Abstract
Hydrocephalus and hydrocephalus-related problems occupy a large part of current neurosurgical activity, accounting for approximately 35–50 % of pediatric neurosurgical practice. At present, the most frequent treatment for hydrocephalus consists of cerebrospinal fluid (CSF) shunting, especially with ventriculoperitoneal (VP) shunts. Other types of CSF derivations, such as lumboperitoneal, ventriculopleural, ventriculo-gallbladder, subgaleal shunting, etc., continue to be in use, although they are less often utilized. Ventriculoatrial valves have almost totally been abandoned due to the severity of their complications. On the contrary, neuroendoscopic procedures, especially endoscopic third ventriculostomy (ETV), are increasingly being used in daily practice for avoiding shunt complications.
Shunt-related complications are numerous and difficult both to manage and to avoid. Shunt failure occurs in a proportion as high as 45–50 % during the first year after surgery and has a subsequent estimated incidence of 4–5 % yearly. At the time of placing a CSF shunt, the neurosurgeon must carefully plan the operation or shunt revision, bearing in mind not only the immediate success of the operation but also taking into account the avoidance of possible future complications.
In this chapter, common clinical manifestations of shunt failure are dealt with, considering the (1) type of complication (mechanical, functional, or infectious), (2) patient’s age at shunt insertion, (3) etiology of the hydrocephalus, (4) early or late appearance of complications, and (5) place of CSF drainage (peritoneum, pleura, jugular vein, etc.).
Standard adjuvant clinical methods for diagnosis of the site and type of complication will be briefly discussed, aimed at planning the most appropriate approach for a shunt revision and at trying to avoid unnecessary surgery. A better knowledge of complications related to shunting and of preventive measures seems to be crucial for increasing technique safety and for improving patients’ outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Subdural Hematoma
- Endoscopic Third Ventriculostomy
- Normal Pressure Hydrocephalus
- Shunt Infection
- Intracranial Hypotension
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction: Hydrocephalus and Shunt Problems
CSF shunts constitute the mainstay of treatment for hydrocephalus of diverse etiologies and are among the most common procedures performed in pediatric neurosurgery [88]. For practical purposes, hydrocephalus can be classified into two main groups: obstructive and communicating (nonobstructive) aimed at indicating the two most popular techniques for its treatment, CSF shunting or endoscopic third ventriculostomy (ETV). Most cases of obstructive hydrocephalus are now treated with neuroendoscopic procedures, mainly ETV, while CSF shunts continue to be utilized for treatment of many cases of both obstructive and nonobstructive hydrocephalus.
At present, the use of shunting procedures is questioned due to the large number of complications with which they are plagued. However, shunts have saved the life of a considerable number of patients, have decreased morbidity and mortality, and have improved the quality of life of many individuals with hydrocephalus. Accordingly, the continued use of CSF shunting procedures and the increasing use of ETV justify an account of the symptoms and signs with which complications are apt of manifesting in this setting.
2 General Scope of Hydrocephalus and CSF Shunt Revisions
According to Bondurant and Jimenez, there are approximately 125,000 hospital discharges with the diagnosis of hydrocephalus each year in the USA, comprising 36,000 shunt-related procedures and 33,000 placement of shunts with an economic cost of 100 millions of USA $ a year. Nearly half of this amount is spent on shunt revisions [7]. Massimi et al. noted a recent change in the epidemiology of hydrocephalus [54]. These authors observed a decrease in the incidence of hydrocephalus related to myelomeningocele, aqueduct stenosis, CNS infections, craniocerebral malformations, and head injuries. The rate of posthemorrhagic hydrocephalus remains stable, while the incidence of tumor-associated hydrocephalus is on the rise [54]. In our mean, approximately 40 % of pediatric neurosurgical operations have to do with hydrocephalus and its complications. We have also observed a steady decrease in the number of new cases of infantile hydrocephalus attributable to better prevention measures, to prenatal diagnosis, and to improvement in neonatal care. There is also a diminution in the rate of surgical revisions probably related to the reduction in the global incidence of hydrocephalus, to the generalized use in our mean of programmable valves, and to the introduction of ETV. We have not appreciated significant changes in the incidence of operations for normal pressure hydrocephalus (NPH).
Wong et al. have recently reported the patterns of neurosurgical adverse events of CSF shunt surgery [88]. Shunt failure constitutes a serious problem with a cost of 1.4–2 billion dollars in hospital charges a year. Wong et al. analyzed 14,683 new ventricular shunts implanted in pediatric and adult patients. Failures during the first year may have an incidence as high as 50–70 % and an estimated rate of 5 % yearly thereafter [87]. Table 2.1 summarizes the distribution of the commonest reported complications of hydrocephalus treatment. Briefly, adverse events of surgical treatment of hydrocephalus can be classified into three groups: (a) mechanical, (b) infectious, and (c) functional. Iatrogenic failures will be dealt with in a separate chapter.
3 Terms, Concepts, and Definitions
Hydrocephalus consists of an excessive accumulation of cerebrospinal fluid (CSF) within the cavities of the brain or around it. Concerning etiology, the causes of hydrocephalus are often grouped into congenital or acquired and may result from a variety of pathological processes such as congenital and malformative conditions, intracranial hemorrhage, infection, trauma, and brain tumors or cysts (Table 2.2). Regarding pathophysiology, hydrocephalus is the result of excessive production, flow obstruction, or impaired reabsorption of CSF. Hydrocephalus may involve different cranial cavities and is often described, mainly by neuroradiologists, as mono-, bi-, tri-, or tetraventricular referring to the number of dilated ventricles proximal to the site of obstruction. External hydrocephalus consists of the extra-axial accumulation of fluid probably by impaired absorption. Arachnoid cysts have also been considered as localized forms of hydrocephalus and communicating hydromyelia as a form of intramedullary hydrocephalus [44, 52]. Normal pressure hydrocephalus (NPH) is a condition of poorly known origin and is now also termed chronic hydrocephalus of the adult [64].
In relation to temporal occurrence, hydrocephalus may present in acute, subacute, or chronic forms. Hydrocephalus may be active or passive and has to be differentiated from brain atrophy. This is particularly true in the case of children afflicted with destructive brain diseases as infections, hemorrhage, or trauma to the central nervous system (CNS). Elderly patients or those with arterial hypertension, atherosclerosis, diabetes mellitus, previous cerebrovascular accidents, or small ischemic brain lesions may also show brain atrophy in imaging studies.
Arrested hydrocephalus means adequately treated (shunted) hydrocephalus. Compensated hydrocephalus refers to all other forms of hydrocephalus at various levels of compensation that often entails some cost to the patient. Uncompensated hydrocephalus in children refers to progressive ventricular enlargement, usually accompanied by macrocephaly. The term uncompensated hydrocephalus is also applied to a situation of stable ventricles associated with developmental delay, cognitive impairment, impaired consciousness, or progressive neurological deficit. The concept of “cure” is rarely applied for shunted hydrocephalus given the current uncertainty for diagnosis even when intracranial pressure (ICP) monitoring or hydrodynamic tests are utilized. According to Rekate, children with communicating hydrocephalus have a probability as high as 50 % of becoming shunt independent at a later age [67]. Patients with doubtful diagnosis of hydrocephalus usually give equivocal results on current tests that justify the exceptional use of the term cure referring to hydrocephalus. Therefore, it seems reasonable to review children with ventriculomegaly if they are neurologically stable and if psychomotor development remains on time. Patients in this situation should be followed up closely even with serial ophthalmologic and psychometric studies.
3.1 Shunt Structure
CSF shunts divert the excess of fluid from the ventricles (or other fluid-filled spaces, as subdural collections and intracranial cysts) to another body cavity. Basically, a shunt is composed of three components: a proximal (ventricular) catheter, the valve, and a distal catheter. These pieces can be manufactured in separated parts that are assembled at the time of insertion or be manufactured in a single kit called unishunt. Most shunts may also contain accessories such as integrated pumping devices or reservoirs, and they may also be supplied with an independent antisiphon device or with a siphon-controlling device integrated in the valve.
Most valves are of differential pressure type, flow regulated, or anti-gravitational. Valves may also be of fixed pressure (low, medium, or high pressure), or they may be externally adjustable (programmable valves). The internal mechanism that regulates CSF flow and pressure of the valve may consist of slits, diaphragm, ball and spring, or miter mechanisms. The components of the shunt are made up of silicone, complemented with other polypropylene or hardened plastic parts or with metallic connectors. Some silicone tubing is cured with silver for increasing resistance to stretching or kinking, while barium impregnation of the tubes is commonly utilized for radiologic identification of the integrity of the shunt.
3.2 Types of CSF Draining Systems
The most popular type of shunting device is the ventriculoperitoneal shunt (VP), followed by ventriculopleural, ventriculoatrial (VA), lumboperitoneal (LP), and more rarely ventriculo-gallbladder (VGB) shunt. Other types of CSF shunt systems are presently considered only of historic interest. External ventricular drainage (EVD) consists of a temporary device endowed with a ventricular (or subdural) catheter that connects with a collecting bag. A variety of CSF drainage is the ventriculo-subgaleal shunt that drains the ventricular CSF to the subgaleal space and is commonly used as a temporizing measure for controlling ICP especially following ventricular hemorrhage or infection. In addition, ETV is a form of internal derivation of CSF that communicates the floor of the third ventricle with the basal cisterns. At present, ETV is more and more utilized for avoiding the innumerable complications of CSF shunting.
3.3 Shunt Failure, Shunt Malfunction, and Complications
The term shunt failure is not well defined in the current literature. The most accepted view is that shunt failure consists of the inability to reach the goal of surgery. In CSF shunting, failure refers to the incapability of accomplishing an appropriate control of hydrocephalus (as opposed to success) indicated by revision, replacement, or removal of the shunt. The term complication refers to any adverse event that interferes with the expected success of the procedure including new insertions, revisions, or replacements of the valve. Complications may or may not be related with the surgical technique or the valve, and may or may not end with shunt revision or replacement. Complications may derive from problems related to the valve, the patient, or the surgery. In many occasions, the terms failure and complication are interchangeably used in the literature. The variety of devices and accessories employed for shunting of CSF attests for the lack of a rigorous knowledge of the mechanisms involved in the pathophysiology of hydrocephalus and the lack of established guidelines for its treatment. In addition, no CSF shunt has demonstrated its superiority over other shunt type.
4 Clinical Manifestations of Shunt Failure
As stated before, shunt failure refers to any condition that ends in revision, replacement, or removal of a CSF valve or even in the patient’s death. Failure may be related to (a) mechanical malfunction, (b) infection, or (c) over- or underdrainage.
4.1 General Clinical Features of Shunt Failure
Shunt failure can show up in several ways and may proceed with a rapid or slow onset and variable evolution. Shunt malfunction may appear acutely, with alarming signs of brain herniation, i.e., rapidly declining level of consciousness, pupillary changes, posturing, apneic spells, and bradycardia, indicating that we are facing an emergent situation [1, 25, 40]. Patients arriving in hospital in this way let little time for reflection and need emergent management. More often, subacute shunt failure appears in a less stressful scenario that permits calm assessment and allows time for planning the appropriate (medical or surgical) management. Shunt malfunction can also present with chronic manifestations such as mild psychomotor retardation, decreased vision, impaired ocular motility, unsteady gait and falls, mood changes, decreased school performance, increased tone and reflexes in the lower limbs, or symptoms and signs of brain stem involvement or of hydromyelia [51, 57]. In patients operated on for NPH, shunt failure is proclaimed by return to their presurgical situation. Patients show slow mental deterioration, urinary urgency or incontinence, and a worsening gait. Headaches, dizziness, and focal symptoms or signs appear exceptionally in NPH patients with shunt malfunction.
4.2 Clinical Features of Mechanical Failures
Mechanical malfunction is the most frequent cause of CSF shunt failure. Its incidence may be as high as 50 % in children [4]. Shunt malfunction may be due to proximal catheter obstruction (the most common), valve obstruction, distal catheter occlusion, disconnections of shunt parts, fracture of the tubing, or migration of the proximal or distal catheters. Brain debris, choroid plexus, blood, or tissue reactions often occlude proximal catheters. Slit ventricles and faulty placement of the catheter within the ventricle may also interfere with the flow through the catheter.
On the contrary, the valve itself appears as the most dependable part of the shunt system. Obstruction of the valve is very rare and, in our experience, it happened in very few cases, and it was almost always produced by blood clots [42]. Breakage of the valve itself may occur without any apparent cause or may follow a cranial traumatism. Obstruction of the distal catheter generally occurs in systems with distal slit valves and very rarely in open-ended catheters [15]. In exceptional occasions, the distal tube may be occluded by fecal contents indicating bowel perforation. In the abdomen, distal catheters may be occluded by ingrowth of mesothelial cells and fibroblasts [17]. Kinking of the tube is also of very rare occurrence and is always due to a defective placement.
Detachment of ventricular catheters is almost exclusively due to a loose ligature or to using absorbable sutures. Separations of distal catheters generally occur at the site of connection to the valve, even in systems with soldered components. Stress rupture of the shunt tubing, in one or several pieces, usually takes place on the anterior neck or upper part of the chest wall and is favored by sustained or repeated stretching or friction. Notable deterioration of drainage systems usually occurs in shunts implanted for more than 5 years [20]. Rupture and disconnections mainly happen when there exist biodegradation and calcification of the outer surface of the tubes (Fig. 2.1) caused by aging of the device [6, 17, 20, 21, 76]. Resistance to rupture perhaps might be improved by incorporating tubes to the shunt device with a greater cross-sectional area [79].
Proximal catheters, reservoirs, and even the entire shunt may migrate into the brain, the ventricle, the scalp, or the subgaleal space. In occasions, catheter migration is accompanied by the valve (Fig. 2.2) or reservoir itself [12, 24, 42, 72]. Hydrogel-coated (BioGlide) catheters (devised to decrease cell adhesion aimed at reducing shunt obstruction and infection) seem to be more prone to disconnection and intracranial migration than standard devices [12]. In the same way, the proximal catheter (and its accompanying reservoir or valve) may be pulled out of the ventricle and be displaced down toward the subcutaneous tract. The distal catheter may be stretched out of the peritoneum too, migrating to the subcutaneous abdominal or thoracic tract or into the pleural space, or it may even follow an upward course and penetrate the skull, ventricle, etc. [43, 78]. Detached or broken distal catheters may totally migrate and lodge into the peritoneal cavity.
There are anecdotal reports on perforations and migrations of the distal tubing into the bowel, stomach, liver and gallbladder, scrotum, urinary bladder, pleural space, bronchi, and heart. Bowel perforation seems to be the most severe form of this complication [16]. Extrusion of shunts may take place through the anus, umbilicus, mouth, vagina, operation scars, midlumbar region, etc. [16, 19, 29].
Distal catheters of VA valves may also break or disconnect and stay in situ or migrate to the right heart ventricle, right atrium, pulmonary arteries, or cava vein [36]. All these mechanical complications habitually show the signs and symptoms reported above and are intimately related to the body cavity where the shunt drains (peritoneum, pleura, heart, lumbar spine, etc.).
4.3 Age as a Risk Factor for Shunt Dysfunction
Complications of CSF shunting are more prevalent in neonates and infants due to the special characteristics these patients possess [18, 19, 48]. There is an increased incidence of both shunt malfunction and shunt infection in this age group in comparison with older children and adults. This vulnerability is due to brain immaturity, skull flexibility, skin fragility (Fig. 2.3a), and compromised immunity. In neonates and infants, irritability, vomiting, decreased appetite, and lethargy, together with bradycardia and apneic episodes, indicate shunt malfunction. Bradycardia and apnea constitute a very reliable indication of shunt failure [40]. Clinical examination findings include abnormal growth of head circumference, bulging of the anterior fontanel (Fig. 2.3a), suture diastasis, sunsetting eyes, and dilated scalp veins (Table 2.3). Palpation of the anterior fontanel may give a reliable estimate of intracranial pressure and is a very dependable sign in children with open skull bones (Fig. 2.3b).
Shunt failure in older children and adults manifests itself with an almost constant triad that consists of headaches, vomiting, and somnolence [4]. Other complaints include blurred or failing vision, squint, loss of appetite, mood changes, and new onset or increase in the number of seizures. Table 2.4 shows some frequent (and less frequent) symptoms and signs of shunt malfunction in older children and adults. Differential diagnosis in children who present with vague symptoms must be made against common infantile diseases, especially gastrointestinal viral diseases or mild upper respiratory tract infections. Children are also prone to experience otitis media, and individuals with myelomeningocele often suffer from repeated urinary infections that may resemble (or mask) those of shunt failure.
Less often, shunt malfunction evolves with chronic and subtle clinical features as failing vision, mood and behavioral changes, gait clumsiness, frequent falls, regression or stagnation of developmental milestones, or even with deficient schooling progression. Clinical examination may show papilledema, spastic paraparesis, hypertonus, hyperreflexia, uni- or bilateral sixth cranial nerve palsy, cervical defense, spinal rigidity, erythema, palpable pseudotumors, or fluid collections along the shunt tract (Fig. 2.4a, b). Rupture or disconnection of the distal catheter can be diagnosed by palpating the entire shunt along the trajectory through the subcutaneous tissue. Partial calcification of distal catheters indicates tube degradation and should raise the suspicion of catheter breakage with or without intra-abdominal migration (Fig. 2.1). In peritoneal shunts, features of shunt malfunction referred to the abdominal cavity are usually the most striking (Table 2.4).
There is a group of pediatric patients who are admitted repeatedly to hospital with repeated shunt failure (“poor-shunt patients”) [81]. The main cause for recurring malfunction is proximal catheter obstruction. Known causative factors for this complication are a younger age at insertion, overdrainage, concurrent other surgeries, and certain causes of hydrocephalus [81].
Bergsneider et al. and Vinchon et al. [5, 84] reported several forms of shunt dysfunction in adults with pediatric-onset hydrocephalus and have also given an account of other related conditions such as adult slit ventricle syndrome, multi-compartmental hydrocephalus, newly diagnosed noncommunicating hydrocephalus, and non-NPH and NPH hydrocephalus [5]. Risk factors for shunt malfunction in adults has been especially described in VA shunts especially after previous multiple external drainages [32]. Adult NPH patients with shunt malfunction often return to hospital with the complaints of deterioration in comparison with the amelioration noted immediately after shunting. Clinical features in this group of patients consist of worsening gait, aggravation of urinary problems, and failing memory. Rarely these patients complain of headaches, mental dullness, dizziness, or seizures. Physical examination shows increased reflexes and Babinski sign, gait ataxia, parkinsonism, and signs of frontal release as sucking and grasping reflexes. Muscle strength is normal and there is no sensory loss. However, the appearance of focal signs, as hemiparesis, should raise the suspicion of a subdural hematoma. In an extensive series of chronic subdural hematomas, six instances (0.6 %) were associated with shunted hydrocephalus and presented with behavioral disturbances or headaches [26].
4.4 Early vs. Late Shunt Failures
The majority of early complications occurs in the first year after shunt insertion and is in relation with patient age and condition, the surgical technique, and the function of the shunt itself. The most frequent reasons for early failure both in children and adults are proximal catheter obstruction and shunt infection [23]. In our experience, technical problems related to valve placement and inadequate selection of valve’s characteristics (pressure and size) may cause early shunt failure too. Late shunt dysfunction is almost exclusively due to proximal or distal catheter block or to fracture or disconnection in relation with problems pertaining to aging of the implanted material and to late infection. The hazard of shunt failure decreases as a function of time in both newly placed and revised shunts: “the longer a shunt functions, the less likely it is to fail” [53]. The rate of shunt infection decreases as a function of time too. Mc Girt reported a 14 % rate of failures for all shunts during the first month after shunt implant and only a 5 % rate beyond 4 years [53]. Obviously, shortening of the distal catheter due to the child’s growth is also a late age-related cause of shunt failure.
4.5 Role of Hydrocephalus Etiology in Shunt Failure
There is no agreement in different studies as to the role played by etiology of hydrocephalus in the rate of subsequent shunt complications. Some find no differences between the diverse causes of hydrocephalus in the occurrence of shunt failures [28, 63]. However, others report an increased rate of shunt malfunction in patients with intraventricular hemorrhage, meningitis, or tumors [37, 39, 63]. Reported risk factors in tumoral hydrocephalus comprise age, tumor histology, and concurrent or prior surgical procedures (external ventricular drains, craniotomy, etc.) [66]. There is a higher rate of severe complications in myelomeningocele patients with the Chiari II malformation at the time of shunt malfunction. These patients may suffer early damage to the brain stem and upper cervical cord that causes breathing problems and quadriparesis [25, 51, 57].
4.6 Malfunction in Different Draining Spaces
Ventriculoperitoneal shunts may fail due to numerous causes as ascites (Fig. 2.5a), hernias, hydrocele, ileus, intussusception, torsion of omental cyst, peritonitis, peritoneal pseudocyst, volvulus, perforation of viscus, peritoneal pseudotumor, and catheter extrusion through several places (umbilicus, rectum, vagina, scrotum, mouth, gastrostomy wound, etc.). Most of these complications usually evolve with features of shunt malfunction and/or with those of infection [16]. An often overlooked cause of shunt malfunction is severe constipation (Fig. 2.5b) that causes increased intra-abdominal pressure thus impairing CSF drainage from the ventricles [49, 54, 71].
Malfunction of ventriculopleural valves is generally due to accumulation of fluid in the pleura (aseptic hydrothorax), pneumothorax, and rarely to pleural empyema or fibrothorax [45, 80]. Symptoms of thoracic involvement include chest pain, cough, shortness of breath, and tachypnea. On examination there may be decreased breath sounds, dullness on percussion, subcutaneous emphysema, pallor, sweating, tachypnea, and cyanosis.
Ventriculoatrial shunts are at present rarely implanted due to the severity of the problems that they may originate. VA valves in children require frequent catheter lengthening due to the patients’ growth. Specific complications of VA valves are often severe and include bland or septic pulmonary embolism, pulmonary hypertension, endocarditis, cor pulmonale, cardiac arrhythmias, and shunt nephritis. Clinical manifestations include chest pain, shortness of breath, low-grade fever, and, in the case of shunt nephritis sepsis, hepatosplenomegaly, arterial hypertension, hematuria, and proteinuria [61, 83].
Lumboperitoneal shunts are used in communicating hydrocephalus, NPH, pseudotumor cerebri, CSF fistulas, and postsurgical pseudomeningocele. LP valves have a high rate of complications [14, 85]. One of their main drawbacks of LP shunts is the difficulty they present for assessing their function. Morbidity of LP shunts may be due to mechanical complications (block, migration), overdrainage (subdural collections), infection, and development of acquired Chiari malformation. Acquired tonsillar herniation is the most feared complication, and it is thought to be more prevalent following the placement of valveless systems. Clinical features include back pain, back stiffness, sciatica, neurological involvement in the lower limbs, scoliosis, and those pertaining to symptomatic tonsillar herniation [14, 85].
Ventriculo-gallbladder shunts are indicated after failure of previously placed VP, VA, or ventriculopleural shunts. Complications comprise malfunction, disconnection, infection, gallbladder atony, gallbladder calculi, peritonitis, and bilious ventriculitis [27, 77].
Subdural-peritoneal shunts are used for draining subdural collections of fluid. Their main complications are blockage, infection, disconnection, migration (including intracranial migration of the entire system), CSF leakage, and skin ulceration, together with overdrainage that may produce cranioencephalic disproportion and proximal catheter adherence to brain surface at removal [22, 34].
Ventriculo-subgaleal shunts are currently used for temporary control of hydrocephalus of intraventricular hemorrhage in premature neonates. These shunts may present with infection, blockage, CSF leakage from the wound, and intracranial hemorrhage and with pressure-related head molding caused by compression from the subgaleal collection of fluid.
5 How Does Shunt Infection Manifest?
Ventricular shunt infection is a common complication of CSF shunting that causes high morbidity and mortality. A recent study on infection after initial CSF shunting in children, comprising 7,071 children, reported 825 shunt infections that produced 4,434 shunt revisions [74]. During a 24-month follow-up, CSF shunt infection rates were 11.7 % per patient and 7.2 % per procedure. Significant risk factors for infection (p < 0.05) included young age, female sex, Afro-American race, public insurance, intraventricular hemorrhage, respiratory complex chronic conditions, subsequent revisions, hospital volume, and surgeon case volume [74]. Predictors of infection in a cohort of 979 shunted patients, 130 (13 %) comprised bacterial growth in CSF, of which 58 (5.9 %) had a final diagnosis of shunt infection [70]. Risk factors for shunt contamination comprise recent (<90 days) shunt surgery, fever, abdominal pain, CSF leak, and erythema or swelling along the shunt tract [70]. For these authors, an important feature discriminating between shunt failure and shunt infection after new placement or valve revision is the presence of fever and leukocytosis (>15,000) [70].
Risk factors for shunt infection in adults include previous CSF leaks, revisions for dysfunction, operation performed late in the day, and longer surgical time [32]. Most clinical manifestations of shunt failure cannot conclusively distinguish between malfunction and infection, except for cases in which clinical features and laboratory tests of shunt infection are clearly identifiable (Table 2.5).
Common symptoms of shunt infection include those of shunt malfunction (headaches, vomiting, etc.) plus those of infection such as feeding problems, fever, malaise, fatigue, and lethargy. Signs include neck rigidity, swelling, erythema or cellulitis around the shunt tract (Fig. 2.4), leakage of CSF, or frank discharge of pus from the surgical incision. Prevention of skin ulceration over the valve involves choosing an adequate cranial site for valve placement and avoiding prominent zones of the head, especially in infants (Fig. 2.6a) and in debilitated or bedridden patients [33]. A scar over the valve reservoir (or other shunt component) suggests the probable infection of the device (Fig. 2.6b). However frank features corresponding to wound or tract contamination are seldom present. Low-grade infections by coagulase-negative staphylococci or other skin microorganisms account for the majority of shunt contamination, but in these cases obvious clinical features of infection, even hyperthermia, are usually lacking.
In peritoneal shunts (VP or LP), symptoms and signs of infection related to the abdomen usually are most prominent. The patients often complain of abdominal pain and distention, nausea and vomiting, or constipation. On examination, there may be abdominal tenderness, guarding and distention in peritonitis or ascites (Fig. 2.5a), or a palpable mass in the case of a pseudocyst. In VA shunts, early or late features of systemic infection, sepsis, or endocarditis may be the hallmark of the presence of valve infection. Shunt nephritis, a late form of VA shunt infection, usually evolves with sepsis, arterial hypertension, hematuria, and proteinuria. In ventriculopleural shunts, infection usually shows up with pleuritic pain, fever, tachypnea, dyspnea, or other features of respiratory tract involvement.
Meticulous surgical technique and extreme measures of asepsis are regarded as crucial for the prevention of shunt contamination [13, 82]. Modifications of surgical techniques include reduced personnel in the operating room, shunting as first case in the day, use of adhesive drapes on the skin, double gloving, no-touching technique, bathing the valve components with an antibiotic solution, meticulous wound closure to prevent CSF leakage, etc. There is some controversy on the role played by hair shaving on the incidence of shunt contamination, but the results are not conclusive [9]. Similarly, there are also unresolved discrepancies about the role played by the factor “experience of the surgeon” (trainees vs. experienced surgeons) on the infection rate [41, 74].
Regarding infection prophylaxis, we consider that the preparation of the surgical field is one of the most important actions to be taken for preventing shunt contamination. We customarily follow Venes’s recommendations for preventing infections [81]. Patients are shaved immediately before operation. The skin is scrubbed with povidone iodine (Betadine) solution for 10 min and then covered with adhesive drapes. We also submerge the shunt components in a solution of vancomycin prior to insertion and use three doses of intravenous prophylactic antibiotics [48]. In a systematic literature review on the subject comprising 5,613 pediatric and adult shunting procedures, antibiotic-impregnated shunts resulted in a significant reduction of shunt infection over standard shunts and in no increase in microbial resistance to antibiotics [60].
6 Functional Complications
In this section of the chapter, we will briefly comment on the clinical features pertaining to diverse syndromes that produce intracranial hypotension or hypertension related with the use of CSF shunting and that are referred to as under- or overdrainage.
6.1 Clinical Manifestations of Underdrainage
Symptoms and signs of CSF underdrainage are those related to intracranial hypertension and that we have been already described (Tables 2.3 and 2.4). Underdrainage is most usually due to using a valve of a higher pressure than the one needed for the patient. Using siphoning-retarding devices or flow-controlled valves, especially in infants and young children, may cause decreased drainage too. Situations with increased pressure at the draining cavity can also lead to underdrainage, for example, increased peritoneal (or pleural) pressure as seen in obesity, pregnancy [87], abdominal tumors, constipation, etc. [49, 55, 71]. The drainage may also be affected by an inadequate absorption of hyperproteic CSF by the peritoneum resulting in ascites as happens with optic-chiasmatic tumors [86]. Multi-compartmental hydrocephalus drained by multiple catheters may lead to symptoms and signs of malfunction by an asymmetrical distribution of forces within the diverse cavities of the CNS.
Isolated fourth ventricle may present one or more of the following clinical features: involvement of cranial nerves sixth and seventh, hoarseness, dysphagia, dysarthria, bradycardia, diplopia, nystagmus, ataxia, irregular breathing, neck pain and rigidity, and hemiparesis as a result of pressure on the brain stem and high spinal cord. Occasionally, these symptoms are produced by a direct injury to the brain stem by a too long catheter [38, 59]. Increased CSF pressure in enlarged and trapped fourth ventricle may contribute to development of cervical spinal cord edema (presyrinx state) or of communicating hydromyelia with symptoms of brain stem involvement and quadriparesis [51, 57].
6.2 Clinical Features in Overdrainage
The most frequent symptom of CSF hyperdrainage is orthostatic headaches that are typically relieved by lying down in horizontal position. Many times orthostatic headaches are seen shortly after shunt insertion or revision and subside with time needing no further treatment. In neonates and infants with excessive drainage of CSF, the fontanel may be sunken and the sutures overlap (Fig. 2.7). The babies may also show decreased appetite and trunk hypotonus. Symptomatic subdural hematomas are exceptional in this age.
Most often, overdrainage in older children and adults induces the formation of subdural collections of fluid or chronic subdural hematomas by excessive drainage of CSF. These extra-axial collections may be asymptomatic or may cause focal neurological deficits. CSF overdrainage is also thought to be the origin of recurring episodes of proximal catheter obstruction with associated shunt malfunction. Enophthalmos may be a late sign of intracranial hypotension related to CSF overshunting [31].
Slit ventricle and slit-ventricle syndrome (SVS) have been the subject of many studies regarding pathogenesis and management [3, 11, 46, 68]. Slit ventricles, often found in routine imaging, are usually asymptomatic and do not require surgical treatment. On the contrary, the SVS produces severe symptoms as incapacitating headaches, vomiting, mood changes, failing vision, syncope, and mental confusion with variable changes in the state of consciousness. In severe cases, there may be impending signs of coma, brain herniation with bradycardia, apnea, fixed pupils, and posturing. SVS has been reported as being exclusive of patients of pediatric age, but features of this condition may also be found in adults [5]. SVS has not been reported in the presence of brain atrophy [19]. SVS may also occur in arachnoid cyst treated with cyst-peritoneal shunts [47, 52]. Another manifestation of CSF shunt overdrainage has been named craniocerebral disproportion and refers to a special form of functional or structural skull synostosis that results in brain compression [47]. Diagnosis and management of overdrainage syndromes will be discussed in a separate chapter.
7 Hemorrhage as a Complication of CSF Shunting
Intraventricular hemorrhage can be produced at the moment of puncturing the ventricle with the proximal catheter. Irrigation of the ventricle with warm saline usually clears the fluid in a few minutes, as CSF fluid clearance usually requires only time and patience. If the hemorrhage does not subside, an EVD is left in place and maintained as long as necessary. Ventricular bleeding constitutes the most dreaded surgical adverse event during proximal catheter revisions. These catheters usually stick to the choroid plexus or to the ventricular walls. Utmost care must be taken at the time of pulling the catheter out of the ventricle. However several maneuvers are apt of decreasing the eventuality of intraventricular bleeding as are gentle rotation of the catheter while slowly pulling it off. Others recommend washing the internal lumen of the tube with saline for disrupting the adhesions or using intraluminal coagulation with a metallic stylet or with a flexible coagulating electrode as those used in neuroendoscopy [50]. A second ventricular catheter must be readily available if clots or cerebral debris would plug again the one that is being implanted. The catheter may also be left in situ if one feels that it is firmly adhered within the ventricle. Others prefer to coagulate with diathermy or laser using through an endoscope. During all the maneuvers for proximal catheter removal, we tightly held its outermost orifice with a straight baby-mosquito forceps to avoid the inadvertent intraventricular migration (a frequent cause of retained ventricular catheters). In case of serious ventricular hemorrhage, the new catheter may become blocked causing a sudden neurological deterioration that usually manifests early after surgery requiring a repeat shunt revision. Gross intraventricular hematomas constitute an extremely infrequent complication of CSF shunting [35] that requires EVD and probably intraventricular injections of urokinase for accelerating the lysis of the clots [50].
Cortical bleeding may also occur during the opening of the dura mater. This hemorrhage can be easily controlled with mono- or bipolar coagulation. However, in some occasions, it requires enlarging the bone orifice of the burr hole to reach the bleeding artery. These superficial hemorrhages usually have no clinical consequences, and the patients remain symptom-free.
Subdural hematomas may be found in patients with large heads and thin parenchyma after shunting as occurs in children with macrocephaly and large ventricles or in the elderly with brain atrophy [2]. Its occurrence is more frequent in adults (4–23 %) than in children (2.8–5.4 %) and in patients with NPH (20–46 %). The proposed mechanism for the development of a subdural hematoma is the stretching and ruptures of bridging veins as a consequence of excessive drainage of CSF. In this context, chronic subdural hematomas are more prevalent than acute subdural hematomas. The clinical picture of subdural hematoma ranges from absence of symptoms to manifestations of shunt failure or of a mass lesion. Treatment is reserved for patients with clinical symptoms or for those who show midline displacement on imaging studies.
Epidural hematomas exceptionally complicate CSF shunt procedures [2, 65]. They predominantly occur in young patients and in those with macrocephaly and large ventricles. They develop as the result of vascular stretching in the presence of a dura mater that is less adherent to the skull than that of older adults. Patients may be asymptomatic or present features of shunt failure or of a mass lesion. Small epidural hematomas are best left untreated as is usually done in epidurals found in accidental trauma. As expected, symptomatic epidurals require craniotomy and valve upgrading.
8 Epilepsy Related to CSF Shunting
Children with hydrocephalus and CSF shunts have a higher incidence of epilepsy than the general population (6–59 %) [8]. Excluding patients with tumoral hydrocephalus, the incidence of seizures in a large series of shunted patients was as high as 30 % [8]. Reported risk factors for epilepsy in this population are the etiology of hydrocephalus (especially congenital causes and brain malformations), antecedent shunt revision, previous valve infection, site of proximal catheter insertion, and perhaps the presence of the shunt by itself that is considered to represent an epileptic focus [8]. This view is supported by the abnormalities found in EEG recordings that show slow waves and epileptic discharges in the hemisphere that harbors the shunt and at especially at the site of catheter insertion. However, this opinion is not generally shared. Epilepsy in hydrocephalic children is associated with a poor intellectual outcome and with problems in behavior. In our experience, seizures are especially seen in association with slit ventricles.
9 Making the Diagnosis of Shunt Failure
As in other neurosurgical conditions, diagnosis of shunt failure must be based on a careful clinical history that includes etiology of hydrocephalus, personal antecedents, age at shunt placement, type of initial valve, prior infections and their outcome, history of previous shunt malfunctions and symptoms when it occurred, and identification of the type and pressure of the last valve. History should record present complaints if any, level of schooling and socialization, concurrent diseases, and present treatments and allergies (latex allergy is frequent in spina bifida patients). Baseline neuroimaging studies must be routinely performed for comparison if shunt malfunction would occur.
Physical examination starts with a complete neurological assessment (including fundoscopy) and with serial measurement of the head circumference, checking that it follows an appropriate growth curve. In neonates and infants, it is followed by inspection of the head, estimating the tension of the fontanel by digital palpation (Fig. 2.3b) and checking the separation of the cranial sutures if any. The position of the eyes (sunsetting eyes, squint) and the size of the epicranial veins are also observed. Then, the integrity of the whole shunt system is ascertained by palpation of its entire trajectory, looking for gaps in the tube, for fluid collections along the tube, and for pseudotumoral masses or calcified zones.
In our experience, pumping of the valve reservoir is a useful adjunct for the diagnosis of shunt failure [69]. When there is no shunt malfunction, the reservoir empties and refills easily. In experienced hands, pumping the reservoir three or four times is sufficient for testing patency of the system. If the reservoir is felt hard to palpation, probably there exists occlusion of the valve or of the distal catheter. When there is a proximal catheter obstruction, the reservoir empties but refills slowly or does not refill at all and remains depressed (“umbilicated”) (Fig. 2.8a). The reliability of this test has been also questioned [62, 67, 69]. Some authors warned on the intracranial hypotension that can be produced by this maneuver and recommend that parents should not be allowed to pumping the chamber [10].
Another valuable tool for testing the valve function is the shunt tap [75]. Under stringent sterile conditions, the reservoir is punctured with a 23G or 25G butterfly system (Fig. 2.8b), and then the pressure is measured. If there is no spontaneous drip, gentle aspiration with a small syringe for encouraging fluid leak can be attempted. When the proximal catheter is obstructed, no fluid will come out either spontaneously of after aspiration. The intraventricular pressure can also be measured attaching a plastic manometer to the needle. Samples of CSF are routinely obtained and sent for biochemical and cytological study and for microbiological culture. Another method for estimating shunt function consists of connecting the needle to a recording system thus obtaining a continuous monitoring of the intracranial pressure from the reservoir (Fig. 2.9). The shunt tap is inexpensive, easy to perform, and repeatable [75]. The shunt puncture has scarce risks of shunt contamination and even less risks of damaging the valve or the reservoir and of causing an intracranial hemorrhage [56, 58]. ICP measurements are also used for diagnosis in doubtful cases of shunt failure [30, 73].
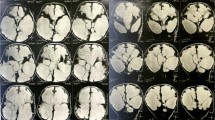
(a) Ventricular pressure recording through the valve reservoir in suspected shunt malfunction. Ventricular pressures were within the ranges of the valve. On the right MRI of the patient depicting the proximal catheter within a middle fossa arachnoid cyst. (b) Distal catheter malfunction: recording during the shunt tap, showing basal pressures of 20 mmHg with waves of up to 40 mmHg. On the right, CT of the same patient showing incipient dilatation of previously collapsed ventricles
Obviously, neuroimaging studies are customarily utilized for the diagnosis of CSF shunt failure. The commonest methods include transfontanelar ultrasonography, shunt series, computerized tomography, cranial magnetic resonance imaging, and Doppler studies. The shuntogram consists of assessing the flow of CSF along the tube after injection of a radio-opaque contrast medium in the reservoir. Isotopic CSF flow studies are presently utilized in only special occasions.
10 Conclusions
The most frequent complications of CSF shunting are mechanical, infectious, or functional. It is not always easy to distinguish which type of complication is causing the shunt dysfunction. Complications may present with a variety of symptoms and signs that must be carefully ascertained before proceeding to surgical revision. These clinical manifestations vary according to the patients’ age, etiology of hydrocephalus, cavity of CSF drainage, and type of valve and according to its occurrence early or late after initial shunt insertion or previous surgical revision.
To conclude, it is very important to bear in mind that the objectives of CSF shunting are (1) to provide control of the raised ICP, (2) to avoid mechanical and functional complications, (3) to prevent infection, and (4) to delay as much as possible shunt revision or replacement.
Appropriate knowledge of the mechanisms that contribute in the production of CSF shunt failures, preventive measures to avoid them, together with forthcoming improvements in shunts’ constructs will doubtless contribute to decrease the number and the severity of these complications.
References
Adderley R (2010) Acute respiratory failure following shunt disconnection. Cerebrospinal Fluid Res 7(Suppl 1):552
Aguiar HA, Shu EBS, Freitas ABR et al (2000) Causes and treatment of intracranial hemorrhage complicating shunting for pediatric hydrocephalus. Childs Nerv Syst 16:218–221
Albright AL, Tyler-Kabara E (2001) Slit-ventricle syndrome secondary to shunt-induced suture ossification. Neurosurgery 48:764–770
Barnes NP, Jones SJ, Hayward RD et al (2002) Ventriculoperitoneal shunt block: what are the best predictive clinical indicators? Arch Dis Child 87:198–201
Bergsneider M, Miller C, Vespa PM, Hu X (2008) Surgical management of adult hydrocephalus. Neurosurgery 62(SHC Suppl 2):SHC643–SHC660
Boch AL, Hermelin E, Sainte-Rose C, Sgouros S (1998) Mechanical dysfunction of ventriculoperitoneal shunts caused by calcification of the silicone rubber catheter. J Neurosurg 88:975–982
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258
Bourgeois M, Sainte-Rose C, Cinalli G et al (1999) Epilepsy in children with shunted hydrocephalus. J Neurosurg 90:274–281
Broekman MLD, Van Beijnum J, Peul WC, Regli L (2011) Neurosurgery and shaving: what’s the evidence? J Neurosurg 115:670–678
Bromby A, Czosnykya Z, Allin D et al (2007) Laboratory study on intracranial hypotension created by pumping the chamber of a hydrocephalus shunt. Cerebrospinal Fluid Res 4:2
Caldarelli M, Novegno F, Di Rocco C (2009) A late complication of CSF shunting: acquired Chiari I malformation. Childs Nerv Syst 25:443–452
Chen HH, Riva-Cambrin J, Brockmeyer DL et al (2011) Shunt failure due to intracranial migration of Bioglide ventricular catheters. J Neurosurg Pediatr 7:408–412
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77:875–880
Chumas PD, Kulkarni AV, Drake JM et al (1993) Lumboperitoneal shunting: a retrospective study in the pediatric population. Neurosurgery 32:376–383
Cozens JW, Chandler J (1997) Increased risk of distal ventriculoperitoneal shunt obstruction associated with slit valves or distal slits in the peritoneal catheter. J Neurosurg 87:682–686
Davidson RI (1976) Peritoneal bypass in the treatment of hydrocephalus: historical review and abdominal complications. J Neurol Neurosurg Psychiatry 39:640–646
Del Bigio MR (1998) Biological reactions to cerebrospinal fluid shunt devices: a review of cellular pathology. Neurosurgery 42:319–326
Di Rocco C, Marchese E, Velardi F (1994) A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst 10:321–327
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs. endoscopic third ventriculostomy in infants: are there different types and/or rates of complications. Childs Nerv Syst 22:1573–1589
Echizenya K, Satoh M, Ueno H et al (1987) Mineralization and biodegradation of CSF shunting systems. J Neurosurg 67:584–591
Elisevich K, Mattar AG, Cheeseman F (1994) Biodegradation of distal shunt catheters. Pediatr Neurosurg 21:71–76
Ersahin Y, Tabur E, Kocaman S, Mutluer S (2000) Complications of subduroperitoneal shunts. Childs Nerv Syst 16:433–436
Ferguson SD, Michael N, Frimm DM (2007) Observations regarding failure of cerebrospinal fluid shunts early after surgery. Neurosurg Focus 22(4):E7
Fukuhara T, Namba Y, Kuyama H (2004) Ventricular reservoir-migration into the lateral ventricle through the endoscopic tract after unsuccessful third ventriculostomy. Pediatr Neurosurg 40:186–189
Galarza M, Gravori T, Lazareff JA (2001) Acute spinal cord swelling in a child with a Chiari II malformation. Pediatr Neurosurg 35:145–148
Gelabert-Gonzalez M, Iglesias Pais M, García-Allut A, Martínez-Rumbo R (2005) Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg 107:223–229
Girotti ME, Singh RR, Rodgers BM (2009) The ventriculo-gallbladder shunt in the treatment of refractory hydrocephalus: a review of current literature. Am Surg 75:734737
Griebel R, Khan M, Tan L (1985) CSF shunt complications: an analysis of contributory failure. Childs Nerv Syst 1:77–80
Gupta A, Ahmad FU, Kumar A et al (2006) Umbilical CSF fistula: a rare complication of ventriculoperitoneal shunt. Acta Neurochir (Wien) 148:1205–1207
Hanlo PW, Gooskens RHJ, Faber JAJ et al (1996) Relationship between anterior fontanelle pressure measurements and clinical signs in infantile hydrocephalus. Childs Nerv Syst 12:200–209
Hwang TN, Rofagha S, McDermontt MW et al (2011) Sunken eyes, sagging brain syndrome: bilateral enophthalmos from chronic intracranial hypotension. Ophthalmology 118:2286–2295
Korinek AM, Fulla-Oler L, Boch AL et al (2011) Morbidity of ventricular cerebrospinal fluid shunt surgery in adults: an 8-year study. Neurosurgery 68:985–995
Kouyialis AT, Stranjalis G, Boviatsis EJ (2005) Selection of cranial site for shunting debilitated patients. Acta Neurochir (Wien) 147:763–765
Kurschel S, Puget S, Borgeois M et al (2007) Factors influencing the complication rate of subduroperitoneal placement for the treatment of subdural hematomas in children. J Neurosurg 106(3 Suppl):172–178
Kuwamura K, Kokunai T (1982) Intraventricular hematoma secondary to a ventriculoperitoneal shunt. Neurosurgery 10:384–386
Langmoen IA, Lundar T, Vatne K, Hovind KH (1992) Occurrence and management of fractured peripheral catheters in CSF shunts. Childs Nerv Syst 8:222–225
Lazareff JA, Peacock W, Holly L et al (1998) Multiple shunt failures: an analysis of relevant factors. Childs Nerv Syst 14:271–275
Lee M, Leahu D, Weiner HL et al (1995) Complications of fourth-ventricular shunts. Pediatr Neurosurg 22:309–314
Liptak GS, McDonald JV (1985) Ventriculoperitoneal shunts in children: factors affecting shunt survival. Pediatr Neurosci 12:289–293
Livingston JH, McCullagh HG, Kooner G et al (2011) Bradycardia without associated hypertension: a common sign of ventriculo-peritoneal shunt malfunction. Childs Nerv Syst 27:729–733
Lund-Johansen M, Svendsen F, Wester K (1994) Shunt failures and complications in adults as related to shunt type, diagnosis, and the experience of the surgeon. Neurosurgery 35:839–844
Martinez-Lage JF, Poza M, Esteban JA (1992) Mechanical complications of the reservoirs and pumping devices in ventricular shunt hydrocephalus. Br J Neurosurg 6:321–325
Martínez-Lage JF, Poza M, Izura V (1993) Retrograde migration of the abdominal catheter as a complication of ventriculoperitoneal shunts: the fishhook sign. Childs Nerv Syst 9:425–427
Martínez-Lage JF, Ruíz-Maciá D, Valentí JA, Poza M (1999) Development of a middle fossa arachnoid cyst. A theory on its pathogenesis. Childs Nerv Syst 15:94–97
Martínez-Lage JF, Torres J, Campillo H et al (2000) Ventriculopleural shunts with new technology valves. Childs Nerv Syst 16:867–871
Martínez-Lage JF, Pérez-Espejo MA, Almagro MJ et al (2005) Síndromes de hiperdrenaje de las válvulas en hidrocefalia infantil. Neurocirugia (Astur) 16:124–133
Martínez-Lage JF, Ruiz-Espejo Vilar A, Pérez-Espejo MA et al (2006) Shunt-related craniocerebral disproportion: treatment with cranial vault expanding procedures. Neurosurg Rev 29:229–235
Martínez-Lage JF, Almagro MJ, Del Rincón IS et al (2008) Management of neonatal hydrocephalus: feasibility of use and safety of two programmable (Sophy and Polaris) valves. Childs Nerv Syst 24:549–556
Martínez-Lage JF, Martos-Tello JM, Ros-de-San Pedro J, Almagro MJ (2008) Severe constipation: an under-appreciated cause of VP shunt malfunction: a case-based update. Childs Nerv Syst 24:431–435
Martínez-Lage JF, Almagro MJ, Ruíz-Espejo A et al (2009) Keeping CSF valve function with urokinase in children with intra-ventricular haemorrhage and CSF shunts. Childs Nerv Syst 25:981–986
Martínez-Lage JF, Alarcón F, López-Guerrero AL et al (2010) Syringomyelia with quadriparesis in CSF shunt malfunction: a case illustration. Childs Nerv Syst 26:1229–1231
Martínez-Lage JF, Pérez-Espejo MA, Almagro MJ, López-Guerrero AL (2011) Hydrocephalus and arachnoid cysts. Childs Nerv Syst 27:1643–1652
McGirt MJ, Leveque JC, Wellons JC 3rd (2003) Cerebrospinal shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg 38:34–40
Massimi L, Paternoster G, Fasano T, Di Rocco C (2009) On the changing epidemiology of hydrocephalus. Childs Nerv Syst 25:795–800
Mirzayan MJ, Koenig K, Bastuerk M, Krauss JK (2006) Coma due to meteorism and increased intra-abdominal pressure subsequent to ventriculoperitoneal shunt dysfunction. Lancet 368:2032
Moghal NE, Quinn MW, Levene MI, Puntis JWL (1992) Intraventricular hemorrhage after aspiration of ventricular reservoirs. Arch Dis Child 67:448–449
Muthukumar N (2012) Syringomyelia as a presenting feature of shunt dysjunction: implications for the pathogenesis of syringomyelia. J Craniovertebr Junction Spine 3:6
Noetzel MJ, Baker RP (1984) Shunt fluid examination: risks and benefits in the evaluation of shunt malfunction and infection. J Neurosurg 61:328–332
O’Hare AE, Brown JK, Minss RA (1987) Specific enlargement of the fourth ventricle after ventriculo-peritoneal shunt for posthemorrhagic hydrocephalus. Arch Dis Child 62:1025–1029
Parker SL, Anderson WN, Lilienffeld S et al (2011) Cerebrospinal fluid shunt infection in patients receiving antibiotic-impregnated versus standard shunts. J Neurosurg Pediatr 8:259–265
Piatt JH Jr, Hoffman HJ (1989) Cor pulmonale: a lethal complication of ventriculoperitoneal CSF diversion. Childs Nerv Syst 5:29–31
Piatt JH (1992) Physical examination of patients with cerebrospinal fluid shunts: is there useful information in pumping the shunt? Pediatrics 89:470–473
Piatt JH Jr, Carlson CV (1993) A search for determinant of cerebrospinal fluid survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg 19:233–242
Poca MA, Sahuquillo J, Mataró M (2001) Actualizaciones en el diagnostico y tratamiento de la hidrocefalia “normotensive” (hidrocefalia crónica del adulto). Neurologia 16:353–369
Power D, Ali-Khan F, Drage M (1999) Contralateral extradural haematoma after insertion of a programmable-valve ventriculoperitoneal shunt. J R Soc Med 92:360–361
Reddy GK, Bollam P, Caldito G et al (2011) Ventriculoperitoneal shunt complications in hydrocephalus patients with intracranial tumors: an analysis of relevant risk factors. J Neurooncol 103:333–342
Rekate HL (1991–1992) Shunt revision: complications and their prevention. Pediatr Neurosurg 17:155–162
Rekate HL (2004) The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg 40:259–263
Rocque BG, Lapsiwala S, Iskandar BJ (2008) Ventricular shunt tap as a predictor of proximal shunt malfunction in children: a prospective study. J Neurosurg Pediatr 1:439–443
Rogers EA, Kimia A, Madsen JR et al (2012) Predictors of ventricular shunt infection among children presenting to a Pediatric Emergency Department. Pediatr Emerg Care 28:405–407
Sahuquillo J, Arikan F, Poca MA et al (2008) Intra-abdominal pressure: the neglected variable in selecting the ventriculoperitoneal shunt for treating hydrocephalus. Neurosurgery 62:143–150
Schueler WB, Mapstone TB, Gross NL (2010) Migration of a ventricular tapping reservoir unto the third ventricle. J Neurosurg Pediatr 6:550–552
Schumann M, Sood S, McAllister JP et al (2008) Value of overnight monitoring of intracranial pressure in hydrocephalic children. Pediatr Neurosurg 44:269–279
Simon TD, Hall M, Riva-Cambrin J et al (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr 4:156–165
Sood S, Kim S, Ham SD et al (1993) Useful components of the shunt tap test for evaluation of shun malfunction. Childs Nerv Syst 9:157–162
Stannard MW, Rollins NK (1995) Subcutaneous catheter calcification in ventriculoperitoneal shunts. AJNR Am J Neuroradiol 16:1276–1278
Stringel G, Turner M, Crase T (1993) Ventriculo-gallbladder shunts in children. Childs Nerv Syst 9:331–333
Taub E, Lavyne MH (1994) Thoracic complications of ventriculoperitoneal shunts: case report and review of the literature. Neurosurgery 34:181–184
Tomes DJ, Hellbusch LC, Albert LR (2003) Stretching and breaking characteristics of cerebrospinal fluid shunt tubing. J Neurosurg 98:578–583
Torres-Lanzas J, Ríos-Zambudio A, Martínez-Lage JF et al (2002) Ventriculopleural shunts to treat hydrocephalus. Arch Bronconeumol 38:511–514
Tuli S, Drake J, Lawless J et al (2000) Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg 92:31–38
Venes JL (1976) Control of shunt infection. J Neurosurg 45:311–314
Vernet O, Campiche R, de Tribolet N (1995) Long-term results after ventriculo-atrial shunting in children. Childs Nerv Syst 11:176–179
Vinchon M, Boroncini M, Delestret I (2012) Adult outcome of pediatric hydrocephalus. Childs Nerv Syst 28:847–854
Wang VY, Barbaro NM, Lawton MT et al (2007) Complications of lumboperitoneal shunts. Neurosurgery 60:1045–1049
West GA, Berger MS, Geyer JR (1994) Childhood optic pathway tumors associated with ascites following ventriculoperitoneal shunt placement. Pediatr Neurosurg 1:254–259
Wisoff JH, Kratzert KJ, Handweker SM et al (1991) Pregnancy in patients with cerebrospinal fluid shunts: report of a series and review of the literature. Neurosurgery 29:827–831
Wong JM, Ziewacz JE, Ho AL et al (2012) Patterns in neurosurgical adverse events: cerebrospinal fluid shunt surgery. Neurosurg Focus 33(5):E13
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martínez-Lage, J.F., López-Guerrero, A.L., Almagro, MJ. (2015). Clinical Manifestations of CSF Shunt Complications. In: Di Rocco, C., Turgut, M., Jallo, G., Martínez-Lage, J. (eds) Complications of CSF Shunting in Hydrocephalus. Springer, Cham. https://doi.org/10.1007/978-3-319-09961-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-09961-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09960-6
Online ISBN: 978-3-319-09961-3
eBook Packages: MedicineMedicine (R0)