Abstract
Experiments in anesthetized, immobile animals have contributed to the classical view that sensory and motor functions in the brain are separated processes. However, under natural conditions, the nervous system and the body of a moving animal interact continuously, and it is from this interaction that neural circuits in the brain form an internal representation of the sensory world. We move our head to detect and localize the source of a sound, we move our eyes to scan a visual scene; likewise, tactile sensation is based on our body’s movement, and olfaction occurs in the context of sniffing. Sensory and motor components of a sensory modality are intimately connected to each other during an active process. How this relation is implemented across sensorimotor circuits, and how motor–sensory coordination improves sensation, are questions that still remain unclear. Recent technological advances have made possible to record neural activity from sensory areas while animals walk or fly—behavioral conditions in which sensation most frequently happens. From these studies, performed both in insects and mammals, it has become apparent that the neural dynamics of primary sensory areas are readily influenced by ongoing locomotion. In this chapter, we discuss work dissecting different components of the locomotion-dependent modulations, focusing on visual circuits in flies and mice. The presence of these locomotive-related signals in early visual centers strongly suggests that motor–sensory coordination is dynamic, diverse, and adaptable to the behavioral situation of the animal.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Sensation typically happens while animals move around and exploit natural habitats. As a consequence, movement affects the temporal and spatial pattern of sensory excitation—the sensory sampling. Motor-driven sensory sampling may jeopardize the detection of events in the world; yet, animals do move their body to uncover the source of the external stimuli. For example, we move our head to localize a sound or an odor source, or move our eyes and body to perceive the 3D structure of a visual landscape, and locate a target within it. The sensory and motor components in every sensory modality are intimately related to each other, and these relations form the bases of an internal representation of the sensory world (Ringach 2009; Wurtz et al. 2011). Therefore, motor–sensory coordination is not detrimental but fundamental to guide an animal’s interaction with its environment. How motor–sensory coordination is implemented within neural circuits in the brain remains unclear.
In order to understand how neural circuits construct an internal representation of the external sensory world, it is important first to consider what challenges the brain faces when sensation occurs in the context of movement. During motor-driven sensation the brain must differentiate sensory signals occurring in the environment, known as exafference (von Holst and Mittelstaedt 1950), from those that inevitably result from an animal’s own movements, so-called reafference (von Holst and Mittelstaedt 1950). To differentiate exafference from reafference, nervous systems across the animal kingdom monitor self-movement. Many sensory systems signal self-movement: the vestibular and proprioceptive systems inform the brain about body and head movements, motion vision conveys information about eye, head or body movements (see below). In addition, premotor circuits route copies of motor commands to different areas of the brain, including sensory pathways. These internally generated signals, known as corollary discharge (CD, Sperry 1950) or efference copies (von Holst and Mittelstaedt 1950) can specifically inhibit the incoming reafference stream (for a review on this topic, we refer the reader to Crapse and Sommer 2008). Such cancelation may happen at early stages of sensory processing (Poulet and Hedwig 2002; Schneider et al. 2014), and as a result, downstream neurons within the circuit only respond to exafference. This type of inhibitory effect by CD is often observed in reflexive systems that must be prevented from self-activation (Davis et al. 1973; Sillar and Roberts 1988; Roy and Cullen 2004; Kim et al. 2015), or sensory systems that must be prevented from self-induced desensitization (Voss et al. 2006; Chagnaud et al. 2015).
Under certain circumstances sensory reafference should not be canceled out (Sommer and Wurtz 2002; Hendricks et al. 2012). In the context of navigation and other oriented behaviors, the brain relies on an accurate internal estimate of self-movement that is based on multisensory reafferent information (Sommer and Wurtz 2002; Whitlock et al. 2008; Franklin and Wolpert 2011; Requarth et al. 2014; Coen et al. 2016). For example, when a stationary prey is detected, a mantis moves its upper body from side to side while keeping the gaze constant. This self-induced motion parallax is combined with proprioceptive information from neck sensors to accurately estimate the distance to the prey, and plan a strike jump (Poteser et al. 1998; Kral 2012). Multimodal reafferent information calculates physical parameters of the environment (in this example, the absolute distance to an object) that can be estimated neither with a single sensory pathway, nor in the absence of movement. In addition to multisensory reafference, CD signals of intended body movements can be also integrated with multimodal information to update internal models of ongoing movement (Franklin and Wolpert 2011; Requarth et al. 2014), and of space (Stackman et al. 2003; Sommer and Wurtz 2006; Whitlock et al. 2008). In summary, the brain monitors ongoing movement from CD signals and/or reafference information; in so doing, the brain maintains the dynamic range of sensory systems despite self-excitation, and constructs internal representations of space and self-movement that command oriented behaviors.
One fundamental and complex oriented behavior is locomotion. Different limbs, the body and the head need all be coordinated together for postural control, and for the control of direction and speed of the movement of the body. Here self-movement estimation during locomotion is not only critical for navigation, but also for planning the next locomotive steps to approach an attractive target, or to runaway from a dangerous source. Vision can provide critical information about the translational and angular movements of the body: the retina of an animal moving about is stimulated by visual flow. The self-generated pattern of visual flow, called optic-flow field, contains information related to the trajectory of the animal (Koenderink 1986). Therefore, the brain could use optic-flow processing networks to estimate the ongoing course: the wide distribution of optic-flow sensitive circuits in both invertebrate and vertebrate species would imply this might be the case (Duffy and Wurtz 1991a, b; Grasse and Cynader 1991; Wall and Smith 2008; Masseck and Hoffmann 2009; Kubo et al. 2014). But vision alone provides ambiguous body-movement information, because optic-flow fields are confounded by changes in gaze and/or by the movement of objects in the world. In primates, optic-flow processing networks also receive vestibular and eye-movement related information (Newsome et al. 1988; Bradley et al. 1996). The convergence of visual and nonvisual information is thought to be critical for the perception of heading (Britten and van Wezel 1998; Angelaki et al. 2011). At present, it is unclear whether other nonvisual signals related to body movement also arrive to these brain areas when the animal is engaged in locomotion. The presence of body-movement related signals in this network could improve the internal estimate of heading, and could also allow for an internal estimate of the velocity of the moving body (Koenderink 1986).
Recent technological advances have made possible to record from sensory areas in walking and flying flies, or running mice. Studies in walking and flying flies have demonstrated that optic-flow processing neurons are modulated by locomotion (Figs. 10.1b and 10.2b, e) (Chiappe et al. 2010; Maimon et al. 2010; Jung et al. 2011; Suver et al. 2012). In rodents, optic-flow processing neurons are found at the accessory optic system, a set of brainstem areas implicated in gaze stabilization (Simpson 1984; Yonehara et al. 2009; Distler and Hoffmann 2011; Dhande et al. 2013). While it is still unclear whether locomotion modulates any of these networks, recent work has shown that visual neurons in the thalamus and the primary visual cortex of the mouse are modulated by movement of the animal (Fig. 10.1a) (Niell and Stryker 2010; Keller et al. 2012; Ayaz et al. 2013; Bennett et al. 2013; Polack et al. 2013; Saleem et al. 2013; Erisken et al. 2014; Fu et al. 2014). Locomotion is always accompanied by an increase of arousal, which broadly changes the state of the brain (Harris and Thiele 2011; McGinley et al. 2015; Nelson and Mooney 2016). Importantly, brain states control the gain of sensory processing, learning, and memory (Lee and Dan 2012); therefore, the modulations associated with locomotion in visual interneurons could result from an arousal-induced change of the state of the network. Indeed, many aspects of the modulations described in V1 during locomotion correlate to changes in arousal levels (McGinley et al. 2015; Vinck et al. 2015). Similarly, locomotion could exert its modulation through changes in the arousal levels of the fly during flight (Suver et al. 2012; Tuthill et al. 2014). On the other hand, locomotion itself, in the form of motor feedback, could send specific signals to visual interneurons about the movement of the body through space (Keller et al. 2012; Saleem et al. 2013; Erisken et al. 2014; Fu et al. 2014; Roth et al. 2016).
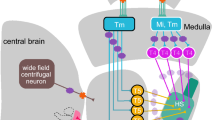
Proposed pathways for locomotion-induced modulations in visual circuits in mice (a), and in flies (b). a Changes in arousal levels during locomotion modulate primary visual cortex (V1) activity through cholinergic (Fu et al. 2014; Lee et al. 2014) and noradrenergic (Polack et al. 2013) pathways. Cholinergic modulations preferentially act on VIP positive cells (Fu et al. 2014). The mesencephalic locomotor related area (MLR) activates the basal forebrain, and nonspecific thalamic nuclei (Lee et al. 2014); therefore, it is thought to mediate the cholinergic-induced activity modulations. The locus coeruleus, housing noradrenergic neurons, controls pupil dilations (Joshi et al. 2016) known to correlate with arousal levels (Vinck et al. 2015). Thalamic projections to V1 such as the dorsal lateral geniculus nucleus (dLGN) and the pulvinar area (LP), also receive motor-related signals during locomotion (Erisken et al. 2014; Roth et al. 2016). b The arousal-induced modulations in optic-flow processing neurons of the fly lobula plate (LoP), the horizontal and vertical system cells (HS- and VS-cells), are directed largely through octopaminergic pathways (Suver et al. 2012), but other pathways may also play a role. In addition, HS-cells selectively receive saccade-related potentials (Kim et al. 2015). These signals precede saccades, suggesting they may be centrally generated (i.e., CD signals). The fly’s central complex (CCX) or lateral accessory lobe (LAL)—areas thought to control turning behavior (Pfeiffer and Homberg 2014; Strauss and Berg 2010), may direct this modulation. HS-cells and VS-cells are known to have direct connections with neck motor neurons in blowflies (Strausfeld et al. 1987; Strausfeld and Sayen 1985). The activity of HS-cells can also drive wing movements (Haikala et al. 2013) and may also drive leg movements (Heisenberg et al. 1978). There is evidence that arousal-like modulations are already present at upstream visual circuits (medulla and lobula circuits, Tuthill et al. 2014). For both schematics, gray arrows indicate visual pathways; solid and the dashed arrows indicate identified and proposed/potential pathways, respectively
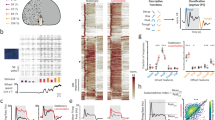
Examples of arousal- and motor feedback-induced phenomena in visual circuits. a–b Arousal- and c–d locomotion-induced phenomena. a Mean local field potential (LFP) power spectrum of mouse V1 during movement (gray) and immobility (black). b (i) Mean temporal frequency tuning curves in Drosophila HS-cells to wide-field motion stimuli as measured with calcium imaging during walking (gray) and stationary (black) trials. (ii) Similar as (i) but from whole-cell electrophysiological recordings from VS-cells during flight. (iii) Similar as in (ii) but from extracellular recordings of H1-cell activity, an optic-flow sensitive cell in blowflies (Lucilia). c Running speed tuning of V1 neurons, measured with calcium imaging in layer 2/3 neurons that are sensitive to a mismatched between behavior and the expected visual feedback. d Example of running speed tuning of neurons from deep layers of V1 obtained with extracellular recordings in dark conditions. e Turning speed tuning of a Drosophila HS-cell to wide-field motion stimulus measured with calcium imaging. Data are adapted with permission from: Niell and Stryker (2010) (a), Chiappe et al. (2010) (b(i) and e); Suver et al. (2012) (b(ii)), Jung et al. (2011) (b(iii)), Keller et al. (2012) (c), Saleem et al. (2013) (d)
What the functional role of these locomotive-related signals is in early visual processing areas in flies and mice remains unclear. Answering this question requires dissecting the mechanisms by which locomotion-related signals affect sensory processing. For this, genetic model organisms such as the fruitfly (Drosophila melanogaster), the mouse (Mus musculus), and the zebrafish (Danio rerio) present a unique opportunity because of the experimental possibility to apply genetic tools in specific, genetically identified population of cells simultaneous with physiology and behavior, either in head-fixed or freely moving conditions (Dombeck et al. 2007; Maimon et al. 2010; Seelig et al. 2010; Niell and Stryker 2010; Schneider et al. 2014; Grover et al. 2016). Here, we review studies looking at locomotion-dependent modulations on visual processing in flies and rodents, where the application of genetic-based approaches has been developing rapidly. With the current experimental evidence, and to clarify the functional implication of these prominent modulations, we first discuss the phenomena caused by changes in the arousal state of an animal, and then we look into the evidence for locomotive-specific phenomena.
2 Arousal State Modulations During Locomotion
2.1 Arousal State Modulations in Rodents
The population dynamics of neurons in the lateral geniculate nucleus of the thalamus (LGN) and the primary visual cortex (V1) are modulated by locomotion (Fig. 10.1a) (Niell and Stryker 2010; McGinley et al. 2015). Concomitant with locomotion is the change in the arousal state of the animal. Therefore the origin of the locomotive-induced modulations may be simply ascribed to changes in the arousal state of the animal rather than by locomotive-related motor feedback (see below). In rodents and other mammals, the animal’s arousal correlates positively with pupil size (Erisken et al. 2014; Reimer et al. 2014; Vinck et al. 2015; McGinley et al. 2015). Pupil dilation precedes the onset of locomotion, and it is sustained after locomotion termination. Furthermore, changes in pupil size can also be triggered by artificial mechanical stimuli, such as a puff of air, which is presumably associated with a startled response of the animal. Using the difference in time between the onset/offset of the pupil dilation versus that of locomotion, and the artificial induction of pupil dilation with no overt movement, Vinck and colleagues identified the aspects of locomotive modulations that were specifically linked to changes in arousal levels (Vinck et al. 2015).
Changes in arousal levels induce a global switch in cortical neural activity associated with changes in the network coding capacity (Harris and Thiele 2011; McGinley et al. 2015). In V1, low-frequency (2–10 Hz) local field potentials (LFPs) prevail during quiet awake states, but are decreased with increases in arousal levels preceding the onset of locomotion (Fig. 10.2a), or when arousal changes are artificially induced (Vinck et al. 2015). This reduction is accompanied by weakened pairwise correlations in firing activity among V1 neurons (Erisken et al. 2014; Vinck et al. 2015). The decorrelation of the firing activity of V1 neurons during high levels of arousal is presumably associated with the development of gamma band oscillations (30–80 Hz) (Niell and Stryker 2010; Bennett et al. 2013; Reimer et al. 2014; McGinley et al. 2015; Vinck et al. 2015), a frequency band thought to be related to activate sensory processing (Poulet and Petersen 2008). Furthermore, spontaneous firing rates of regular (presumably excitatory) and fast (likely inhibitory) spiking neurons also decline with the increment of arousal levels (Vinck et al. 2015). The decline of spontaneous activity may be related to both a decrease in variance of the membrane potential of the cells (Bennett et al. 2013; Polack et al. 2013), as well as an overall depolarization of the baseline membrane potential level preceding the onset of locomotion (Polack et al. 2013). Importantly, a decrease in spontaneous activity may be advantageous to improve the signal-to-noise ratio (the ratio between evoked/spontaneous cell activity) of the neuron’s responses, which can impact cortical coding of sensory stimuli, as well as the performance of an animal during a sensory discrimination task (Cohen and Maunsell 2009; Goard and Dan 2009; Pinto et al. 2013). Altogether, these studies show that changes in arousal levels modify the activity of visual cortical networks in mice in a manner that resembles the physiological correlates of attention in primates: augmenting the sensory processing capacity of the network (Cohen and Maunsell 2009; Goard and Dan 2009; Harris and Thiele 2011).
The mesencephalic reticular formation (MRF) in the brainstem has long been implicated in the control of arousal, REM sleep and initiation of locomotion (Moruzzi and Magoun 1949; Shik et al. 1966; Steriade et al. 1990; Kobayashi and Isa 2002; Goetz et al. 2016). Artificial activation of this brainstem area in anesthetized animals induces desynchronization of low-frequency oscillations of the electroencephalogram (EEG) signal (Moruzzi and Magoun 1949), a phenomenon that resembles the physiological correlates of alertness (Steriade et al. 1993). Within the MRF is the mesencephalic locomotor region (MLR). MLR sends projections to descending motor pathways (Garcia-Rill and Skinner 1987a, b). Accordingly, electrical activation of MLR induces locomotion (Shik et al. 1966, Mori et al. 1978). In addition, MLR sends projections to the thalamus and basal forebrain (BF) (Nauta and Kuypers 1958), and these ascending projections may partially elicit the physiological correlates of alertness mediated by MRF (Fig. 10.1a) (Moruzzi and Magoun 1949; Goard and Dan 2009). In a recent study, Lee and colleagues used optogenetic tools to stimulate glutamatergic neurons in MLR (Lee et al. 2014). As expected, they showed that the activation of these neurons induces running (Fig. 10.1a) (Lee et al. 2014; Roseberry et al. 2016). In addition, optogenetic activation of MLR increases the gain of visual responses and enhances gamma oscillations in V1 (Lee et al. 2014). Importantly, under certain experimental conditions, stimulation of MLR induces similar effects in V1 without overt movement. Strikingly, this result parallels the modulation of V1 neurons by increased arousal levels (Vinck et al. 2015). Furthermore, optogenetic activation of axon terminals of BF–projecting MLR neurons, or of cholinergic neurons within BF mimics cortical enhancement in V1 (Pinto et al. 2013; Lee et al. 2014), suggesting that a pathway including MLR and BF may mediate the arousal effect in V1 neurons during locomotion (Fig. 10.1a). In support of this idea, it was recently shown that electric stimulation of BF activates vasoactive intestinal peptide (VIP) positive cells in V1 via nicotinic receptors (Fig. 10.1a) (Alitto and Dan 2013). Indeed, the activity of VIP neurons in V1 correlate with running, and this correlation declines after application of nicotinic receptor antagonists (Fu et al. 2014). VIP neurons in auditory and somatosensory cortices are similarly activated upon locomotion (Eggermann et al. 2014; Nelson and Mooney 2016). Therefore, the modulation observed in the visual cortex by increases in arousal state seems to share common mechanisms across different sensory cortices. However, cholinergic activity does not account for all the phenomena associated with changes in arousal levels. For example, the baseline membrane potential boost, and the decrease in membrane potential variance upon running onset depend on noradrenergic inputs to V1 (Fig. 10.1a) (Polack et al. 2013).
Cholinergic activity in sensory cortices has been linked to arousal, attention, memory encoding (Hasselmo and Sarter 2011), and performance in visual discrimination tasks (Cohen and Maunsell 2009; Goard and Dan 2009; Pinto et al. 2013). Locomotion also improves the performance of a discrimination task in conditions of low contrast (Bennett et al. 2013). This improvement in behavioral performance is likely originating from the increase in the signal-to-noise ratio of the activity of V1 neurons (Vinck et al. 2015). Alternatively, changes in the spatial integration properties of the receptive fields of V1 neurons induced by locomotion could also improve behavioral performance, and broadening spatial receptive fields might be a way to adaptively coordinate spatial attention during active movement (Ayaz et al. 2013). However, it remains unclear whether the changes in the spatial properties of V1 neurons receptive fields can be ascribed to arousal-related modulations or to phenomena associated with motor feedback.
2.2 Arousal State Modulations in Visual Circuits in Flies
Octopamine (OA) in insects increases the general level of arousal of the animal (Roeder 2005). Many of the modulations of neural activity observed during the transition from quiescence into movement can be reproduced with OA agonists in non-behaving preparations. This may imply that such modulatory activity is associated with changes in arousal levels. However, in insects, it has not been possible to decouple arousal-related modulations from modulations induced by overt movement yet. Therefore, it is still possible that some aspect of OA-based modulations in visual circuits may be related to locomotion-induced motor feedback.
Motion vision is critical for the detection of moving objects in the world, and for monitoring self-movement (Lappe et al. 1999). During flight or walking, the retina is excited with visual flow, known as optic-flow. Optic-flow processing neurons are thought to be important for monitoring self-movement to correct deviations from the intended course. In flies, optic-flow processing neurons are located in the Lobula Plate (LoP) of the fly brain. Because of their large dendritic arbors, expanding across the retinotopical organization of the LoP, these neurons are called Lobula Plate Tangential cells (LPTCs) (Borst 2014; Silies et al. 2014). In each hemisphere of the Drosophila brain, there are three horizontal system cells (HS-cells) (Scott et al. 2002; Schnell et al. 2010), and six vertical system cells (VS-cells) processing optic-flow along the yaw, or the roll (and pitch) axis, respectively (Hausen 1982a, b; Joesch et al. 2008). These LPTCs show graded membrane potential changes upon wide-field visual motion stimuli, they are direction selective, and they are tuned to a preferred velocity of the moving visual stimuli. For example, HS-cells depolarize with front to back motion, and hyperpolarize with back to front motion, and the direction-selective response (the difference between the magnitude of the preferred- and the nulled-direction response) peaks at a best stimulus velocity (Joesch et al. 2008; Schnell et al. 2010). Likewise, VS-cells depolarize with downward motion and hyperpolarize with upward motion, and they also display temporal tuning properties. Recently, it has become possible to record from these neurons while the fly is walking (Seelig et al. 2010) or flying (Maimon et al. 2010). Experiments performed under these conditions have shown that locomotion modulates the activity of HS- and VS-cell. Flight induces a boost in the membrane potential of VS-cells in the absence of visual motion stimuli (Maimon et al. 2010; Suver et al. 2012). Walking and flight also induce an increase in the magnitude of the response of HS- and VS-cells to visual motion stimuli (Fig. 10.2b). Furthermore, during walking, this response increase scales monotonically with walking speed (Fig. 10.2e) (Chiappe et al. 2010; Longden et al. 2014). Last but not least, the temporal tuning properties of LPTCs are modified during locomotion. Walking and flight induce a shift in the sensitivity of HS- and VS-cells towards larger stimulus velocities (Chiappe et al. 2010; Suver et al. 2012), a phenomenon also observed in LPTCs of other fly species (Fig. 10.2b) (Jung et al. 2011; Longden et al. 2014), which may be related to the role of these networks during locomotion.
The prime candidate for these behavioral-state dependent changes in the activity of LPTCs has been OA, a neuromodulator released during flight in insects (Fig. 10.1b) (Orchard et al. 1993). Consistent with this idea, electrophysiological recordings from LPTCs and other early visual neurons in the presence of OA or OA agonists (chlordimeform, CDM) produce increased responses to visual stimuli resembling the modulation induced by locomotion in these neurons (Longden and Krapp 2009, 2010; Rien et al. 2012; Tuthill et al. 2014). This phenomenon could be partly explained by a decrease in the adaptation to motion stimuli in motion-sensitive circuits upstream from LPTCs (Longden and Krapp 2010; de Haan et al. 2012; Rien et al. 2012; Lüders and Kurtz 2015). Indeed, T4 and T5 cells, cognate presynaptic neurons to LPTCs, show flight-induced increased response to visual stimuli (Schnell et al. 2014).
OA neurons project to LoP and other areas of the visual system of the fly (Busch et al. 2009). Therefore, the activity of these OA neurons could mediate the modulations observed during locomotion. Indeed, Drosophila OA neurons show an increase in activity during flight (Suver et al. 2012). Using Drosophila’s genetic tools, Suver and colleagues artificially activated OA neurons, while they recorded the activity of VS-cells in flying and non-flying flies (Suver et al. 2012). Activation of OA neurons reproduced some of the flight effects; it induced an increased visual response in VS-cells (Suver et al. 2012). Conversely, silencing OA neurons by overexpressing an inward-rectified potassium channel (Kir2.1) abolishes the flight-induced increased visual response. Under this experimental condition, the flight-induced baseline membrane potential depolarization remains intact. This result, together with the observation that the time course of the baseline shift, and of the increased response amplitude are different, supports the idea that these locomotion-induced phenomena originate from different mechanisms. What the source of the locomotion-induced fast increase in baseline membrane potential is remains unclear (Fig. 10.1b).
The observed locomotion-induced modulations in LPTCs, i.e., the depolarization of the baseline membrane potential, the increased response to visual stimuli, and the change in the temporal sensitivity of the neuron, are in line with the idea that locomotion—perhaps through a change in arousal state, controls the gain and the temporal properties of visual processing. LPTCs have been thought to be responsible for a stabilization reflex known as the optomotor response. Imagine that a sudden gust of wind pushes a flying fly into an unintended course. Optic-flow resulting from this passive body drift will excite HS- or VS-cells depending on the direction of movement. Activity in these neurons are thought to compensate for the detour, bringing the fly back to her intended course. Although it is unclear how exactly the activity of these neurons controls compensatory body movements, there is some evidence indicating that HS-cells may control ipsilateral head and body yaw movements (Fig. 10.1b) (Blondeau and Heisenberg 1982; Haikala et al. 2013). Consistent with this proposed function, Drosophila mutants that lack LPTCs, or flies with ablated LPTCs show an attenuated optomotor reflex (Heisenberg et al. 1978; Geiger and Nässel 1981; Hausen and Wehrhahn 1983). Therefore, the locomotion-induced modulations on the activity of LPTCs are likely controlling the gain of the transformation of visual motion stimuli into a body compensatory movement. The observation that the gain of the head optomotor response is increased during flight supports this idea (Haag et al. 2010). Moreover, it has been recently shown that the activity of OA neurons may control flight acceleration upon visual motion stimulation (van Breugel et al. 2014).
The temporal properties of visual motion processing in LPTCs are modulated by the behavioral state of the fly (Fig. 10.2b). This locomotion-induced modulation may be beneficial for the function of LPTCs in the control of locomotion. The statistics of motion visual stimuli during locomotion versus quiescence are different. During quiescence, motion stimuli will be elicited by movement of objects in the world, or by movements of the surface where the fly is landed, usually elicited by a wind breeze. At the onset of locomotion, motion visual stimuli will be largely generated by the animal’s own movement. Electrophysiological recordings in non-behaving preparations from LPTCs have shown that the temporal sensitivity of these neurons to stimuli that elicits robust optomotor response could not account for the temporal properties of the behavior: the optimal stimulus velocity for the behavior is at least 4X faster than the optimal stimulus velocity for the neuron in quiescence (Götz and Wenking 1973; Schnell et al. 2010). Therefore, it has been difficult to reconcile these disparate results until the recent discovery of the locomotion-induced temporal modulation of visual processing in LTPCs and/or in upstream neurons (Seelig et al. 2010; Chiappe et al. 2010; Tuthill et al. 2014).
The modulations in the activity of LPTCs by the transition from quiescence into locomotion could be related to changes in the arousal state of the animal, or by specific locomotion-related motor feedback. While it has not been possible to decouple these two phenomena in insects, for example, there is still no “pupil dilation”—like measurements monitoring changes in arousal independent of overt movement, several lines of evidence indicate that the described modulations could be related to changes in fly alertness. Increases in OA levels have been associated with increases in arousal levels (Roeder 2005). The activity of OA neurons increases during flight (Suver et al. 2012). Artificial activation of OA reproduces many of the effects triggered by flight, and silencing these neurons reduces the response amplitude of LPTCs to visual stimuli without affecting flight per se. What makes OA neurons active during flight, and whether these neurons are also involved in the walking-induced modulations of HS-cells are questions that remain poorly understood.
3 Locomotion-Specific Modulations
3.1 Locomotion-Specific Modulations in Rodents
Recent evidence indicates that early visual circuits in mice receive locomotion-related motor feedback. However, because the majority of the work has been performed in conditions that did not decouple a change in arousal from overt movement, the interpretation of the results from these studies cannot exclude the possibility that some of the phenomena noted below could still relate to a change in the arousal level of the animal.
Locomotion induces increased evoked responses in layer 2/3 V1 neurons (Niell and Stryker 2010), and increased spontaneous activity in both regular and fast-spiking neurons across layers in V1 (Vinck et al. 2015). The latter effect was decoupled from changes in the arousal state of the mouse that accompanies the onset of locomotion (Vinck et al. 2015). Other studies confirmed these initial observations, and importantly, revealed a high level of heterogeneity in the effect of locomotion on V1 activity (Andermann et al. 2011; Keller et al. 2012; Bennett et al. 2013; Polack et al. 2013; Saleem et al. 2013; Erisken et al. 2014; Fu et al. 2014). For example, some neurons are excited while others are suppressed by locomotion (Polack et al. 2013; Saleem et al. 2013; Erisken et al. 2014; Fu et al. 2014). This variability could be partially accounted by differences in the effect of modulation across different cortical layers (Andermann et al. 2013; Erisken et al. 2014), or among different cell types (Polack et al. 2013; Fu et al. 2014). Nevertheless, such heterogeneity would not be expected if the source of the modulation were related to a global change in arousal levels. Interestingly, V1 neurons respond to running even in darkness (Fig. 10.2c, d) (Keller et al. 2012; Andermann et al. 2013; Saleem et al. 2013; Erisken et al. 2014), with firing rates that can be modulated monotonically with the increase or decrease of running speed, or can display a bell-shaped running-speed tuning (Saleem et al. 2013; Erisken et al. 2014). Such nonlinear speed tuning property indicates the presence of a motor-related specific feedback signal rather than a global arousal-related modulation. Other evidence for locomotion-related motor feedback comes from virtual reality experiments in mice. Keller and colleagues adopted a closed-loop virtual reality system where the visual-motor coupling is interrupted by a brief halt of the visual feedback (Keller et al. 2012). In this environment, a subset of V1 neurons selectively responds to a mismatch between running and the expected visual-flow feedback, revealing potential predictive motor-related signals in the visual cortex.
It is still unclear what the source of these motor-related feedback signals is. Both lower-order thalamic projections to V1, through the dorsal lateral geniculus (dLGN) (Erisken et al. 2014; Roth et al. 2016), or higher-order thalamic projections to V1, through the lateral posterior nucleus (LP, the pulvinar equivalent in mice), contain neurons sensitive to the running speed of the animal (Roth et al. 2016). These observations are not surprising given that both dLGN and LP receive inputs from motor-related areas (Grieve et al. 2000; Baldwin et al. 2011; Wurtz et al. 2011). Considering that MLR can initiate locomotion, and can activate thalamic and BF circuits (Nauta and Kuypers 1958), it is tempting to speculate that MRL could also deliver locomotion-related motor feedback to V1 (Fig. 10.1a). Another possibility could be feedback from other cortical areas to V1. These feedback signals have been characterized in the somatosensory system (Lee et al. 2008; Petreanu et al. 2009, 2012; Lee et al. 2013; Zagha et al. 2013) in the context of active whisking, and in primary auditory cortex (Nelson et al. 2013; Nelson and Mooney 2016). Therefore, it could be the case that V1 receives locomotor signals from cortico-thalamic feedback (Sillito et al. 2006). In addition, the motor-related signals could arrive from the superior colliculus, through its projection to dLGN (Erisken et al. 2014; Liang et al. 2015; Roth et al. 2016). These potential different sources of modulations might explain the heterogeneous sensitivity of V1 neurons to the locomotion-induced modulations.
The effect of locomotion-related modulations is diverse among V1 neurons even within the same cortical layers (Keller et al. 2012; Saleem et al. 2013; Erisken et al. 2014). In some cases, locomotion motor feedback-related signals are not simply gating or modulating visual processing, these signals induce well-defined running speed tuning curves in V1 neurons in darkness (Fig. 10.2d). In addition, some neurons in V1 selectively respond to the mismatch between running and the expected visual feedback (Keller et al. 2012; Roth et al. 2016), suggesting that self-generated visual inputs might be actively suppressed in V1. This might be a specific property of locomotion-related feedback; however, Saleem and collegues, found that many V1 neurons code the sum of running and visual-flow speeds instead of their mismatch (Saleem et al. 2013; see also Roth et al. 2016). The integration of running speed and visual feedback may be a useful mechanism for an internal estimate of the animal’s own speed during navigation (Whitlock et al. 2008; Kropff et al. 2015). It remains to be seen how these apparent contradictory interactions between visual and motor-related signals, a positive integration versus a sensitivity to a mismatch, interrelate within networks in V1, and what the specific function of these interactions is for visuomotor processing and visually guided behaviors.
3.2 Locomotion-Specific Modulations in Flies
Much less is known about locomotion-related motor feedback in visual circuits in flies. During walking, the response amplitude of HS-cells to visual stimuli increases monotonically with walking speed (Fig. 10.2e) (Chiappe et al. 2010; in blowflies: Longden et al. 2014), suggesting that these neurons may receive quantitative walking-related information.
More recently, an intriguing locomotion-specific modulation was found in a subclass of HS-cells, the HSN cell, while a fly was flying. Explorative flight is characterized by segments of straight course interrupted by abrupt changes in heading, known as saccades (Schilstra and Hateren 1999; Muijres et al. 2014). In the absence of visual stimuli, recordings from HSN showed that when the fly made a saccade the membrane potential of the neuron was depolarized or hyperpolarized. The direction of the modulation depended on the direction of the saccade, suggesting the presence of extra-retinal, direction-selective signals (Kim et al. 2015). These direction-selective signals were opposite to the visual direction-selective responses of the cell, such that, in the presence of visual motion stimuli, saccades provoked a cancelation of the response of the HSN to visual stimuli (Kim, et al. 2015). Because the saccade-related potentials precede the saccades, these signals are thought to represent CD signals with an apparent cancelation function.
LPTCs are thought to trigger the optomotor reflex to stabilize the course of the fly during segments of straight, forward movement. A long-standing question has been how the flies can escape from the optomotor reflex during voluntary turns, for example, during saccades (von Holst and Mittelstaedt 1950). Here, a CD signal predicting the sensory consequence of the abrupt saccade may be used to silence optomotor inducing networks (von Holst and Mittelstaedt 1950). The motor-related signals in HSNs could serve such a cancelation function, blocking the stabilization reflex specifically when the fly desires to change course. It is still not clear what the source of these signals is; however, reasonable candidates are the central complex and the lateral accessory lobe of the fly brain, premotor areas shown to be associated to turning behavior (Strauss and Berg 2010; Pfeiffer and Homberg 2014). Indeed, visual responses in central complex neurons are also modulated during locomotion (Weir et al. 2014; Seelig and Jayaraman 2015; Weir and Dickinson 2015). It will be interesting to investigate if there is any causal relation between the motor-related modulations observed in HS-cells and the ones observed in such higher brain centers.
4 Conclusion
The dynamics of visual neurons is influenced by the behavioral context of an animal (Fig. 10.1). Movement of the body through space generates modulatory signals such that the activity of sensory circuits change in correspondence with the arousal level of the animal. In rodents, many aspects of this arousal-induced modulation resemble the attention-related phenomena described in homolog circuits in primates. Both processes involve a similar control of activity oscillations and correlations among cortical neurons, which are mediated by common receptors and neuromodulatory systems (Fig. 10.1a) (Harris and Thiele 2011). This may seem at first surprising because arousal is a global state while spatial attention is a local process, highlighting one location of the visual field over others. However, broadly speaking, locomotion and attention can be both considered as active internal states. As such, these behavioral contexts may share basic cognitive factors, for example, the commonly observed changes in cortical state. More striking is the fact that some commonalities in state-dependent modulations on visual activity are beginning to emerge from studies in flies (Fig. 10.1b). Like attention in primates, and locomotion in rodents, walking and flight in flies induce changes in the response gain and stimulus selectivity of visual interneurons (Chiappe et al. 2010; Maimon et al. 2010; Jung et al. 2011), and these phenomena are also under the control of neuromodulation (Suver et al. 2012). Thus, modulation by behavioral context is a common feature of visual processing.
One interesting aspect of the state-dependent modulations is how multiple coexisting active internal states may interact to control the dynamics of visual or other sensory circuits. Studies of attention in monkeys have been exclusively focused on eye movements. Because more ethological relevant behaviors, such as locomotion, are difficult to control in a laboratory setting, it is still not clear whether and how locomotion modulates visual activity in monkeys, and if so, how attention and locomotion interact to control the dynamics of visual circuits, and the performance of visual guided behaviors. On the other hand, it is possible that in mice (Gill et al. 2000), and even perhaps in insects (Hoy 1989; van Swinderen 2007; Wiederman and O’Carroll 2013), one could test the interaction of attention-like and locomotion-induced phenomena in visual circuits with the development of suitable behavioral paradigms. Using modern techniques, such as specific labeling, and recording and perturbing neural activity in a genetically identified population of neurons, these experiments would allow for a detailed understanding of what aspects of the modulations are shared between alertness and attention, and which ones are not. Moreover, these experiments could also test what the function of either the arousal- or the attention-induced modulations is for visuomotor processing, and for animal behavior: two important elements that are currently unsatisfactorily understood.
Equally prominent in rodents are the locomotion-related motor feedback signals arriving to visual circuits from bottom-up and top-down inputs (Fig. 10.2c–e). These signals were uncovered while mice run in darkness, and showed a variety of modulations on visual activity. It is still unclear what the origin of these signals is. Both proprioceptive information as well as CD signals could deliver such modulations to V1 neurons. During running, some V1 neurons are excited, others are inhibited, and yet others are “band-pass” modulated by the speed of the mouse. Moreover, some neurons display a cooperative interaction between visual stimuli and running speed, while others display mismatching sensitivity between the two signals, suggesting a predictive component of the modulation. Although it is not known what the function of these motor feedback signals is for V1 computation, the variety in motor feedback modulations suggest that in V1 both multimodal self-movement estimation, and error-detection functions may be coexisting. Importantly, these functions may reflect neural processes related to an internal monitoring of ongoing movement, and/or an update of internal models of the ongoing movement. Indeed, these two computations are critical to drive visually guided spatial oriented behaviors. In Drosophila, there is some evidence supporting the idea that visual neurons in this species also receive motor feedback signals in the context of walking (Fig. 10.2e). Future work performing electrophysiology in flies walking in darkness should determine if this is indeed the case. Interestingly, in the context of a saccade during flight, optic-flow processing neurons receive CD-like signals that cancel the visual responses of the neurons. This cancelation function may be another example of how the brain in a moving animal controls reflexes that can be self-induced by voluntary behavior. It is of high priority is to determine what the source of these modulations is. Circuits thought to be related to control voluntary turning behavior, such as the central complex and the lateral accessory lobe, would be the prime candidates for testing their role in directing motor-related signals to visual circuits. Using the ever-expanding genetic tools of Drosophila, and taking advantage of her relatively numerically simpler brain than that of mice or primates, one could apply a systematic strategy to perturb the activity of different populations of neurons and examine their role in generating the motor-related signals in visual neurons.
Modern techniques have uncovered that locomotion induces changes in the state of sensory circuits, and have determined that the state of the brain follows a continuing from sleep, to quiescence, to behavior. To understand the function of this state-dependent modulation, it is crucial to decouple arousal components from those generated by motor feedback during locomotion. Because neural circuits may be sensitive to the relation between self-movement and self-generated sensory stimuli, fine control of the properties of the sensory stimuli, as well as a more detailed characterization of the behavior is required to understand the mechanisms and function of locomotion-induced modulation. In addition, recording from more naturalistic situations (i.e., in closed-loop conditions) would be also critical to understand how neural circuits process self-generated versus externally generated stimuli while animals interact with an environment. All these preconditions are addressable by combining head-fixed preparations with virtual reality technology (Dombeck and Reiser 2012; see also: Minderer et al. 2016).
In summary, recording from, and perturbing the activity of genetically identified populations of neurons while animals behave in controlled environments will be essential to test how downstream circuits in the brain read out motor–sensory coordination to ultimately construct an internal representation of the sensory world that guides animal behavior. In this manner, a multidisciplinary effort combining realistic models of sensory circuit function, and experiments in genetic model organisms, will pave the way to solve mechanistic questions, and to generate hypothesis about the principles of motor–sensory coordination that can be tested in other animals, and in other scenarios.
References
Alitto HJ, Dan Y (2013) Cell type specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci 6:79
Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC (2011) Functional specialization of mouse higher visual cortical areas. Neuron 72:1025–1039
Andermann ML, Gilfoy NB, Goldey GJ, Sachdev RN, Wolfel M, McCormick DA, Reid RC, Levene MJ (2013) Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron 80:900–913
Angelaki DE, Gu Y, Deangelis GC (2011) Visual and vestibular cue integration for heading perception in extrastriate visual cortex. J Physiol 589:825–833
Ayaz A, Saleem AB, Scholvinck ML, Carandini M (2013) Locomotion controls spatial integration in mouse visual cortex. Curr Biol 23:890–894
Baldwin MK, Wong P, Reed JL, Kaas JH (2011) Superior colliculus connections with visual thalamus in gray squirrels Sciurus carolinensis: evidence for four subdivisions within the pulvinar complex. J Comp Neurol 519:1071–1094
Bennett C, Arroyo S, Hestrin S (2013) Subthreshold mechanisms underlying state dependent modulation of visual responses. Neuron 80:350–357
Blondeau J, Heisenberg M (1982) The three-dimensional optomotor torque system of Drosophila melanogaster. J Comp Physiol 145:321–329
Borst A (2014) Fly visual course control behaviour algorithms and circuits. Nat Rev Neurosci 15:590–599
Bradley DC, Maxwell M, Andersen RA, Banks MS, Shenoy KV (1996) Mechanisms of heading perception in primate visual cortex. Science 273:1544–1547
Britten KH, van Wezel RJ (1998) Electrical microstimulation of cortical area MST biases heading perception in monkeys. Nat Neurosci 1:59–63
Busch S, Selcho M, Ito K, Tanimoto H (2009) A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol 513:643–667
Chagnaud BP, Banchi R, Simmers J, Straka H (2015) Spinal corollary discharge modulates motion sensing during vertebrate locomotion. Nat Commun 6:7982
Chiappe ME, Seelig JD, Reiser MB, Jayaraman V (2010) Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol 20:1470–1475
Coen P, Xie M, Clemens J, Murthy M (2016) Sensorimotor transformations underlying variability in song intensity during Drosophila courtship. Neuron 89:629–644
Cohen MR, Maunsell JH (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12:1594–1600
Crapse TB, Sommer MA (2008) Corollary discharge across the animal kingdom. Nat Rev Neurosci 9:587–600
Davis WJ, Siegler MVS, Mpitos GJ (1973) Distributed neuronal oscillators and efference copy in the feeding system of Pleurobranchaca. J Neurophysiol 36:258–274
de Haan R, Lee YJ, Nordstrom K (2012) Octopaminergic modulation of contrast sensitivity. Front Integr Neurosci 6:55
Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD (2013) Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J Neurosci 33:17797–17813
Distler C, Hoffmann KP (2011) Visual pathway for the optokinetic reflex in infant macaque monkeys. J Neurosci 31:17659–17668
Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW (2007) Imaging large scale neural activity with cellular resolution in awake mobile mice. Neuron 56:43–57
Dombeck DA, Reiser MB (2012) Real neuroscience in virtual worlds. Curr Opin Neurobiol 22:3–10
Duffy CJ, Wurtz RH (1991a) Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large field stimuli. J Neurophysiol 65:1329–1345
Duffy CJ, Wurtz RH (1991b) Sensitivity of MST neurons to optic flow stimuli II Mechanisms of response selectivity revealed by small field stimuli. J Neurophysiol 65:1346–1359
Eggermann E, Kremer Y, Crochet S, Petersen CC (2014) Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep 9:1654–1660
Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, Busse L (2014) Effects of locomotion extend throughout the mouse early visual system. Curr Biol 24:2899–2907
Franklin DW, Wolpert DM (2011) Computational mechanisms of sensorimotor control. Neuron 72:425–442
Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP (2014) A cortical circuit for gain control by behavioral state. Cell 156:1139–1152
Garcia-Rill E, Skinner RD (1987a) The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res 411:13–20
Garcia-Rill E, Skinner RD (1987b) The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res 411:1–12
Geiger G, Nässel DR (1981) Visual orientation behaviour of flies after selective laser beam ablation of interneurones. Nature 293:398–399
Gill TM, Sarter M, Givens B (2000) Sustained visual attention performance-associated prefrontal neural activity: evidence for cholinergic activity. J Neurosci 20:4745–4757
Goard M, Dan Y (2009) Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci 12:1444–1449
Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, Chabardès S (2016) On the role of the pedunculopontine nucleus and mesencephalic reticular formation in locomotion in nonhuman primates. J Neurosci 36:4917–4929
Götz KG, Wenking H (1973) Visual control of locomotion in the walking fruit fly Drosophila. J Comp Physiol 85:235–266
Grasse KL, Cynader MS (1991) The accessory optic system in frontal-eyed animals. Macmillan, New York, pp 111–139
Grieve KL, Acuna C, Cudeiro J (2000) The primate pulvinar nuclei vision and action. Trends Neurosci 23:35–39
Grover D, Katsuki T, Greenspan RJ (2016) Flyception: imaging brain activity in freely walking fruit flies. Nat Methods 13:569–572
Haag J, Wertz A, Borst A (2010) Central gating of fly optomotor response. Proc Nat Acad Sci U S A 107:20104–20109
Haikala V, Joesch M, Borst A, Mauss AS (2013) Optogenetic control of fly optomotor responses. J Neurosci 33:13927–13934
Harris KD, Thiele A (2011) Cortical state and attention. Nat Rev Neurosci 12:509–523
Hasselmo ME, Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36:52–73
Hausen K (1982a) Motion sensitive interneurons in the optomotor system of the fly. I. The horizontal cells: structure and signals. Biol Cybern 45:143–156
Hausen K (1982b) Motion sensitive interneurons in the optomotor system of the fly. II. The horizontal cells: receptive field organization and response characteristics. Biol Cybern 46:67–79
Hausen K, Wehrhahn C (1983) Microsurgical lesion of horizontal cells changes optomotor yaw responses in the blowfly Calliphora erythrocephala. Proc R Soc Lond B 219:211–216
Heisenberg M, Wonneberger R, Wolf R (1978) Optomotor-blindh31—a Drosophila mutant of the lobula plate giant neurons. J Comp Physiol 124:287–296
Hendricks M, Ha H, Maffey N, Zhang Y (2012) Compartmentalized calcium dynamics in a C elegans interneuron encode head movement. Nature 487:99–103
Hoy RR (1989) Startle, categorical response, and attention in acoustic behavior of insects. Annu Rev Neurosci 12:355–375
Joesch M, Plett J, Borst A, Reiff DF (2008) Response properties of motion sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr Biol 18:368–374
Jung SN, Borst A, Haag J (2011) Flight activity alters velocity tuning of fly motion sensitive neurons. J Neurosci 31:9231–9237
Keller GB, Bonhoeffer T, Hubener M (2012) Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74:809–815
Kim AJ, Fitzgerald JK, Maimon G (2015) Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci 18:1247–1255
Kobayashi Y, Isa T (2002) Sensory motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15:731–741
Koenderink JJ (1986) Optic flow. Vision Res 26:161–179
Kral K (2012) The functional significance of mantis peering behaviour. Eur J Entomol 109:295–301
Kropff E, Carmichael JE, Moser MB, Moser EI (2015) Speed cells in the medial entorhinal cortex. Nature 523:419–424
Kubo F, Hablitzel B, Maschio MD, Driever W, Baier H, Arrenberg AB (2014) Functional architecture of an optic flow responsive area that drives horizontal eye movements in zebrafish. Neuron 81:1344–1359
Lappe M, Bremmer F, van den Berg AV (1999) Perception of self motion from visual flow. Trends Cogn Sci 3:329–336
Lee SH, Dan Y (2012) Neuromodulation of brain states. Neuron 76:209–222
Lee S, Carvell GE, Simons DJ (2008) Motor modulation of afferent somatosensory circuits. Nat Neurosci 11:1430–1438
Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B (2013) A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16:1662–1670
Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM (2014) Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83:455–466
Liang F, Xiong XR, Zingg B, Ji XY, Zhang LI, Tao HW (2015) Sensory cortical control of a visually induced arrest behavior via corticotectal projections. Neuron 86:755–767
Longden KD, Krapp HG (2009) State dependent performance of optic flow processing interneurons. J Neurophysiol 102:3606–3618
Longden KD, Krapp HG (2010) Octopaminergic modulation of temporal frequency coding in an identified optic flow processing interneuron. Front Syst Neurosci 4:153
Longden KD, Muzzu T, Cook DJ, Schultz SR, Krapp HG (2014) Nutritional state modulates the neural processing of visual motion. Curr Biol 24:890–895
Lüders J, Kurtz R (2015) Octopaminergic modulation of temporal frequency tuning of a fly visual motion sensitive neuron depends on adaptation level. Front Integr Neurosci 9:36
Maimon G, Straw AD, Dickinson MH (2010) Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci 13:393–399
Masseck OA, Hoffmann KP (2009) Comparative neurobiology of the optokinetic reflex. Ann N Y Acad Sci 1164:430–439
McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, McCormick DA (2015) Waking state: rapid variations modulate neural and behavioral responses. Neuron 87:1143–1161
Minderer M, Harvey CD, Donato F, Moser E (2016) Neuroscience: virtual reality explored. Nature 533:324–325
Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M (1978) Controlled locomotion in the mesencephalic cat distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol 41:1580–1591
Moruzzi G, Magoun HW (1949) Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1:455–473
Muijres FT, Elzinga MJ, Melis JM, Dickinson MH (2014) Flies evade looming targets by executing rapid visually directed banked turns. Science 344:172–177
Nauta WJH, Kuypers HGJM (1958) Some ascending pathways in the brain stem reticular formation. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT (eds) Reticular formation of the brain (pp. 3-30), Little, Brown: Oxford, England, 766 pp
Nelson A, Mooney R (2016) The basal forebrain and motor cortex provide convergent yet distinct movement related inputs to the auditory cortex. Neuron 90:635–648
Nelson A, Schneider DM, Taktoh J, Sakurai K, Wang F, Mooney R (2013) A circuit for motor cortical modulation of auditory cortical activity. J Neurosci 33:14342–14353
Newsome WT, Wurtz RH, Komatsu H (1988) Relation of cortical areas MT and MST to pursuit eye movements II Differentiation of retinal from extraretinal inputs. J Neurophysiol 60:604–620
Niell CM, Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65:472–479
Orchard I, Ramirez JM, Lange AB (1993) A multifunctional role for octopamine in locust flight. Annu Rev Entomol 38:227–249
Petreanu L, Mao T, Sternson SM, Svoboda K (2009) The subcellular organization of neocortical excitatory connections. Nature 457:1142–1145
Petreanu L, Gutnisky DA, Huber D, Xu NL, O’Connor DH, Tian L, Looger L, Svoboda K (2012) Activity in motor sensory projections reveals distributed coding in somatosensation. Nature 489:299–303
Pfeiffer K, Homberg U (2014) Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59:165–184
Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y (2013) Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16:1857–1863
Polack PO, Friedman J, Golshani P (2013) Cellular mechanisms of brain state dependent gain modulation in visual cortex. Nat Neurosci 16:1331–1339
Poteser M, Pabst MA, Kral K (1998) Proprioceptive contribution to distance estimation by motion parallax in praying mantid. J Exp Biol 201:1483–1491
Poulet JF, Hedwig B (2002) A corollary discharge maintains auditory sensitivity during sound production. Nature 418:872–876
Poulet JF, Petersen CC (2008) Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454:881–885
Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS (2014) Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84:355–362
Requarth T, Kaifosh P, Sawtell NB (2014) A role for mixed corollary discharge and proprioceptive signals in predicting the sensory consequences of movements. J Neurosci 34:16103–16116
Rien D, Kern R, Kurtz R (2012) Octopaminergic modulation of contrast gain adaptation in fly visual motion sensitive neurons. Eur J Neurosci 36:3030–3039
Ringach DL (2009) Spontaneous and driven cortical activity: implications for computation. Curr Opin Neurobiol 19:439–444
Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50:447–477
Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC (2016) Cell type specific control of brainstem locomotor circuits by basal ganglia. Cell 164:526–537
Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB (2016) Thalamic nuclei convey diverse contextual information to layer of visual cortex. Nat Neurosci 19:299–307
Roy JE, Cullen KE (2004) Dissociating self generated from passively applied head motion neural mechanisms in the vestibular nuclei. J Neurosci 24:2102–2111
Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M (2013) Integration of visual motion and locomotion in mouse visual cortex. Nat Neurosci 16:1864–1869
Schilstra C, Hateren JH (1999) Blowfly flight and optic flow. I. Thorax kinematics and flight dynamics. J Exp Biol 202:1481–1490
Schneider DM, Nelson A, Mooney R (2014) A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513:189–194
Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF (2010) Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol 103:1646–1657
Schnell B, Weir PT, Roth E, Fairhall AL, Dickinson MH (2014) Cellular mechanisms for integral feedback in visually guided behavior. Proc Nat Acad Sci U S A 111:5700–5705
Scott EK, Raabe T, Luo L (2002) Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J Comp Neurol 454:470–481
Seelig JD, Jayaraman V (2015) Neural dynamics for landmark orientation and angular path integration. Nature 521:186–191
Seelig JD, Chiappe ME, Lott GK, Dutta A, Osborne JE, Reiser MB, Jayaraman V (2010) Two photon calcium imaging from head fixed Drosophila during optomotor walking behavior. Nat Methods 7:535–540
Shik ML, Severin FV, Orlovsky GN (1966) Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics 11:756–765
Joshi S, Yin Li, Rishi M. Kalwani, Joshua I. Gold (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89:221–234
Silies M, Gohl DM, Clandinin TR (2014) Motion detecting circuits in flies coming into view. Annu Rev Neurosci 37:307–327
Sillito AM, Cudeiro J, Jones HE (2006) Always returning feedback and sensory processing in visual cortex and thalamus. Trends Neurosci 29:307–316
Sillar KT, Roberts A (1988) A neuronal mechanism for sensory gating during locomotion in a vertebrate. Nature 331:262–265
Simpson JI (1984) The accessory optic system. Annu Rev Neurosci 7:13–41
Sommer MA, Wurtz RH (2002) A pathway in primate brain for internal monitoring of movements. Science 296:1480–1482
Sommer MA, Wurtz RH (2006) Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444:374–377
Sperry RW (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43:482–489
Stackman RW, Golob EJ, Bassett JP, Taube JS (2003) Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90:2862–2874
Steriade M, Datta S, Pare D, Oakson G, Dossi RC (1990) Neuronal activities in brain–stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci 10:2541–2559
Steriade M, McCormick DA, Sejnowski TJ (1993) Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685
Strauss R, Berg C (2010) The central control of oriented locomotion in insects—towards a neurobiological model. IEEE world congress on computational intelligence, pp 3919–3926
Suver MP, Mamiya A, Dickinson MH (2012) Octopamine neurons mediate flight induced modulation of visual processing in Drosophila. Curr Biol 22:2294–2302
Tuthill JC, Nern A, Rubin GM, Reiser MB (2014) Wide field feedback neurons dynamically tune early visual processing. Neuron 82:887–895
van Breugel F, Suver MP, Dickinson MH (2014) Octopaminergic modulation of the visual flight speed regulator of Drosophila. J Exp Biol 217:1737–1744
van Swinderen B (2007) Attention-like processes in Drosophila require short-term memory genes. Science 315:1590–1593
Vinck M, Batista-Brito R, Knoblich U, Cardin JA (2015) Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86:740–754
von Holst E, Mittelstaedt H (1950) Das Reafferenzprinzip. Wechselwirkungen zwischen Zentralnervensystem und Peripherie. Naturwissenschaften 37:464–476
Voss M, Ingram JN, Haggard P, Wolpert DM (2006) Sensorimotor attenuation by central motor command signals in the absence of movement. Nat Neurosci 9:26–27
Wall MB, Smith AT (2008) The representation of egomotion in the human brain. Curr Biol 18:191–194
Weir PT, Dickinson MH (2015) Functional divisions for visual processing in the central brain of flying Drosophila. Proc Nat Acad Sci U S A 112:E5523–E5532
Weir PT, Schnell B, Dickinson MH (2014) Central complex neurons exhibit behaviorally gated responses to visual motion in Drosophila. J Neurophysiol 111:62–71
Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI (2008) Navigating from hippocampus to parietal cortex. Proc Nat Acad Sci U S A 105:14755–14762
Wiederman SD, O’Carroll DC (2013) Selective attention in an insect neuron. Curr Biol 23:156–161
Wurtz RH, McAlonan K, Cavanaugh J, Berman RA (2011) Thalamic pathways for active vision. Trends Cogn Sci 15:177–184
Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M (2009) Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS ONE 4:e4320
Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA (2013) Motor cortex feedback influences sensory processing by modulating network state. Neuron 79:567–578
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Fujiwara, T., Chiappe, E. (2017). Motor-Driven Modulation in Visual Neural Circuits. In: Çelik, A., Wernet, M. (eds) Decoding Neural Circuit Structure and Function. Springer, Cham. https://doi.org/10.1007/978-3-319-57363-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-57363-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57362-5
Online ISBN: 978-3-319-57363-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)