008). Initiation of locomotion in lampreys. Brain Research Reviews, 57(1), 172–182. doi:10.1016/j.brainresrev.2007.07.016.
Eaton, R. C., Bombardieri, R. A., & Meyer, D. L. (1977). The Mauthner-initiated startle response in teleost fish. Journal of Experimental Biology, 66(1), 65–81.
Eaton, R. C., Lee, R. K., & Foreman, M. B. (2001). The Mauthner cell and other identified neurons of the brainstem escape network of fish. Progress in Neurobiology, 63(4), 467–485.
Eccles, R. M., & Lundberg, A. (1958). Significance of supraspinal control of reflex actions by impulses in muscle afferents. Experientia, 14(6), 197–199.
Eccles, J. C., Eccles, D. M., & Fatt, P. (1956). Pharmacological investigations on a central synapse operated by acetylcholine. Journal of Physiology, 131(1), 154–169.
Eccles, J. C., Eccles, R. M., & Lundberg, A. (1957a). Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. Journal of Physiology, 138(2), 227–252.
Eccles, J. C., Eccles, R. M., & Lundberg, A. (1957b). The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. Journal of Physiology, 137(1), 22–50.
Edgerton, V. R., Courtine, G., Gerasimenko, Y. P., Lavrov, I., Ichiyama, R. M., Fong, A. J., et al. (2008). Training locomotor networks. Brain Research Reviews, 57(1), 241–254. doi:10.1016/j.brainresrev.2007.09.002.
Eidelberg, E., Walden, J. G., & Nguyen, L. H. (1981). Locomotor control in macaque monkeys. Brain: A Journal of Neurology, 104(4), 647–663.
Fetcho, J. R., & Mclean, D. L. (2010). Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications. Annals of the New York Academy of Sciences, 1198, 94–104. doi:10.1111/j.1749-6632.2010.05539.x.
Fetz, E. E., Jankowska, E., Johannisson, T., & Lipski, J. (1979). Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. Journal of Physiology, 293, 173–195.
Flexor reflex afferents reset the step cycle during fictive locomotion in the cat (1998). Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research (Experimentelle Hirnforschung. Expérimentation Cérébrale), 122(3), 339–350.
Frank, K., & Fuortes, M. (1959). Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Federation Proceedings, 16, 39–40.
Franklin, D. W., & Wolpert, D. M. (2011). Computational mechanisms of sensorimotor control. Neuron, 72(3), 425–442. doi:10.1016/j.neuron.2011.10.006.
Fry, S. N., Rohrseitz, N., Straw, A. D., & Dickinson, M. H. (2008). TrackFly: Virtual reality for a behavioral system analysis in free-flying fruit flies. Journal of Neuroscience Methods, 171(1), 110–117. doi:10.1016/j.jneumeth.2008.02.016.
Frye, M. A. (2010). Multisensory systems integration for high-performance motor control in flies. Current Opinion in Neurobiology, 20(3), 347–352. doi:10.1016/j.conb.2010.02.002.
Gabriel, J. P., Ausborn, J., Ampatzis, K., Mahmood, R., Eklöf-Ljunggren, E., & Manira, El. A. (2011). Principles governing recruitment of motoneurons during swimming in zebrafish. Nature Publishing Group, 14(1), 93–99. doi:10.1038/nn.2704.
Gao, X. J., Potter, C. J., Gohl, D. M., Silies, M., Katsov, A. Y., Clandinin, T. R., et al. (2013). Specific kinematics and motor-related neurons for aversive chemotaxis in Drosophila. Current Biology: CB, 23(13), 1163–1172. doi:10.1016/j.cub.2013.05.008.
Garcia-Rill, E., Kinjo, N., Atsuta, Y., Ishikawa, Y., Webber, M., & Skinner, R. D. (1990). Posterior midbrain-induced locomotion. Brain Research Bulletin, 24(3), 499–508.
Ghazanfar, A. A., & Schroeder, C. E. (2006). Is neocortex essentially multisensory? Trends in Cognitive Sciences, 10(6), 278–285. doi:10.1016/j.tics.2006.04.008.
Ghysen, A. A., & Dambly-Chaudière, C. C. (2007). The lateral line microcosmos. Genes & Development, 21(17), 2118–2130. doi:10.1101/gad.1568407.
Gorostiza, P., Volgraf, M., Numano, R., Szobota, S., Trauner, D., & Isacoff, E. Y. (2007). Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 10865–10870. doi:10.1073/pnas.0701274104.
Gosgnach, S., Lanuza, G. M., Butt, S. J. B., Saueressig, H., Zhang, Y., Velasquez, T., et al. (2006). V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature, 440(7081), 215–219. doi:10.1038/nature04545.
Gossen, M., & Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proceedings of the National Academy of Sciences of the United States of America, 89(12), 5547–5551.
Goulding, M. (2009). Circuits controlling vertebrate locomotion: Moving in a new direction. Nature Reviews Neuroscience, 10(7), 507–518. doi:10.1038/nrn2608.
Gradinaru, V., Thompson, K. R., & Deisseroth, K. (2008). eNpHR: A natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biology, 36(1–4), 129–139. doi:10.1007/s11068-008-9027-6.
Green, A. M., & Angelaki, D. E. (2010). Multisensory integration: Resolving sensory ambiguities to build novel representations. Current Opinion in Neurobiology, 20(3), 353–360. doi:10.1016/j.conb.2010.04.009.
Grienberger, C., & Konnerth, A. (2012). Imaging calcium in neurons. Neuron, 73(5), 862–885. doi:10.1016/j.neuron.2012.02.011.
Grillner, S. (2003). The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience, 4(7), 573–586. doi:10.1038/nrn1137.
Grillner, S., Williams, T., & Lagerbäck, P. A. (1984). The edge cell, a possible intraspinal mechanoreceptor. Science, 223(4635), 500–503.
Grillner, S., Manira, El. A., Kiehn, O., Rossignol, S., & S G Stein, P. (2008). Networks in motion. Brain Research Reviews, 57(1), 1. doi:10.1016/j.brainresrev.2007.11.005.
Guertin, P. A. (2013). Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. 1–15. doi:10.3389/fneur.2012.00183/abstract.
Guertin, P., Angel, M. J., Perreault, M. C., & McCrea, D. A. (1995). Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. Journal of Physiology, 487(Pt 1), 197–209.
Hale, M. E. M., Ritter, D. A. D., & Fetcho, J. R. J. (2001). A confocal study of spinal interneurons in living larval zebrafish. The Journal of Comparative Neurology, 437(1), 1–16. doi:10.1002/cne.1266.
Harkema, S., Gerasimenko, Y., Hodes, J., Burdick, J., Angeli, C., Chen, Y., et al. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet, 377(9781), 1938–1947. doi:10.1016/S0140-6736(11)60547-3.
Harvey, C. D., Collman, F., Dombeck, D. A., & Tank, D. W. (2010). Intracellular dynamics of hippocampal place cells during virtual navigation. Nature, 461(7266), 941–946. doi:10.1038/nature08499.
Hägglund, M., Borgius, L., Dougherty, K. J., & Kiehn, O. (2010). Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nature Neuroscience, 13(2), 246–252. doi:10.1038/nn.2482.
Higashijima, S., Hotta, Y., & Okamoto, H. (2000). Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(1), 206–218.
Higashijima, S.-I., Masino, M. A., Mandel, G., & Fetcho, J. R. (2003). Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. Journal of Neurophysiology, 90(6), 3986–3997. doi:10.1152/jn.00576.2003.
Higashijima, S.-I., Masino, M. A., Mandel, G., & Fetcho, J. R. (2004). Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(25), 5827–5839. doi:10.1523/JNEUROSCI.5342-03.2004.
Hultborn, H. (1972). Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiologica Scandinavica (Supplementum), 375, 1–42.
Hultborn, H. (2006). Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Progress in Neurobiology, 78(3–5), 215–232. doi:10.1016/j.pneurobio.2006.04.001.
Jalalvand, E., Robertson, B., Wallén, P., Hill, R. H., & Grillner, S. (2014). Laterally projecting cerebrospinal fluid-contacting cells in the lamprey spinal cord are of two distinct types. The Journal of Comparative Neurology. doi:10.1002/cne.23542.
Jankowska, E. (1992). Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology, 38(4), 335–378.
Jankowska, E., & Edgley, S. A. (2010). Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. European Journal of Neuroscience, 32(6), 881–893. doi:10.1111/j.1460-9568.2010.07354.x.
Jiang, W., & Drew, T. (1996). Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. Journal of Neurophysiology, 76(2), 849–866.
Jordan, L. M., Liu, J., Hedlund, P. B., Akay, T., & Pearson, K. G. (2008a). Descending command systems for the initiation of locomotion in mammals. Brain Research Reviews, 57(1), 183–191. doi:10.1016/j.brainresrev.2007.07.019.
Jordan, L. M., Liu, J., Hedlund, P. B., Akay, T., & Pearson, K. G. (2008b). Descending command systems for the initiation of locomotion in mammals. Brain Research Reviews, 57(1), 183–191. doi:10.1016/j.brainresrev.2007.07.019.
Kato, M. (1987). Motoneuronal activity of cat lumbar spinal cord following separation from descending or contralateral impulses. Central Nervous System Trauma: Journal of the American Paralysis Association, 4(4), 239–248.
Kawakami, K., Shima, A., & Kawakami, N. (2000). Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proceedings of the National Academy of Sciences of the United States of America, 97(21), 11403–11408. doi:10.1073/pnas.97.21.11403.
Kiehn, O. (2006). Locomotor circuits in the mammalian spinal cord. Annual Review of Neuroscience, 29(1), 279–306. doi:10.1146/annurev.neuro.29.051605.112910.
Kiehn, O. (2011). Development and functional organization of spinal locomotor circuits. Current Opinion in Neurobiology, 21(1), 100–109. doi:10.1016/j.conb.2010.09.004.
Kiehn, O., Quinlan, K. A., Restrepo, C. E., Lundfald, L., Borgius, L., Talpalar, A. E., et al. (2008). Excitatory components of the mammalian locomotor CPG. Brain Research Reviews, 57(1), 56–63. doi:10.1016/j.brainresrev.2007.07.002.
Kimmel, C. B., Warga, R. M., & Kane, D. A. (1994). Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development, 120(2), 265–276.
Kimura, Y. (2006). Alx, a Zebrafish homolog of chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(21), 5684–5697. doi:10.1523/JNEUROSCI.4993-05.2006.
Kimura, Y., Satou, C., Fujioka, S., Shoji, W., Umeda, K., Ishizuka, T., et al. (2013). Hindbrain V2a neuronsin the excitation of spinal locomotor circuits during zebrafish swimming. Current Biology, 23(10), 843–849. doi:10.1016/j.cub.2013.03.066.
Knikou, M. (2008). The H-reflex as a probe: Pathways and pitfalls. Journal of Neuroscience Methods, 171(1), 1–12. doi:10.1016/j.jneumeth.2008.02.012.
Kohashi, T., & Oda, Y. (2008). Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(42), 10641–10653. doi:10.1523/JNEUROSCI.1435-08.2008.
Kohashi, T. T., Nakata, N. N., & Oda, Y. Y. (2012). Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(17), 5810–5820. doi:10.1523/JNEUROSCI.6169-11.2012.
Korn, H., & Faber, D. S. (2005). The mauthner cell half a century later: A neurobiological model for decision-making? Neuron, 47(1), 13–28. doi:10.1016/j.neuron.2005.05.019.
Koster, R. W., & Fraser, S. E. (2001). Tracing transgene expression in living zebrafish embryos. Developmental Biology, 233(2), 329–346.
Körding, K. P., & Wolpert, D. M. (2004). Bayesian integration in sensorimotor learning. Nature, 427(6971), 244–247.
Körding, K. P., & Wolpert, D. M. (2006). Bayesian decision theory in sensorimotor control. Trends in Cognitive Sciences, 10(7), 319–326. doi:10.1016/j.tics.2006.05.003.
Lanuza, G. M., Gosgnach, S., Pierani, A., Jessell, T. M., & Goulding, M. (2004). Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron, 42(3), 375–386.
Latorre, R., Levi, R., & Varona, P. (2013). Transformation of context-dependent sensory dynamics into motor behavior. PLOS Computational Biology. doi:10.1371/journal.pcbi.1002908.s002.
Lavrov, I., Courtine, G., Dy, C. J., Brand, R. V. D., Fong, A. J., Gerasimenko, Y., et al. (2008). Facilitation of stepping with epidural stimulation in spinal rats: Role of sensory input. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(31), 7774–7780. doi:10.1523/JNEUROSCI.1069-08.2008.
Levitz, J., Pantoja, C., Gaub, B., Janovjak, H., Reiner, A., Hoagland, A., et al. (2013). Optical control of metabotropic glutamate receptors. Nature Neuroscience, 16(4), 507–516. doi:10.1038/nn.3346.
Lewis, K. E., & Eisen, J. S. (2003). From cells to circuits: Development of the zebrafish spinal cord. Progress in Neurobiology, 69(6), 419–449.
Li, W.-C., Soffe, S. R., & Roberts, A. (2002). Spinal inhibitory neurons that modulate cutaneous sensory pathways during locomotion in a simple vertebrate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(24), 10924–10934.
Li, W.-C., Soffe, S. R., & Roberts, A. (2004). Dorsal spinal interneurons forming a primitive, cutaneous sensory pathway. Journal of Neurophysiology, 92(2), 895–904. doi:10.1152/jn.00024.2004.
Li, X., Gutierrez, D. V., Hanson, M. G., Han, J., Mark, M. D., Chiel, H., et al. (2005). Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proceedings of the National Academy of Sciences of the United States of America, 102(49), 17816–17821. doi:10.1073/pnas.0509030102.
Li, W.-C. W., Roberts, A. A., & Soffe, S. R. S. (2010). Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(49), 16609–16620. doi:10.1523/JNEUROSCI.3695-10.2010.
Liao, J. C., & Fetcho, J. R. (2008). Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(48), 12982–12992. doi:10.1523/JNEUROSCI.3330-08.2008.
Liu, K. S., & Sternberg, P. W. (1994). Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron, 14(1), 79–89. doi:10.1016/0896-6273(95)90242-2.
Liu, K. S., & Fetcho, J. R. (1999). Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron, 23(2), 325–335. doi:10.1016/S0896-6273(00)80783-7.
Loquet, G. (2013). Multisensory integration in non-human primates during a sensory-motor task. 1–15. doi:10.3389/fnhum.2013.00799/abstract.
Lundberg, A. (1979). Multisensory control of spinal reflex pathways. Progress in Brain Research, 50, 11–28. doi:10.1016/S0079-6123(08)60803–1.
Marder, E. (2011). Variability, compensation, and modulation in neurons and circuits. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl. 3), 15542–15548. doi:10.1073/pnas.1010674108.
Marder, E. (2012). Neuromodulation of neuronal circuits: Back to the future. Neuron, 76(1), 1–11. doi:10.1016/j.neuron.2012.09.010.
Marder, E., & Taylor, A. L. (2011). Multiple models to capture the variability in biological neurons and networks. Nature Neuroscience, 14(2), 133–138. doi:10.1038/nn.2735.
Martial, F. P., & Hartell, N. A. (2012). Programmable Illumination and high-speed, multi-wavelength, confocal microscopy using a digital micromirror. PloS ONE, 7(8), e43942. doi:10.1371/journal.pone.0043942.
Martin, J.-R., Rogers, K. L., Chagneau, C., & Brûlet, P. (2007). In vivo Bioluminescence imaging of Ca2+ signalling in the brain of drosophila. PloS ONE, 2(3), e275. doi:10.1371/journal.pone.0000275.s009.
Martinez, M., & Rossignol, S. (2013). A dual spinal cord lesion paradigm to study spinal locomotor plasticity in the cat. Annals of the New York Academy of Sciences, 1279(1), 127–134. doi:10.1111/j.1749-6632.2012.06823.x.
Martinez, M., Delivet-Mongrain, H., Leblond, H., & Rossignol, S. (2012). Incomplete spinal cord injury promotes durable functional changes within the spinal locomotor circuitry. Journal of Neurophysiology, 108(1), 124–134. doi:10.1152/jn.00073.2012.
McClellan, A. D., & Grillner, S. (1984). Activation of “fictive swimming” by electrical microstimulation of brainstem locomotor regions in an in vitro preparation of the lamprey central nervous system. Brain Research, 300(2), 357–361.
McCrea, D. A. (2001). Spinal circuitry of sensorimotor control of locomotion. Journal of Physiology, 533(1), 41–50.
McCrea, D. A., Pratt, C. A., & Jordan, L. M. (1980). Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. Journal of Neurophysiology, 44(3), 475–488.
Mclean, D. L., Fan, J., Higashijima, S.-I., Hale, M. E., & Fetcho, J. R. (2007). A topographic map of recruitment in spinal cord. Nature, 446(7131), 71–75. doi:10.1038/nature05588.
Mclean, D. L., Masino, M. A., Koh, I. Y. Y., Lindquist, W. B., & Fetcho, J. R. (2008). Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nature Neuroscience, 11(12), 1419–1429. doi:10.1038/nn.2225.
Mirat, O., Sternberg, J. R., & Severi, K. E. (2013). ZebraZoom: An automated program for high-throughput behavioral analysis and categorization. Frontiers in Neural. doi:10.3389/fncir.2013.00107/abstract.
Miyawaki, A., Llopis, J., Heim, R., McCaffery, J. M., Adams, J. A., Ikura, M., et al. (1997). Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature, 388(6645), 882–887. doi:10.1038/42264.
Moran-Rivard, L., Kagawa, T., Saueressig, H., Gross, M. K., Burrill, J., & Goulding, M. (2001). Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron, 29(2), 385–399.
Multisensory control of spinal reflex pathways (1979). Multisensory control of spinal reflex pathways. Progress in Brain Research, 50, 11–28. doi:10.1016/S0079-6123(08)60803-1.
Musienko, P., Brand, R. V. D., Märzendorfer, O., Roy, R. R., Gerasimenko, Y., Edgerton, V. R., et al. (2011). Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(25), 9264–9278. doi:10.1523/JNEUROSCI.5796-10.2011.
Muto, A., Ohkura, M., Kotani, T., Higashijima, S., Nakai, J., & Kawakami, K. (2011). Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 108(13), 5425–5430. doi:10.1073/pnas.1000887108/-/DCSupplemental.
Nagai, T., Sawano, A., Park, E. S., & Miyawaki, A. (2001). Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proceedings of the National Academy of Sciences of the United States of America, 98(6), 3197–3202.
Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 13940–13945. doi:10.1073/pnas.1936192100.
Nakai, J., Ohkura, M., & Imoto, K. (2001). A high signal-to-noise Ca(2 +) probe composed of a single green fluorescent protein. Nature Biotechnology, 19(2), 137–141. doi:10.1038/84397.
Nakayama, H. H., & Oda, Y. Y. (2004). Common sensory inputs and differential excitability of segmentally homologous reticulospinal neurons in the hindbrain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(13), 3199–3209. doi:10.1523/JNEUROSCI.4419-03.2004.
Naumann, E. A., Kampff, A. R., Prober, D. A., Schier, A. F., & Engert, F. (2010). Monitoring neural activity with bioluminescence during natural behavior. Nature Neuroscience, 13(4), 513–520. doi:10.1038/nn.2518.
Neural control and modulation of swimming speed in the larval zebrafish (2014). Neural control and modulation of swimming speed in the larval zebrafish. Neuron, 83(3), 692–707. doi:10.1016/j.neuron.2014.06.032.
O’Malley, D. M. D., Kao, Y. H. Y., & Fetcho, J. R. J. (1996). Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron, 17(6), 1145–1155. doi:10.1016/S0896-6273(00)80246-9.
Olszewski, J., Haehnel, M., Taguchi, M., & Liao, J. C. (2012). Zebrafish larvae exhibit rheotaxis and can escape a continuous suction source using their lateral line. PloS ONE, 7(5), e36661. doi:10.1371/journal.pone.0036661.g004.
Orger, M. B., Kampff, A. R., Severi, K. E., Bollmann, J. H., & Engert, F. (2008). Control of visually guided behavior by distinct populations of spinal projection neurons. Nature Neuroscience, 11(3), 327–333. doi:10.1038/nn2048.
Oron, D., Papagiakoumou, E., Anselmi, F., & Emiliani, V. (2012). Two-photon optogenetics. Progress in Brain Research, 196, 119–143. doi:10.1016/B978-0-444-59426-6.00007-0.
O’Doherty, J. E., Lebedev, M. A., Ifft, P. J., Zhuang, K. Z., Shokur, S., Bleuler, H., et al. (2012). Active tactile exploration using a brain–machine–brain interface. Nature, 479(7372), 228–231. doi:10.1038/nature10489.
Papagiakoumou, E., Anselmi, F., Bègue, A., de Sars, V., Glückstad, J., Isacoff, E. Y., et al. (2010). Scanless two-photon excitation of channelrhodopsin-2. Nature Methods, 7(10), 848–854. doi:10.1038/nmeth.1505.
Pietri, T., Manalo, E., Ryan, J., Saint-Amant, L., & Washbourne, P. (2009). Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Developmental Neurobiology, 69(12), 780–795. doi:10.1002/dneu.20741.
Pirri, J. K., & Alkema, M. J. (2012). The neuroethology of C. elegans escape. Current Opinion in Neurobiology, 22(2), 187–193. doi:10.1016/j.conb.2011.12.007.
Portugues, R. (2011). Adaptive locomotor behavior in larval zebrafish. 1–11. doi:10.3389/fnsys.2011.00072/abstract.
Portugues, R., Severi, K. E., Wyart, C., & Ahrens, M. B. (2013). Optogenetics in a transparent animal: Circuit function in the larval zebrafish. Current Opinion in Neurobiology, 23(1), 119–126. doi:10.1016/j.conb.2012.11.001.
Pouget, A., & Snyder, L. H. (2000). Computational approaches to sensorimotor transformations. Nature Neuroscience, 3 Suppl(supp), 1192–1198. doi:10.1038/81469.
Pouget, A. A., Deneve, S. S., & Duhamel, J.-R. J. (2002). A computational perspective on the neural basis of multisensory spatial representations. Nature Reviews Neuroscience, 3(9), 741–747. doi:10.1038/nrn914.
Quinlan, K. A., & Kiehn, O. (2007). Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(24), 6521–6530. doi:10.1523/JNEUROSCI.1618-07.2007.
Renshaw, B. (1946). Central effects of centripetal impulses in axons of spinal ventral roots. Journal of Neurophysiology, 9, 191–204.
Reyes, R., Haendel, M., Grant, D., Melancon, E., & Eisen, J. S. (2003). Slow degeneration of zebrafish Rohon-Beard neurons during programmed cell death. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 229(1), 30–41. doi:10.1002/dvdy.10488.
Ritter, D. A., Bhatt, D. H., & Fetcho, J. R. (2001). In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 21(22), 8956–8965.
Roberts, A. A., Li, W.-C. W., & Soffe, S. R. S. (2009). How neurons generate behavior in a hatchling amphibian tadpole: An outline. Frontiers in Behavioral Neuroscience, 4, 16–16. doi:10.3389/fnbeh.2010.00016.
Rogers, K. L., Stinnakre, J., Agulhon, C., Jublot, D., Shorte, S. L., Kremer, E. J., et al. (2005). Visualization of local Ca2+ dynamics with genetically encoded bioluminescent reporters. European Journal of Neuroscience, 21(3), 597–610. doi:10.1111/j.1460-9568.2005.03871.x.
Rogers, K. L., Picaud, S., Roncali, E., Boisgard, R., Colasante, C., Stinnakre, J., et al. (2007). Non-invasive in vivo imaging of calcium signaling in mice. PloS ONE, 2(10), e974. doi:10.1371/journal.pone.0000974.
Rohrseitz, N., & Fry, S. N. (2010). Behavioural system identification of visual flight speed control in Drosophila melanogaster. Journal of the Royal Society Interface, 8(55), 171–185. doi:10.1038/417359a.
Rossignol, S. (1996). Visuomotor regulation of locomotion. Canadian Journal of Physiology and Pharmacology, 74(4), 418–425.
Rossignol, S., & Frigon, A. (2011). Recovery of locomotion after spinal cord injury: Some facts and mechanisms. Annual Review of Neuroscience, 34, 413–440. doi:10.1146/annurev-neuro-061010–113746.
Rossignol, S., & Gauthier, L. (1980). An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Research, 182(1), 31–45.
Rossignol, S., Dubuc, R., & Gossard, J.-P. (2006). Dynamic sensorimotor interactions in locomotion. Physiological Reviews, 86(1), 89–154. doi:10.1152/physrev.00028.2005.
Rudomin, P. (2009). In search of lost presynaptic inhibition. Experimental Brain Research (Experimentelle Hirnforschung. Expérimentation Cérébrale), 196(1), 139–151. doi:10.1007/s00221-009-1758-9.
Rudomin, P., & Schmidt, R. F. (1999). Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research (Experimentelle Hirnforschung. Expérimentation Cérébrale), 129(1), 1–37.
Sankrithi, N. S., & O’Malley, D. M. (2010). Activation of a multisensory, multifunctional nucleus in the zebrafish midbrain during diverse locomotor behaviors. Neuroscience, 166(3), 970–993. doi:10.1016/j.neuroscience.2010.01.003.
Satou, C., Kimura, Y., Kohashi, T., Horikawa, K., Takeda, H., Oda, Y., et al. (2009). Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(21), 6780–6793. doi:10.1523/JNEUROSCI.0801-09.2009.
Satou, C., Kimura, Y., Hirata, H., Suster, M. L., Kawakami, K., & Higashijima, S.-I. (2013). Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development, 140(18), 3927–3931. doi:10.1242/dev.099531.
Schobert, B., & Lanyi, J. K. (1982). Halorhodopsin is a light-driven chloride pump. The Journal of Biological Chemistry, 257(17), 10306–10313.
Schomburg, E. D., Petersen, N., Barajon, I., & Hultborn, H. (1998). Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale, 122(3), 339–350.
Schoonheim, P. J., Arrenberg, A. B., Del Bene, F., & Baier, H. (2010). Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(20), 7111–7120. doi:10.1523/JNEUROSCI.5193-09.2010.
Schroeder, C. E., & Foxe, J. (2005). Multisensory contributions to low-level, “unisensory” processing. Current Opinion in Neurobiology, 15(4), 454–458. doi:10.1016/j.conb.2005.06.008.
Schuster, S. (2012). Fast-starts in hunting fish: Decision-making in small networks of identified neurons. Current Opinion in Neurobiology, 22(2), 279–284. doi:10.1016/j.conb.2011.12.004.
Scott, E. K., Mason, L., Arrenberg, A. B., Ziv, L., Gosse, N. J., Xiao, T., et al. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nature Methods, 4(4), 323–326. doi:10.1038/nmeth1033.
Seelig, J. D., & Jayaraman, V. (2011). Studying sensorimotor processing with physiology in behaving drosophila. International Review of Neurobiology, 99, 169–189. doi:10.1016/B978-0-12-387003-2.00007-0
Severi, K. E., Portugues, R., Marques, J. C., O’’Malley, D. M., Orger, M. B., & Engert, F. (2014). Neural control and modulation of swimming speed in the larval zebrafish. Neuron, 83(3), 692–707. doi:10.1016/j.neuron.2014.06.032
Severi, K. E., Portugues, R., Marques, J. C., O’Malley, D. M., Orger, M. B., & Engert, F. (2014). Neural control and modulation of swimming speed in the larval zebrafish. Neuron, 83(3), 692–707. doi:10.1016/j.neuron.2014.06.032
Shik, M. L., Severin, F. V., & Orlovsky, G. N. (1969). Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalography and Clinical Neurophysiology, 26(5), 549.
Shimomura, O., Johnson, F. H., & Saiga, Y. (1962). Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. Journal of Cellular and Comparative Physiology, 59, 223–239.
Sillar, K. T., Combes, D., Ramanathan, S., Molinari, M., & Simmers, J. (2008). Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Research Reviews, 57(1), 94–102. doi:10.1016/j.brainresrev.2007.07.018.
Slimko, E. M., McKinney, S., Anderson, D. J., Davidson, N., & Lester, H. A. (2002). Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(17), 7373–7379.
Suli, A., Watson, G. M., Rubel, E. W., & Raible, D. W. (2012). Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PloS ONE, 7(2), e29727. doi:10.1371/journal.pone.0029727.g003.
Szobota, S. S., Gorostiza, P. P., Del Bene, F. F., Wyart, C. C., Fortin, D. L. D., Kolstad, K. D. K., et al. (2007). Remote control of neuronal activity with a light-gated glutamate receptor. Neuron, 54(4), 11–11. doi:10.1016/j.neuron.2007.05.010.
Tabot, G. A., Dammann, J. F., & Berg, J. A. (2013). Restoring the sense of touch with a prosthetic hand through a brain interface. Presented at the Proceedings of the United States of America. doi:10.1073/pnas.1221113110/-/DCSupplemental.
Talpalar, A. E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., & Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature, 500(7460), 85–88. doi:10.1038/nature12286.
Tazerart, S., Vinay, L., & Brocard, F. (2008). The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(34), 8577–8589. doi:10.1523/JNEUROSCI.1437-08.2008.
Thiele, T. R., Donovan, J. C., & Baier, H. (2014). Descending control of swim posture by a midbrain nucleus in zebrafish. Neuron, 83(3), 679–691. doi:10.1016/j.neuron.2014.04.018
Tian, L., Hires, S. A., Mao, T., Huber, D., Chiappe, M. E., Chalasani, S. H., et al. (2009). imaging neural activity in worms, flies and mice with improved GcamP calcium indicators. Nature Methods, 6(12), 875–881. doi:10.1038/nmeth.1398.
Trotter, Y., & Celebrini, S. (1999). Gaze direction controls response gain in primary visual-cortex neurons. Nature, 398(6724), 239–242. doi:10.1038/18444.
Viana Di Prisco, G., Ohta, Y., Bongianni, F., Grillner, S., & Dubuc, R. (1995). Trigeminal inputs to reticulospinal neurones in lampreys are mediated by excitatory and inhibitory amino acids. Brain Research, 695(1), 76–80.
Wannier, T., Deliagina, T. G., Orlovsky, G. N., & Grillner, S. (1998). Differential effects of the reticulospinal system on locomotion in lamprey. Journal of Neurophysiology, 80(1), 103–112.
Whelan, P. J. (1996). Control of locomotion in the decerebrate cat. Progress in Neurobiology, 49(5), 481–515.
Windhorst, U. (2007). Muscle proprioceptive feedback and spinal networks. Brain Research Bulletin, 73(4–6), 155–202. doi:10.1016/j.brainresbull.2007.03.010.
Wolpert, D. M. (2007). Probabilistic models in human sensorimotor control. Human Movement Science, 26(4), 511–524. doi:10.1016/j.humov.2007.05.005.
Wyart, C., Del Bene, F., Warp, E., Scott, E. K., Trauner, D., Baier, H., et al. (2009). Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature, 461(7262), 407–410. doi:10.1038/nature08323.
Ye, H., Morton, D. W., & Chiel, H. J. (2006). Neuromechanics of multifunctionality during rejection in Aplysia californica. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(42), 10743–10755. doi:10.1523/JNEUROSCI.3143-06.2006.
Yemini, E., Jucikas, T., Grundy, L. J., Brown, A. E. X., & Schafer, W. R. (2013). A database of Caenorhabditis elegans behavioral phenotypes. Nature Methods, 10(9), 877–879. doi:10.1038/nmeth.2560.
Zajac, F. E. (1989). Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering, 17(4), 359–411.
Zhang, F., Aravanis, A. M., Adamantidis, A., de Lecea, L., & Deisseroth, K. (2007a). Circuit-breakers: Optical technologies for probing neural signals and systems. Nature Reviews Neuroscience, 8(8), 577–581. doi:10.1038/nrn2192.
Zhang, F., Wang, L.-P., Brauner, M., Liewald, J. F., Kay, K., Watzke, N., et al. (2007b). Multimodal fast optical interrogation of neural circuitry. Nature, 446(7136), 633–639. doi:10.1038/nature05744.
Zhu, P., Narita, Y., Bundschuh, S. T., Fajardo, O., Schärer, Y.-P. Z., Chattopadhyaya, B., et al. (2009). Optogenetic dissection of neuronal circuits in zebrafish using viral gene transfer and the Tet system. Frontiers in Neural Circuits, 3, 21. doi:10.3389/neuro.04.021.2009.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wyart, C., Knafo, S. (2015). Sensorimotor Integration in the Spinal Cord, from Behaviors to Circuits: New Tools to Close the Loop?. In: Douglass, A. (eds) New Techniques in Systems Neuroscience. Biological and Medical Physics, Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-12913-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-12913-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12912-9
Online ISBN: 978-3-319-12913-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)