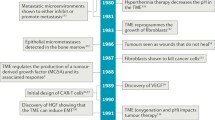
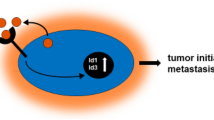
Abstract
As solid tumors progress, the surrounding microenvironment is altered dramatically. This microenvironment contains stromal and immune cells, some resident and some newly recruited, that are often activated due to factors released by the tumor cells themselves. These activated cells then release soluble factors that feed forward on the tumour cells in a symbiotic manner. Activated cells also alter the deposition and processing of extracellular matrix molecules in the microenvironment which further affects both the genotype and the phenotype of tumor cells. More specifically, these microenvironmental alterations can have profound effects on the genome and epigenome of tumor cells as well as their signal transduction pathways, both biochemical and mechanical. All of these effects contribute to the invasion and progression of the metastatic tumor organ.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Given that the overwhelming majority of cancer victims succumb to the formation and expansion of metastatic lesions, an overarching goal of modern cancer research is to determine how changes in defined cohorts of oncogenes and tumor suppressors facilitate the emergence of the malignant phenotype within individual tumor cells and their progeny. As a result, identifying actionable targets that are the products of such dysregulated genes has become the cornerstone of rational cancer therapeutics. However, even when they are directed against bona fide targets, such therapies are often only partially effective and they are invariably susceptible to acquired resistance as the tumor evolves towards full blown malignancy. The recognition that many non-tumor cell autonomous events contribute to this progressive evolution, together with data generated by genome-wide profiling both at the transcriptomic and epigenomic levels, are starting to give us a systems-based picture of why this is the case. What is becoming increasingly clear from such studies is that the tumor microenvironment is a major multifactorial contributor to this progressive evolution [23].
In early in situ lesions that have been initiated by mutagenic insult, the microenvironment still closely resembles the normal tissue that the lesion arises in. In many cases, notably the breast [48], the prostate [56, 60], and the thyroid gland [25], this near ‘normal’ tissue microenvironment actually acts to suppress the further expansion of microcarcinomatous lesions. However, as the tumor progresses, the surrounding tissue microenvironment is replaced by an ever-changing milieu that is itself abnormal. Importantly, this abnormal microenvironment co-evolves with the tumor cells as part of the malignant tumor ‘organ’. Rather than being suppressive, the microenvironment of the malignant tumor organ instead functions to promote the invasion and metastatic spread of the expanding lesion [5].
In addition to cellular differences, the microenvironment of the malignant tumor organ also differs in its extracellular components compared to the near normal microenvironment that surrounds early tumorigenic lesions. For example, factors released into the extracellular milieu by the tumor cells themselves can act to ‘activate’ nearby stromal cells in the surrounding microenvironment. The responding stromal cells can consist of those that already reside within the tissue affected or they can be recruited from other sites, most notably the bone marrow. The latter cells often have an ability to expand due to their progenitor characteristics which can further expand the activated stromal cell pool. This expansion and activation has been best documented in the case of cancer-associated fibroblasts (CAF’s; [24]), although many other stromal cell types can also be ‘activated’ by factors in the tumor microenvironment.
CAF’s are capable of modifying the acellular architecture of the tumor microenvironment by altering its insoluble extracellular matrix (ECM). This is achieved through changes in the production and deposition of matrix molecules as well as the alteration of the structure and interaction of those matrix molecules already present in the ECM. Examples include CAF-mediated changes in the deposition of collagens, their extracellular processing by metalloproteases and their cross-linking by lysyl oxidases [11, 31]. CAF’s also release soluble factors into the tumor microenvironment that act in a feed-forward way to further stimulate the growth of, or alter the phenotype of, tumor cells and to further recruit and activate more stromal cells. Tumor cell proliferation and survival can be facilitated by the CAF-mediated release of factors such as IGF-1, EGF, HGF and IL6, while a major tumor cell phenotype modifier that can be released by CAFs is transforming growth factor beta (TGF-β) (see epithelial-mesenchymal transformation section, below; [64]). Stromal cell recruitment and their feed forward activation can be initiated by the CAF-mediated release of cytokines such as CCL5 and SDF-1 [32, 35]. Importantly, the cytokines and growth factors that are released into the primary tumor microenvironment can act as short range paracrine factors as described above or they can also travel to distant sites and sow the microenvironmental soil to facilitate the expansion and local invasion of secondary metastatic lesions [76].
Acting in concert, soluble factors released by both the tumor cells and the activated stromal cells in the microenvironment can act over considerable distances to recruit immune cells to the lesion site. This immune infiltration, which has many of the hallmarks of a chronic inflammatory state, consists of varying numbers and ratios of lymphoid and myeloid cells, the precise nature of which depends on the tumor site involved and the malignant state of the lesion [59]. These infiltrates produce their own growth factors and cytokines that then further influence nearby and tumor and stromal cells in the manner described above. They also secrete proteolytic enzymes that can remodel the ECM [42]. Such remodeling can have profound effects on the tissue microenvironment as it can release and/or activate soluble factors that are normally sequestered within the insoluble portion of the ECM.
Soluble factors and ECM fragments released into the tumor microenvironment by proteolytic degradation can also act in a paracrine manner on nearby vascular endothelial cells and their surrounding pericyte stem-like population to initiate an angiogenic response. A critical factor that helps drive this angiogenic response is vascular endothelial growth factor (VEGF); [61], although VEGF-independent factors come to the fore as the malignant tumor organ evolves which has been confounding to targeted anti-angiogenic therapy development [63]. Regardless, the resulting formation of new blood vessels leads to an increase in blood supply that is critical to the malignant tumor organ’s survival. Concurrently, the angiogenic process itself further alters the landscape of the tumor microenvironment because the resulting newly formed blood vessels are often tortuous, porous and ‘leaky’ which increases the hydrostatic pressure within the tissue. In addition, tumor cells are subjected to widely varying oxygen tensions depending how far they are from these new vessels [7, 19]. In areas of low oxygen, the resulting hypoxia induces metabolic alterations and a suite of gene expression changes in both the tumor and the stromal cells that lead to further microenvironmental changes in soluble factor production and ECM production and modulation that can affect a number of tumor characteristics including genome stability (see below). It is becoming increasingly clear that a wide variety of responses to metabolic alterations within the malignant tumor microenvironment are also mediated by the activated stromal cells. For example, in response to the release of reactive oxygen species by tumor cell, nearby CAFs upregulate the expression of glycolytic enzymes which leads to their increased production and secretion of pyruvate and lactic acid that are then secreted and utilized by the surrounding tumor cells as a critical alternative energy source for their continued rapid proliferation [55].
While the cellular and acellular aspects of the microenvironment broadly influence tumor and stromal cell phenotypes, they also impinge upon specific processes that are critical for malignant progression. These include alterations to genomic stability, the epigenome, non-coding RNAs, immunomodulation, mesenchymal transformation and mechanotransduction. We provide specific examples below and expand on individual processes in the chapters that follow.
Hypoxia-Induced Genomic Instability
In normal tissues, the microenvironmental oxygen tension can be as high as 10 %. In contrast, the oxygen tension within rapidly expanding locally advanced solid tumors is often less than 1 % due in large part to their high metabolic rate [71]. The resulting hypoxia leads to the increased production of factors by both tumor and stromal cells that are transcriptional regulators, the prototype of which is hypoxia inducible factor 1alpha (HIF1α). These regulators both stimulate and suppress the expression of a wide variety of genes whose products modify the microenvironment to facilitate metastatic progression. These include the afore-mentioned angiogenic factor VEGF as well as osteopontin, an ECM protein that facilitates tumor cell invasion. The latter factor facilitates the movement of tumor cell cohorts even farther away from blood vessels which further exacerbates the hypoxic state locally within the lesion [43].
Hypoxia also acts to suppress the expression of a number of homologous recombination (HR) genes involved in repairing the DNA double strand breaks caused by ionizing radiation and radiomimetic drugs [44]. In some contexts this suppression is HIF1α-dependent [35] while in others it is not [4]. Interestingly, in some cases these two means of hypoxia-induced suppression can act on the same HR gene. This is the case, for example, with the suppression of BRCA1. Hypoxia also suppresses the expression of genes involved in DNA mismatch repair. This leads to an increase in spontaneous mutations that are associated with microsatellite instability (MSI) during experimental colorectal carcinoma progression [17, 62]. Furthermore, in human colorectal tumors HIF1α expression, which is used as an indication of hypoxia, and MSI are associated are associated with poor outcome/progression [21].
There is some evidence that hypoxia can also affect chromosome segregation during mitosis. Mechanistically, it appears that this occurs due to an alteration of the centrosome that leads to a defect in mitotic spindle formation [46]. Taken together, these and many other observations [43] indicate that hypoxia-mediated defects in DNA repair and chromosome segregation accelerate the genomic instability that is already intrinsic to the growing tumor organ, thereby facilitating the continued evolution of malignant progression [9].
Epigenetic Dysregulation
The most widely studied epigenomic change that is correlated with tumorigenesis is the CpG island hypermethylation phenotype (CIMP). Essentially, this is a broad measure of suppressive promoter methylation that has been observed in bladder, breast, endometrial, gastric, colorectal, hepatocellular, lung, ovarian, pancreatic, renal cell and prostate carcinomas as well as leukemias, melanomas and gliomas. Drivers of this phenotype, including mutations in the isocitrade dehydrogenase-1 gene that result in the accumulation of the hypermethylating oncometabolite 2HG [70], are now being identified. However, while it is clear that the CIMP phenotype contributes to tumor formation, its role in tumor progression is less clear. For example, in colorectal cancer CIMP functionally contributes to the initial tumor formation but not tumor progression. Instead, a subsequent trend towards hypomethylation becomes more prominent as lesions progress from adenomas to invasive cancers [67]. In addition to contributing to a general increase in genomic instability, this hypomethylation has been shown to specifically trigger the production and release of soluble growth factors and modulators including insulin-like growth factor-2 (IGF2) and IGF2 binding protein-3 into the tumor microenvironment [29, 41]. These factors act in an autocrine fashion to increase tumor cell proliferation and invasion and they act in a paracrine fashion to activate stromal cells which, as was described above, have feed forward effects on tumour cells that contribute to malignant progression.
LINE-1 hypomethylation within the long interspersed nucleotide element-1 (LINE-1) often occurs in metastatic prostate [74] and metastatic endocrine pancreatic [10] carcinomas. While this epigenetic mark is often used as a general indicator of hypomethylation, it is also known to be functionally significant in tumor progression in a number of ways. Specifically, it can lead to the activation of adjacent genes as well as an increase in chromosomal instability [18, 65, 72] as well as genomic instability [1, 50].
Immunomodulation
Cytokines such as interleukin-4 and -13, produced by malignant and stromal cells within the tumor microenvironment in a manner that mimics the end stages of wound healing, cause an immune suppression that is tumor promoting. This is initiated, in large part, by the cytokine-mediated recruitment of monocytes to the lesion. These new recruits then differentiate into alternatively activated tumor-associated macrophages (TAMs) that skew towards an ‘M2’ phenotype that is immunosuppressive [47, 59]. More specifically, alternatively activated TAMs do not exhibit the cytotoxicity of typical macrophages [54]. Instead, they release paracrine-acting factors such as the chemokine CCL22 [12] and they generate nitrogen species, particularly under hypoxic microenvironmental conditions [14], that suppress the infiltration and proliferation of T-lymphocytes into the tumor microenvironment. Immunosuppressive TAM’s also secrete VEGF-A [40] which augments the hypoxia-induced increase in angiogenesis within the tumor microenvironment described above. Alternatively activated TAM’s are an attractive anti-metastatic therapeutic target given their profound ability to facilitate tumor progression by contributing to an escape from immune surveillance while simultaneously promoting angiogenesis. Experimentally, TAMs can be targeted by blocking the cytokine CSF-1 [13, 45], which is required for the proliferation and differentiation all macrophage populations. Unfortunately, this approach is a very broad one and is likely to have long term side effects in patients. A more specific approach would be the reprogramming of TAM’s into more conventional ‘antigen-presenting’ immune response-promoting macrophages that are known to have anti-tumor effect. Experimentally, this has been achieved using histidine-rich glycoprotein [57].
Interestingly, some cytotoxic drugs (eg. paclitaxel) can suppress the M2 TAM phenotype and skew it towards a pro-inflammatory M1 phenotype that is then antitumoral [34]. Thus, a goal of the field has been to identify mediators that drive this proinflammatory M1 TAM skewing in a predictable manner. One such mediator is the microRNA miR-511–3p [66]. Thus, non-coding RNA’s are capable of modulating effectors in the microenvironment that play a critical role in tumor progression.
Immunomodulatory changes during tumor progression are discussed in more detail by Gregor Reid in Chap. 8 of this volume.
Mesenchymal Transformation
A major driver of tumor cell invasion is the epithelial-to-mesenchymal transformation (EMT; [69]). During the EMT process there is a breakdown in apical-basal polarity followed by the loss of adhesive junctions between stationary epithelial cells. The resulting individual cells acquire an anterior-posterior polarity and they become motile and mesenchymal [22]. Therefore, classical markers of this transformation are the loss of the epithelial cell-cell adhesion molecule E-Cadherin and the upregulation the mesenchymal intermediate filament protein vimentin. These changes, particularly at expanding tumor fronts, are often used as an indicator of invasive progression and the onset of the metastatic process [30].
During normal development, EMTs contribute to organogenesis and the formation of the body plan in a manner that is tightly regulated, both spatially and temporally [26]. For example, this occurs during gastrulation where a precisely controlled EMT leads to the production of invasive mesenchymal cells that move into the embryo and later re-aggregate to form the mesodermal condensations during primary germ layer formation [39]. While microenvironmental organizing centers (eg. the primitive knot, Spemann’s organizer) that regulate the position and timing of developmental EMT’s have been identified, the precise nature of the instructive paracrine factors they release have still not been well characterized. In contrast, the core transcriptional machinery that acts to initiate the gene expression changes that initiate an EMT has been determined. This includes the Snail, Zeb and Twist transcription factors which act on the E-cadherin promoter to inhibit the gene’s expression as well as stimulate the expression of secreted factors that further stimulate an EMT [52]. An example of the latter is platelet-derived growth factor (PDGF) which itself stimulates the localized production and activation of metalloproteases that degrade the ECM in the microenvironment to facilitate the migration and invasion of mesenchymal cells produced by the EMT [16].
During malignant tumor progression the precise spatial and temporal control of EMT is disrupted, most often because of an inappropriate accumulation and/or activation of EMT-inducing factors within the tumor microenvironment. One such factor is TGF-ß which, when it acts on epithelial-derived tumor cells (but not normal epithelial cells) stimulates the activity of the core EMT transcription factor complex [33]. TGF-ß is often produced and secreted by cancer-associated fibroblasts in the microenvironment [8]. Interestingly, TGF-ß that is produced by platelets and released into the microenvironment due to the leakiness of new vessels formed by angiogenesis can also contribute to transient tumor cell EMT at sites of thrombosis [36]. This has important implications for the movement of tumor cells from the stroma into the vasculature by a process known as intravasation. Additionally, it may also help explain why circulating tumor cells themselves can be mesenchymal [75]. The latter point is not trivial in terms of survival in the circulation as mesenchymally-transformed cells tend to be resistant to the suspension-mediated apoptosis that normally occurs when epithelial cells are detached from the ECM [27].
Other factors found in the tumor microenvironment that can act to stimulate the core EMT transcriptional machinery including the afore-mentioned PDGF produced by CAF’s as well as WNT or WNT-like factors produced by recruited mesenchymal stem cells and interleukin-6 (IL-6) produced by TAMs [49]. Importantly, the removal of such factors can shift the tumor cell phenotype from the mesenchymal back to the epithelial in a process known as mesenchymal-epithelial transformation (MET). This often occurs during the later stages of metastatic progression where an MET is proposed to contribute to the colonization of distant sites after tumor cells have left the vasculature by extravasation. Such transient shifts between epithelial and mesenchymal phenotypes can also be regulated by the oxygen tension in the microenvironment given that hypoxia upregulates the core EMT transcriptional complex via the actions of HIF-1α [73]. Thus, the mesenchymal phenotype is often plastic, unless mutations within the E-cadherin gene and/or stable, epigenetically-driven changes in E-cadherin expression occur. As such, there are varying tumor microenvironment-driven metastable and stable states of mesenchymal transformation within tumor lesions that have important implications for therapeutic treatment strategies bent on reversing the process [68].
Mechanotransduction
Once they have acquired the ability to become invasive, either by varying degrees of mesenchymal transformation or by other means that can include either collective or single-celled amoeboid migration [20], tumor cells move through the tumor microenvironment by interacting with the ECM, the components of which are highly modified due to changes in component deposition, molecular cross-linking, and proteolytic processing within that microenvironment [58]. Ultimately, the molecular composition of the ECM greatly contributes to changes in motile phenotype of the invading tumor cells based on, for example, the soluble factors it sequesters and the specific nature of the cell surface integrins that it engages [28]. However, it is becoming increasingly clear that the mechanical properties of the ECM also play an important role in regulating the phenotype of the invading tumor cells. In this case, physical changes in the ECM can dramatically alter mechanical signals within tumor cells that influence proliferation, survival and the invasive phenotype itself [15]. Experimentally, this can be achieved by artificially crosslinking ECM components, particularly collagens, to increase the stiffness of the matrix which increases intracellular tension and integrin-mediated biochemical signaling within the tumor cell [38]. Thus, mechanical cues in the ECM are translated intracellularly by the cytoskeleton and signaling moieties that are modulated by tension applied through integrin-containing adhesion complexes. Such collagen cross-linking can be achieved by the actions lysyl-oxidase which is released into the tissue microenvironment by CAFs. In yet another example of a feed forward mechanism, lysyl oxidase-dependent collagen crosslinking will further activate CAF’s themselves in an integrin signaling-dependent manner [3] and this effect can be so strong that it can facilitate tumor invasion and metastatic progression even when TGF-ß is removed [53].
Importantly, collagen crosslinking-dependent increases in radiologically observable mammographic ‘density’ is a major risk factor for breast carcinoma formation and progression [6]. The latter effect may be facilitated by the fact that ECM stiffness-mediated mechanotransduction augments the ability of soluble factors sequestered within the tumor microenvironment to efficiently induce an EMT [37].
Mechanotransduction events that contribute to tumor progression are discussed in more detail by Celeste Nelson’s group in Chap. 7 of this volume.
Summary
It is now clear that the microenvironment that a tumor cell finds itself within can greatly affect its phenotype regardless its genotype. These microenvironmental effects are mediated by surrounding stromal cells, soluble factors, and the extracellular matrix all of which act together, with tumor cells, to form the tumor organ. Therefore, given the molecular and cellular complexity within the tumor organ, it is very difficult to predict the response of any one component of the organ to a particular therapeutic treatment when that component is viewed in isolation. While this complexity can be extremely problematic when viewed from a reductionist point of view, particularly when it contributes to the failure of agents targeted against specific tumor cell-intrinsic oncogenes or tumor suppressors, it also provides myriad new therapeutic opportunities to halt the emergence of those microenvironment-dependent tumour progression phenotypes that contribute to the overwhelming majority of cancer deaths due to metastasis.
Abbreviations
- CAFs:
-
Cancer-associated fibroblasts
- CIMP:
-
CpG island hypermethylation phenotype
- DNA:
-
Deoxyribonucleic acid
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial to mesenchymal transition
- HIF1α:
-
Hypoxia inducible factor 1alpha
- MET:
-
Mesenchymal to epithelial transition
- MSI:
-
Microsatellite instability
- TAMs:
-
Tumor-associated macrophages
- TGF-β:
-
Transforming growth factor beta
- VEGF:
-
Vascular endothelial growth factor
References
Baba Y, Huttenhower C, Nosho K, Tanaka N, Shima K, Hazra A, Schernhammer ES, Hunter DJ, Giovannucci EL, Fuchs CS, Ogino S (2010a) Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol Cancer 9:125–134
Baba Y, Nosho K, Shima K, Huttenhower C, Tanaka N, Hazra A, Giovannucci EL, Fuchs CS, Ogino S (2010b) Hypomethylation of the IGF2 DMR in colorectal tumors, detected by bisulfite pyrosequencing, is associated with poor prognosis. Gastroenterology 139(6):1855–1864
Barker HE, Bird D, Lang G, Erler JT (2013) Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res 11(11):1425–1436
Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM (2005) Alterations in DNA repair gene expression under hypoxia: elucidating the mechanisms of hypoxia-induced genetic instability. Ann N Y Acad Sci 4:184–195
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329
Boyd NF, Martin LJ, Yaffe MJ, Minkin S (2011) Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 13(6):223
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564
Chan N, Koch CJ, Bristow RG (2009) Tumor hypoxia as a modifier of DNA strand break and cross-link repair. Curr Mol Med 4:401–410
Choi IS, Estecio MR, Nagano Y, Kim do H, White JA, Yao JC, Issa JP, Rashid A (2007) Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors). Mod Pathol 20:802–810
Cirri P, Chiarugi P (2012) Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 31(1–2):195–208
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942–949
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1(1):54–67
Doedens AL1, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70(19):7465–7475
DuFort CC, Paszek MJ, Weaver VM (2011) Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 12:308–319
Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J (2011) Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19:372–386
Edwards RA, Witherspoon M, Wang K, Afrasiabi K, Pham T, Birnbaumer L, Lipkin SM (2009) Epigenetic repression of DNA mismatch repair by inflammation and hypoxia in inflammatory bowel disease-associated colorectal cancer. Cancer Res 4:6423–6429
Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, Schroder K, Cloonan N, Steptoe AL, Lassmann T, Waki K, Hornig N, Arakawa T, Takahashi H, Kawai J, Forrest AR, Suzuki H, Hayashizaki Y, Hume DA, Orlando V, Grimmond SM, Carninci P (2009) The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 41:563–571
Folkman J, Watson K, Ingber D, Hanahan D (1989) Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339(6219):58–61
Friedl P1, Locker J, Sahai E, Segall JE (2012) Classifying collective cancer cell invasion. Nat Cell Biol 14(8):777–783
Furlan D, Sahnane N, Carnevali I, Cerutti R, Bertoni F, Kwee I, Uccella S, Bertolini V, Chiaravalli AM, Capella C (2008) Up-regulation of the hypoxia-inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum Pathol 4:1483–1494
Greenburg G, Hay E D (1982). Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95:333–339
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Harach HR, Franssila KO, Wasenius VM (1985) Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer 56:531–538
Hay E D (2005) The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn 233:706–720
Huang RY, Wong MK, Tan TZ, Kuay KT, Ng AH, Chung VY, Chu YS, Matsumura N, Lai HC, Lee YF, Sim WJ, Chai C, Pietschmann E, Mori S, Low JJ, Choolani M, Thiery JP (2013) An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis 4:e915
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219
Ito Y1, Koessler T, Ibrahim AE, Rai S, Vowler SL, Abu-Amero S, Silva AL, Maia AT, Huddleston JE, Uribe-Lewis S, Woodfine K, Jagodic M, Nativio R, Dunning A, Moore G, Klenova E, Bingham S, Pharoah PD, Brenton JD, Beck S, Sandhu MS, Murrell A (2008) Somatically acquired hypomethylation of IGF2 in breast and colorectal cancer. Hum Mol Genet 17(17):2633–2643
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563
Katsuno Y, Lamouille S, Derynck R (2013) TGF-ß signaling and EMT in cancer progression. Curr Opin Oncol 25(1):76–84
Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY (2010) A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 16(18):4583–4594
Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A 107(46):20009–20014
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–590
Lee K, Chen QK, Lui C, Cichon MA, Radisky DC, Nelson CM (2012) Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol Biol Cell 23(20):4097–4108
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5):891–906
Lim J, Thiery JP (2012) Epithelial-mesenchymal transitions: insights from development. Development 139(19):3471–3486
Lin EY, Li JF, Bricard G, Wang W, Deng Y, Sellers R, Porcelli SA, Pollard JW (2007) Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol 1(3):288–302
Lochhead P, Imamura Y, Morikawa T, Kuchiba A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA, Fuchs CS, Ogino S (2012) Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur J Cancer 48(18):3405–3413
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196(4):395–406
Luoto KR, Kumareswaran R, Bristow RG (2013) Tumor hypoxia as a driving force in genetic instability. Genome Integr 4(1):5
Meng AX, Jalali F, Cuddihy A, Chan N, Bindra RS, Glazer PM, Bristow RG (2005) Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol 76:168–176
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73(3):1128–1141
Moser SC, Bensaddek D, Ortmann B, Maure JF, Mudie S, Blow JJ, Lamond AI, Swedlow JR, Rocha S (2013) PHD1 Links cell-cycle progression to oxygen sensing through hydroxylation of the centrosomal protein Cep192. Dev Cell 4:381–392
Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70(14):5728–5739
Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA (1987) Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer 56:814–819
Nieto MA (2011) The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 27:347–376
Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Meyerhardt JA, Fuchs CS (2009) Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 27(27):4591–4598
Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Meyerhard JA, Fuchs CS (2009) Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 26:5713–5720
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM, Moses HL (2013) Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res 73(17):5336–5346
Qian BZ1, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51
Rattigan YI1, Patel BB, Ackerstaff E, Sukenick G, Koutcher JA, Glod JW, Banerjee D (2012) Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res 318(4):326–335
Rich AR (2007) On the frequency of occurrence of occult carcinoma of the prostrate. Int J Epidemiol 36:274–277
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Åkerud P, De Mol M, Salomäki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19(1):31–44
Rubashkin MG, Ou G, Weaver VM (2014) Deconstructing signaling in three dimensions. Biochemistry 53(13):2078–2090
Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM (2012) Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 109(8):2796–2801
Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD (1993) The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol 150:379–385
Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12:303–324
Shahrzad S, Quayle L, Stone C, Plumb C, Shirasawa S, Rak JW, Coomber BL (2005) Ischemia-induced K-ras mutations in human colorectal cancer cells: role of microenvironmental regulation of MSH2 expression. Cancer Res 4:8134–8141
Sitohy B, Nagy JA, Dvorak HF (2012) Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res 72(8):1909–1914
Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 4(4):e4992
Speek M (2001) Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes. Mol Cell Biol 21:1973–1985
Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M (2012) miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep 1(2):141–154
Sunami E, de Maat M, Vu A, Turner RR, Hoon DS (2011) LINE-1 hypomethylation during primary colon cancer progression. PLoS ONE 6(4):e18884
Tam WL, Weinberg RA (2013) The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19(11):1438–1449
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, Viale A, Riggins GJ, Chan TA (2013) Efficient inductino of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT inhibitor decitabine. Oncotarget 4:1729–338
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 4(Suppl 5):4–9
Yamada Y, Jackson-Grusby L, Linhart H, Meissner A, Eden A, Lin H, Jaenish R (2005) Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci U S A 102:13580–13585
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 10(3):295–305
Yegnasubramanian S, Haffner MC, Zhang Y Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese TL, Isaacs WB, Bova GS, DeMarzo AM, Nelson WG (2008) DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res 68:8954–8967
Yu M1, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339(6119):580–584
Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massagué J (2013) Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154(5):1060–1073
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Roskelley, C. (2015). Microenvironmental Control of Metastatic Progression. In: Maxwell, C., Roskelley, C. (eds) Genomic Instability and Cancer Metastasis. Cancer Metastasis - Biology and Treatment, vol 20. Springer, Cham. https://doi.org/10.1007/978-3-319-12136-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-12136-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12135-2
Online ISBN: 978-3-319-12136-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)