Abstract
Background
Increased intra-abdominal pressure has been reported to result in raised intracranial pressure in a variety of conditions such as obesity and pregnancy, and it also constitutes an infrequent cause of ventriculoperitoneal (VP) shunt malfunction. Patients with neurological deficits, as those with myelomeningocele or cerebral palsy, are prone to developing a neurogenic bowel and to suffer chronic constipation. Although previously recognized, VP shunt failure attributed to constipation has only recently been described. We briefly review the etiopathogenesis, diagnosis and management of severe constipation leading to VP shunt malfunction. Our aim is to draw the attention of pediatric neurosurgeons towards severe constipation as a possible cause of VP shunt failure thus avoiding unnecessary surgical valve revisions, to which children with hydrocephalus are often submitted to.
Illustrative cases
We report two children that developed transient VP shunt failure because of intense constipation that caused exacerbation of previously shunted hydrocephalus. One of the patients constitutes the first description of this complication associated with an anteriorly placed anus and the other with intestinal paresis after ileostomy. Conservative treatment aimed at alleviating the increased intra-abdominal pressure resulted in complete resolution of the children’s manifestations of VP shunt failure, without having to resort to surgical revision of the VP shunt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In our mean, lumbosacral myelomeningocele and periventricular haemorrhage still constitute a frequent cause of hydrocephalus. Current management of most patients with neonatal hydrocephalus includes the insertion of a ventriculoperitoneal (VP) shunt. A large number of mechanical, infectious and functional causes may lead to VP shunt failure [4]. Correct functioning of cerebrospinal fluid (CSF) valves requires a gradient of pressure between the ventricular and the abdominal cavities. Increased intra-abdominal pressure (IAP) may induce shunt failure by decreasing CSF drainage from the ventricles to the peritoneal cavity. Patients with neurological deficits, as those with myelomeningocele or cerebral palsy, are prone to develop chronic constipation because of neurogenic bowel. Constipation, usually regarded as a trivial condition, may result in decompensation of previously controlled hydrocephalus in patients harbouring a VP or lumboperitoneal shunt. Severe constipation causing VP shunt malfunction has been only recently described [3, 10, 12].
VP shunts and abdominal complications
Many children, especially premature babies and children with communicating hydrocephalus, are treated with VP shunts in spite of the revival of third ventriculostomy [4]. The peritoneal cavity constitutes the preferred place for CSF drainage because of its large surface and capacity for fluid re-absorption and to technical reasons (easier technique for placement of VP shunts and reduced rate and severity of complications). There are several commonly reported problems with peritoneal shunts [4]. Distal shunt block accounts for approximately 20% of cases of early failure. Inguinal hernia or hydrocele constitutes the commonest of these complications, especially in children under 1year of age. The small surface of the peritoneum for absorbing CSF, raised IAP and a patent processus vaginalis are relevant factors in the production of these complications. Peritoneal CSF pseudocysts and ascites are localized or diffuse collections of fluid within the peritoneum of aseptic or infective origin [8]. Diminished absorption can also be motivated by an excessive amount of CSF or by a hyperproteic fluid [8]. Visceral perforation (bowel, bladder, stomach, scrotum, vagina liver, or gallbladder) are now rarely seen. Grosfeld et al. [7] published a large series of intra-abdominal complications of VP shunts, including inguinal hernia, colonic or bladder perforation, CSF cysts, ascites and bowel obstruction with intestinal volvulus, which occurred in 45 of 185 shunted children.
Role of intra-abdominal pressure
Correct functioning of VP drainage systems requires the existence of a difference of pressure between the cranial and abdominal cavities. Normal IAP has been scarcely investigated in relation with VP shunt function. IAP at rest has been reported to be in the range of 0 ± 2cm H2O, although this figure is presently unknown in normal children [9, 16]. Methods to assess IAP include direct intraperitoneal, esophageal, gastric or bladder pressure recordings [14, 15]. A physiological increase in IAP has been documented during pregnancy and labor [2, 17]. Several cases of shunt failure during gestation and delivery have been reported [2, 17]. The causes for shunt dysfunction during these physiological processes have been attributed to raised IAP caused by the enlarged uterus and by blockage of venous outflow or to the forceful uterine contractions that occur during labor [2, 17].
Obesity constitutes another example of increased IAP that may lead to increased intracranial pressure (ICP) and pseudo-tumour cerebri [14, 15]. Obesity increases pleural pressure and cardiac filling pressure that, in turn, produce obstruction of venous return from the brain. In fact, Sugerman et al. [15] have treated pseudo-tumour cerebri associated with severe obesity by gastric (bariatric) surgery. Laparoscopic surgery has been blamed too for inducing CSF shunt failure [1, 16]. Pierro et al. [11] have also documented an increase in VP shunt malfunction in children with necrotizing enterocolitis and hydrocephalus.
George et al. [6] reported 19 patients with spina bifida that presented 22 episodes of acute abdominal symptoms, of which 10 of 22 were attributed to neurogenic bladder and 3 of 22 to neurogenic bowel, being the remaining cases ascribed to other causes.
There is a recent interest in so-called abdominal compartment syndrome [9, 13]. Scalea et al. [13] have studied increased IAP, intrathoracic and ICP after severe brain injury that they have termed multiple compartment syndrome. Fluid administration and/or acute pulmonary injury may increase intrathoracic and IAP leading to raised ICP in patients with head injury. In a series of 102 severely injured patients, they performed decompressive craniectomies in 78 instances and combined decompressive craniectomy plus decompressive laparotomy in 24. Mean ICP decreased significantly after both procedures. They suggest that both cranial and abdominal decompressive procedures reduce ICP and can be used in sequence for this purpose when intracranial hypertension does not respond to other therapies [13]. Abdominal compartment syndrome has been reported in a few pediatric patients [9].
In 1994, Bragg et al. [2] first suggested that VP shunt malfunction in children could be due to constipation. There are scarce publications on constipation causing VP dysfunction, probably because of the unimportance given to this complication of VP shunts when it is transient and mild in nature [2, 10, 12]. However, we think that recognition of this complication may be useful to avoid unnecessary surgical shunt revisions in these patients that too often require repeated operations.
Clinical presentation
Clinical manifestations of VP malfunction because of severe constipation consist of: (1) symptoms and signs of shunt failure, such as headache, somnolence, vomiting and a bulging anterior fontanel, and (2) abdominal manifestations of severe constipation (distended abdomen with dilated superficial veins, abnormal peristalsis etc.) that closely remind the clinical features of the abdominal compartment syndrome. This syndrome has been defined as the adverse physiologic effects that occur in response to a severe increase in IAP and is identified by cardiovascular, pulmonary, renal, splanchnic and intracranial disturbances regardless of the cause [9]. The level at which raised IAP occurs in children is presently unknown [9, 16]. However, the syndrome can be recognized clinically by an intensely distended abdomen together with cyanosis of the lower limbs, decreased femoral pulse, oliguria and hypoxia because of increased intrathoracic pressure.
Diagnosis
Symptoms and signs of shunt dysfunction are readily recognized by parents and physicians prompting an emergency consultation. The diagnosis of shunt failure often involves performing a radiographic shunt series, a computerized tomography (CT) scan or even a shunt tap. The results of these investigations often lead to surgical revision of the shunting device.
The diagnosis of transient shunt blockage because of the intense abdominal distension in our two patients was mainly made on a clinical basis. Complete blood count, erythrocyte sedimentation rate and C-reactive protein together with a urinalysis are routinely obtained. Palpation of the shunt tubing and of the reservoir may help in assessing the integrity and function of the valve. Radiographs and ultrasound study of the abdomen are mandatory to rule out other causes of intraperitoneal affections amenable at surgical treatment [6]. Head ultrasounds or CT can be used to compare ventricular size with previous studies. When valve infection is suspected, a reservoir tap with pressure measurement and CSF cytology and culture should be obtained.
Management
Our cases were managed conservatively with measures aimed at relieving the cause of the increased IAP as were the use of laxatives and enemas [5]. In addition, patient 1 required faecal disimpaction and anoplasty with rectal dilatations. Patient 2 was also treated with medical measures for her constipation. This child also benefited of the use of a programmable shunt, as decompensation of hydrocephalus was also managed by down-regulating the valve pressure setting until the resolution of symptoms. During treatment, children with clinical features of shunt malfunction attributed to constipation must be kept under close in-hospital observation. In this way, the proposed conservative attitude can be changed if the patients show signs of neurological deterioration.
Outcome
The two exemplary cases with whom we have illustrated the consequences of severe constipation resolved satisfactorily with conservative measures aimed at treating the underlying condition in each case, including medical treatment of constipation and neurological observation. However, we have the impression that there exist milder forms of the condition that might be under-recognized or neglected. Preventive measures to secure a normal intestinal function should be customarily instituted in children with neurological conditions, as happens with those diagnosed with spina bifida or with cerebral palsy [5].
Illustrative cases
Patient 1
This patient was operated on as a neonate of a lumbosacral myelomeningocele and given a VP shunt with a programmable valve (Sophy, Sophysa, France). The girl had also been diagnosed with anterior-displaced anus and stenosis and treated with repeated anal dilatations; in spite of which, the child suffered chronic constipation. She was regularly followed up at the outpatient clinic with good control of her hydrocephalus.
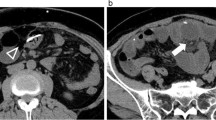
At age 16 months, the girl was admitted to the hospital with fever, irritability and vomiting for the previous 24h. On examination, the child appeared severely ill and had a markedly distended abdomen (Fig. 1). Signs of severe VP shunt malfunction (macrocephaly, a bulging anterior fontanel and engorged epicranial veins) were observed. The child was fully conscious but had trunk and lower limbs rigidity that were not present previously. The subcutaneous tract of the valve was normal, and the reservoir refilled easily.
A urinary tract infection because of Escherichia coli was demonstrated in the urine culture that was treated with cefotaxime. Radiographs of the abdomen disclosed faecal retention and a striking distension of the left colon. Abdominal ultrasounds ruled out the presence of ascites, peritoneal CSF cysts or other masses. Transfontanelar ultrasonography revealed marked dilatation of the cerebral ventricles in comparison with previous studies.
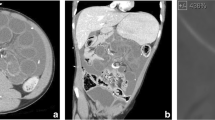
The patient’s manifestations of shunt malfunction were attributed to her severe abdominal distension. Accordingly, she was initially given oral lactulose and enemas. During this admission, the child was finally submitted, under general anaesthesia, to faecal disimpaction, anoplasty and rectal dilatation that produced total resolution of all her manifestations of VP shunt failure. A CT head scan showed a decrease in the size of the ventricles that were asymmetric (Fig. 2). The girl has now been followed up for 1year at the outpatient clinic and has not experienced new episodes of shunt malfunction.
Several factors appear to have lead to VP shunt malfunction in this patient. The girl had a neurogenic bladder and bowel paresis with chronic constipation [5, 6]. In addition, her urinary tract infection and, probably, worsening of her pre-existing anal anomaly resulted in severe constipation that, by increasing the IAP, would have led to the temporary blockage of the normal outflow of CSF from the ventricles to the peritoneum. The appropriate management of the child’s constipation by faecal disimpaction and anoplasty restored a normal CSF drainage thus resolving the distal shunt malfunction [5, 6]. Given the stable neurological condition of the child, we chose not to act on her programmable valve and preferred to watch the child’s evolution during her hospital stay. Needless to say that this conservative attitude would have changed should the patient have shown signs of neurological worsening.
Patient 2
A pre-term female neonate (birth weight 1,570g) was diagnosed of post-haemorrhagic hydrocephalus and treated with serial lumbar taps and two ventricular punctures. At age of 2weeks, the child underwent an ileostomy for necrotizing enterocolitis. At age of 45days, the girl was given a VP shunt (Polaris, Sophysa, France) initially set at a pressure of 70mm H2O that was followed by resolution of her hydrocephalus. Ten days after shunt insertion, the girl developed a severely distended abdomen and symptoms and signs of decompesated hydrocephalus (Fig. 3). A tap of the valve reservoir produced normal CSF. Repeat abdominal ultrasonography studies disclosed signs of intestinal paresis that were managed with conservative treatment [5]. Simultaneously, the valve pressure was temporarily down-regulated to 30mm H2O. Both measures lead to complete resolution of the baby’s symptoms of shunt malfunction.
The cause of shunt malfunction in this patient was attributed to persistent intestinal paresis owing to the child’s immaturity and to previous abdominal surgery for necrotizing enterocolitis.
Conclusions
We have reported two instances of reversible VP shunt failure related to severe constipation (caused by an ano-rectal malformation and to post-surgical bowel paresis, respectively). We have briefly reviewed several clinical conditions that can result in increased IAP as pregnancy, labor, obesity and abdominal compartment syndrome. We have also discussed diagnostic and management strategies in cases of VP shunt failure related to serious constipation. We wanted to catch the attention of the attending physician (be it paediatrician, neurosurgeon or paediatric surgeon) to this condition as a cause of VP shunt malfunction to avoid unnecessary surgical shunt revisions.
References
Baskin JJ, Vishtech AG, Wesche DE, Rekate HL, Carrion CA (1998) Ventriculoperitoneal shunt failure as a complication of laparoscopic surgery. J Soc Laparoendosc Surg 2:177–180
Bradley NC, Liakos AM, McAllister JP II, Magram G, Kinsman S, Bardley MK (1998) Maternal shunt dependency: implications for obstetric care, neurosurgical management, and pregnancy outcomes and a review of selected literature. Neurosurgery 43:448–461
Bragg CL, Edwards-Beckett J, Eckle N, Principe K, Terry D (1994) Ventriculoperitoneal shunt dysfunction and constipation: a chart review. Neurosci Nurs 26:265–269
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs. endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Child’s Nerv Syst 22:1573–1589
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (2006) Evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 43:e1–e13
George TM, Fuchs HE, Mackey JF, Fitch RD, Oldham KT (2001) Acute abdominal symptoms and signs in children and young adults with spina bifida: ten years’ experience. J Pediatr Surg 36:1381–1386
Grosfeld JL, Cooney DR, Smith J, Campbell RL (1976) Intra-abdominal complications following ventriculoperitoneal shunt procedures. Pediatrics 54:791–796
Kariyattil R, Steinbok, P, Shingai A, Cochrane DD (2007) Ascites and abdominal pseudocysts following ventriculoperitoneal shunt surgery: variations on the same theme. J Neurosurg 106(5 Suppl Pediatrics):350–353
Kawar B, Siplovich L (2003) Abdominal compartment syndrome in children: the dilemma of treatment. Eur J Pediatr Surg 13:330–333
Muzumdar D, Ventureyra ECG (2007) Transient ventriculoperitoneal shunt malfunction after chronic constipation: case report and review of the literature. Child’s Nerv Syst 23:455–458
Pierro A, Manalang LR, May PL, Cooke RW, Cudmore RE, Lloyd DA (1993) Necrotizing enterocolitis complicating the management of posthemorrhagic hydrocephalus. J Pediatr Surg 28:982–985
Powers CJ, George T, Fuchs HE (2006) Constipation as a reversible cause of ventriculoperitoneal shunt failure. J Neurosurg (Pediatrics) 105:227–230
Scalea TM, Bochichio GV, Habashi N, McCunn M, Shih D, McQuillan K, Aarabi B (2007) Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: multiple compartment syndrome. J Trauma 62:647–656
Sugerman HJ, DeMaria EJ, Felton WL 3rd, Nakatsuka M, Sismanis A (1977) increased intra-abdominal pressure and cardiac filling pressure in obesity-associated pseudotumor cerebri. Neurology 49:507–511
Sugerman HJ, Felton WL 3rd, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL (1999) Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg 229:634–642
Uzzo RG, Bilsky M, Mininberg DT, Poppas DP (1997) Laparoscopic surgery in children with ventriculoperitoneal shunts: effect of pneumoperitoneum on intracranial pressure—preliminary experience. Urology 49:753–757
Wisoff JH, Kratzert KJ, Handwerker SM, Young BK, Epstein F (1991) Pregnancy in patients with cerebrospinal fluid shunts: report of a series and review of the literature. Neurosurgery 29:827–831
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Lage, J.F., Martos-Tello, J.M., Ros-de-San Pedro, J. et al. Severe constipation: an under-appreciated cause of VP shunt malfunction: a case-based update. Childs Nerv Syst 24, 431–435 (2008). https://doi.org/10.1007/s00381-007-0514-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-007-0514-3