Abstract
Programmed cell death (PCD) is a genetically controlled biological process involved in defense, development, and stress response. Generally, the characters of plant PCD are similar to animal apoptosis, for instance cytoplasm shrinkage, chromatin condensation, and DNA fragmentation. An important signaling molecule, nitric oxide (NO) has been implicated in environmental-induced plant PCD, but its signaling and controlling network is still unknown. Whether NO promotes or suppresses PCD depends on NO sources and concentration in different plant species and environmental conditions. The effects of NO on developmental PCD were extensively studied. NO not only plays a crucial role in hypersensitive response (HR) during plant-pathogen interactions, but is also involved in abiotic stress-induced PCD including heat shock, salt, drought, cold, UV radiation, ozone, and heavy metals (mainly cadmium, aluminum). Previous studies showed the mitochondrion as a modulating center of PCD and also control NO level in planta. Vacuole processing enzyme (VPE) and caspase-like protein are involved in PCD. NO regulates the expression of PCD-associated genes via mitogen-activated protein kinase (MAPK) cascade, S-nitrosylation, and cGMP-dependent pathway. In addition, there are diverse interactions between NO and other signals such as hydrogen peroxide, calcium, ethylene, and salicylic acid (SA) during PCD. Based on understanding of related knowledge, NO signaling network in response to PCD in higher plants is presented in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Programmed cell death (PCD) occurs in various forms throughout the plant life cycle, probably with both common and specific aspects. PCD is a genetically controlled biological process which activates an intrinsic suicide program of cells. It not only controls the degradation of intracellular components, but facilitates removal of unwanted, incorrect, or damaged cells from multicellular organisms. It plays an important role in defense response, development, and environmental stress. Leaf senescence, hypersensitive response (HR), lysigenous aerenchyma formation, and aleurone degradation are all the forms of PCD in pants.
Generally, the characters of plant PCD are similar to animal apoptosis, such as cytoplasm shrinkage, chromatin condensation, membrane blebbing, DNA fragmentation, and selective cleavage of proteins. During HR development, plant also can form apoptotic bodies. Although individual processes differ in the triggering factors such as vacuole collapse, releasing sequestered hydrolases, may be the universal trigger of plant PCD. It is indicated that the molecular machinery underlying PCD is well conserved in eukaryotic organisms. The executive phases and typical hallmarks of PCD differ under different occasions. Cleavage of genomic DNA during apoptotic PCD is divided into two subsequent steps; an early cleavage into high molecular weight fragments, whose sizes coincidence with chromatin loop domains, and later an intense fragmentation, usually forming oligonucleosomal fragments (Brotner et al. 1995), that can be detected by DNA electrophoresis in the whole tissue or cell population, also visualized by TUNEL reaction (TdT-mediated dUTP nick-end labeling) in individual cells (Gavrieli et al. 1992; Zhan et al. 2013).
Nitric oxide (NO) a simple diatomic, diffusible, gaseous free radical, involved in many physiological processes such as PCD, seed germination, lateral root initiation, flowering, stomatal closure, and responses to stress in plants. Moreover, as an important signaling molecule, NO has been implicated in environmental-induced plant PCD, but its signaling network is still unknown. Whether NO promotes or suppresses PCD is dependent on sources and concentration of NO in different plant species.
2 Evolution of NO and Dual Function During Plant Programmed Cell Death
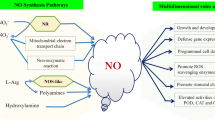
Owing to the essential function of NO in plant signaling network, its endogenous source is very important. There are two ways to generate NO in plants viz. l-arginine-dependent nitric oxide synthase (NOS) pathway and nitrite-dependent nitrate reductase (NR) pathway. Although NOS-like activity has been detected in plants, this enzyme remains enigmatic. No gene or protein with sequence homology to known mammalian type NOS has been found (Crawford 2006). NR works as a major enzymatic source of NO production in plants. It can convert nitrite to NO in vitro and in vivo (Desikan et al. 2002). In Arabidopsis, NR is encoded by two genes, NIA1 and NIA2, which contribute differently to the synthesis of NO in different tissues.
Another is nonenzymatic conversion of nitrite to NO in the apoplast. NO produced in plants at low concentration may rapidly eliminate lipid peroxyl radicals, alter the species and components of reactive oxygen species (ROS), and block the injuries from ROS, induce the expression of antioxidant genes and the activity of antioxidant enzymes (Lamattina et al. 2003).
3 Effects of NO on Developmental PCD
The effects of NO on developmental PCD have been extensively studied (Table 17.1). Gibberellin (GA)-induced PCD in barley aleurone layers is mediated by ROS, because GA greatly reduces the amount of CAT (catalase) and SOD (superoxide dismutase). NO donors, SNP (sodium nitroprusside) and SNAP (S-nitroso-N-acetylpenicillamine) delay the loss of two enzymes and PCD in barley aleurone layers treated with GA, but stimulate slightly the secretion of α-amylase. It is suggested that NO may be an endogenous modulator of PCD in barley aleurone layers (Beligni et al. 2002).
Leaf senescence is a highly coordinated process that involves PCD. Early stages of leaf senescence occurring during normal leaf ontogenesis, but not triggered by stress factors, are poorly known. Kolodziejek et al. (2007) found that both nDNA fragmentation and chromatin condensation occurred quite early during barley leaf senescence and always in the same order. NO was localized in vivo and in situ within the cytoplasm, mainly in mitochondria, in leaves at the same stage as those in which chromatin condensation was observed. The highest concentration of NO was found in the cytoplasm of mesophyll cells in the earliest stage of senescence, and lower concentrations were found during later stages that might suggest that NO plays an inductive role in PCD in leaf senescence.
During the seed development, the cells of the nucleus suffer a degenerative process early after fertilization as the cellular endosperm expands and accumulates reserves. Nuclear cell degeneration has been characterized as a form of developmental PCD. Lombardi et al. (2010) showed that nucleus PCD is accompanied by a considerable production of both NO and hydrogen peroxide (H2O2), and each of the two molecules is able to induce the production of the other and to cause PCD when applied to a living nucleus. Xylem cells have to be killed so as to facilitate the formation of rigid hollow tubes specialized for water transport. NO is also a key factor regulating PCD and lignification during xylem formation (Neill et al. 2005).
4 Role of NO in Hypersensitive Response
NO plays a crucial role in HR during plant-pathogen interactions. NO and H2O2 function in combination with each other all along HR cell death (Table 17.1).
Administration of NO donors or recombinant mammalian NOS to tobacco plants or tobacco suspension cells triggered expression of the defense-related genes encoding pathogenesis-related 1 protein and phenylalanine ammonia lyase (PAL). These genes were also induced by cyclic guanosine monophosphate (cGMP) and cyclic ADP-ribose, two molecules that can serve as secondary messengers for NO signaling in mammals. Consistent with cGMP acting as a secondary messenger in tobacco, NO treatment induced dramatic and transient increases in endogenous cGMP levels. Unregulated NO levels drive a diffusion limited reaction with O2 − to generate peroxynitrite (ONOO−), which is a mediator of cellular injury in many biological systems but not a mediator of HR. The HR is triggered only by balanced production of NO and reactive oxygen intermediates. Increasing the level of O2 − reduces NO-mediated toxicity. HR is activated after interaction of NO not with O2 − but with H2O2. During the HR, SOD accelerates O2 − dismutation to H2O2 to minimize the loss of NO by reaction with O2 − and to trigger HR through NO/H2O2 cooperation. The rates of production and dismutation of O2 − generated during the oxidative burst play a crucial role in the modulation and integration of NO/H2O2 signaling in the HR (Delledonne et al. 2001). The researches on the kinetics of NO production and hypersensitive cell death showed that NO accumulation contributed to HR. NO was first seen as punctate foci at the cell surface. Subsequent NO accumulation patterns were consistent with NO being an intercellular signal that functions in cell-to-cell spread of the HR (Zhang et al. 2003).
Arabidopsis suspension cultures generate elevated levels of NO in response to challenge by avirulent bacteria, and NO are sufficient to induce cell death in Arabidopsis cells independently of ROS. NO-induced cell death is a form of PCD, requiring gene expression, and has a number of characteristics of PCD such as chromatin condensation and caspase-like activity in Arabidopsis cells (Clarke et al. 2000). Phytotoxin fusicoccin induces another form of cell death in sycamore (Acer pseudoplatanus L.) cultured cells, likely mediated by NO and independent of cytochrome c release, and they make it tempting to speculate that changes in actin cytoskeleton are involved in this form of PCD (Malerba et al. 2008).
5 Involvement of NO in Abiotic Stress-Induced PCD
NO is also involved in PCD induced by abiotic stress including heat shock, salt, drought, cold, UV radiation, ozone, and heavy metals (mainly cadmium, aluminum) (Table 17.1).
Arabidopsis thaliana cell suspension cultures underwent a PCD process when exposed to 100 and 150 mM CdCl2. As suggested by the expression of the marker senescence-associated gene12 (SAG12), this process resembled an accelerated senescence. CdCl2 treatment was accompanied by a rapid increase in NO and phytochelatin (PC) synthesis, which continued to be high as long as cells remained viable. NO is actually required for Cd2+-induced cell death, because the inhibition of NO synthesis by NG-monomethylarginine monoacetate (l-NMMA) resulted in partial prevention of H2O2 increase, SAG12 expression, and mortality. NO also modulated the extent of PC content and their function by S-nitrosylation (De Michele et al. 2009). Tobacco BY-2 cells exposed to 150 μM CdCl2 underwent PCD with TUNEL-positive nuclei, significant chromatin condensation and the increasing expression of a PCD-related gene Hsr203J. Accompanied with the PCD, the production of NO increased significantly. NO played a positive role in CdCl2-induced PCD by modulating Cd2+ uptake and thus promoting Cd2+ accumulation in BY-2 cells (Ma et al. 2010). The roots of 3-day-old yellow lupine seedlings exposed to 89 mM CdCl2 resulted in PCD starting from 24 h of stress duration. Cd-induced PCD was preceded by a relatively early burst of NO localized mainly in the root tips. Above changes were accompanied by the NADPH-oxidase-dependent superoxide anion (O .−2 ) production. NADPH-oxidase inhibitor and NO-scavenger significantly reduced O .−2 and NO production, respectively, as well as diminished the pool of cells undergoing PCD (Arasimowicz-Jelonek et al. 2012).
Tobacco leaves, exposed to moderate high light, dramatically potentiated NO-mediated cell death in catalase-deficient (CAT1AS) but not in wild-type plants. The results consolidate significant crosstalk between NO and H2O2, and provide new insight into the early transcriptional response of plants to increased NO and H2O2 levels, and identify target genes of the combined action of NO and H2O2 during the induction of plant cell death (Zago et al. 2006). Lin et al. (2011) identified an NO accumulation mutant noe1 (nitric oxide excess 1) in rice and analyzed its role in NO-mediated leaf cell death. The NOE1, encoded a rice catalase OsCATC, that increased the H2O2 in the leaves, which consequently promoted NO production via activation of NR. Removal of excess NO reduced cell death in both leaves and suspension cultures derived from noe1 plants, implicating NO as an important endogenous mediator of H2O2-induced leaf cell death.
Ozone (O3) induced a rapid accumulation of NO, which started from guard cells, spread to adjacent epidermal cells and eventually moved to mesophyll cells. NO production coincided with the formation of HR-like lesions. SNP and O3 individually induced a large set of defense-related genes; however, in a combined treatment SNP attenuated the O3 induction of salicylic acid (SA) biosynthesis and other defense-related genes. SNP treatment decreased O3-induced SA accumulation. The O3-sensitive mutant rcd1 was found to be an NO overproducer; in contrast, Atnoa1/rif1 (Arabidopsis nitric oxide associated 1/resistant to inhibition by FSM1), a mutant with decreased production of NO, was also O3 sensitive. NO can modify signaling, hormone biosynthesis and gene expression in plants during O3 exposure. NO is an important signaling molecule, which production is needed for a proper O3 response (Ahlfors et al. 2009a, b).
The involvement of cellular antioxidant metabolism in the signal transduction triggered by these bioactive molecules has been investigated. NO and ROS levels were singularly or simultaneously increased in tobacco (Nicotiana tabacum cv Bright-Yellow 2) cells by the addition of NO and/or ROS generators to the culture medium. The generation of NO did not cause an increase in PAL activity or induction of cellular death. It only induced minor changes in ascorbate (ASC) and glutathione (GSH) metabolisms. An increase in ROS induced oxidative stress in the cells, causing an oxidation of the ASC and GSH redox pairs; however, it had no effect on PAL activity and did not induce cell death at low concentrations. In contrast, the simultaneous increase of NO and ROS activated a process of death with the typical cytological and biochemical features of hypersensitive PCD and a remarkable rise in PAL activity. Under the simultaneous generation of NO and ROS, the cellular antioxidant capabilities were also suppressed (De Pinto et al. 2002). Treatment of tobacco protoplasts with SNP resulted in a rapid [Ca2+]cyt accumulation and decrease in mitochondrial membrane potential (potential (ΔΨm) before the appearance of PCD. NO-induced PCD could be largely prevented not only by cPTIO, but also by Ca2+ chelator, EGTA (ethylene glycol tetraacetic acid), Ca2+-channel blocker LaCl3 (Lanthanum chloride) or CsA (a specific mitochondrial permeability transition pore inhibitor, which also inhibit Ca2+ cycling by mitochondria). NO-induced PCD is mediated through mitochondrial pathway and regulated by Ca2+ (Wang et al. 2010a, b). The effects of different NO-donors releasing NO with either NO+ (SNP) or NO− (SNAP, GSNO, NOC-18) character have been compared in plant cells. SNP behaves differently than the other NO-donors tested; indeed, SNP induces accumulation of ferritin transcripts in Arabidopsis, whereas SNAP (S-nitroso-N-acetylpenicillamine) inhibits its accumulation. Only SNP caused PCD and suppression of ROS-scavenging systems (Murgia et al. 2004). Artificial NO donors are widely used as tools to study the role of NO in plants. However, reliable and reproducible characterizations of metabolic responses induced by different NO donors are complicated by the variability of their NO release characteristics. NO release characteristics of the donors SNP, S-nitrosoglutathione (GSNO) and NOS, both in vitro and in planta (Nicotiana tabacum L. cv. BelW3) were evaluated and their effects on NO dependent processes such as the transcriptional regulation of the mitochondrial alternative oxidase (AOX) gene, accumulation of H2O2 and induction of cell death were assessed. Contrary to NOS and SNP, GSNO is not an efficient NO generator in leaf tissue. In spite of the different NO release signatures by SNP and NOS in tissue, the NO-dependent responses examined were similar, suggesting that there is a critical threshold for the NO response (Ederli et al. 2009).
6 Regulation of NO on PCD–Associated Genes Expression
Vacuole processing enzymes (VPEs) are a vacuole-localized cysteine protease, which exhibit caspase-1-like protein activity. It can mediate the activation of caspase-3-like protein to provoke PCD and is involved in virus-induced hypersensitive cell death in tobacco (Hatsugai et al. 2004). VPE activity is also required for aluminum (Al)-induced PCD in plants. Ced-9 inhibited both the Al-induced activity of caspase-like VPE and Al-induced PCD in tobacco (Wang et al. 2009). Senescence-associated gene 12 (SAG12) is considered the best molecular marker of senescence. The expression of SAG12 increased at 2 and 3 d after 100 μM CdCl2 treatment (De Michele et al. 2009). Al-induced PCD was promoted by AhSAG, a senescence-associated gene in Arachis hypoganea (Zhan et al. 2013). As one of the few endogenous cell death inhibitors in plants, bax inhibitor-1 (BI-1) is potentially a core regulator of PCD (Huckelhoven 2004). PpBI-1 can attenuate Al-induced PCD and enhance Al tolerance in transgenic yeast (Zheng et al. 2007). The programmed cell death 5 (PDCD5) gene encodes a protein that shares significant homology with the corresponding proteins of species ranging from yeast to mice (Liu et al. 1999). Overexpression of OsPDCD5 gene induces PCD in rice (Attia et al. 2005).
In tobacco, mechanical wounding induced the rapid transcript accumulation and activation of wound-induced protein kinase (WIPK) (Seo et al. 1995). Transgenic tobacco plants ectopically expressing AhMPK3 exhibited enhanced resistance to first and second instar larvae of Spodoptera litura (Kumar et al. 2009). The conditional overexpression of AhMPK6 resulted in HR-like cell death in tobacco (Kumar and Kirti 2010). MPK kinase 6-mediated activation of VPE modulates heat shock-induced PCD in Arabidopsis (Li et al. 2012). NO promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana PCD (Ye et al. 2012). Over-expression of OsGSNOR reduced intracellular SNO levels, which regulates global levels of protein S-nitrosylation, alleviated leaf cell death in noe1 plants (Lin et al. 2011).
Cytochorme c gets to the cytoplasm at least via two mechanisms. One is via formation of a transient mitochondrial permeability transition pore (MPTP), which is produced by the voltage-dependent anion channel (VDAC) on the outer membrane, the adenine nucleotide transporter (ANT) from the inner membrane and cyclophilin D in the matrix (Green and Reed 1998). Another is directly via the VDAC (Shimizu et al. 1999). Because the expression of AOX, the unique respiratory terminal oxidase in plants, can scavenge excess superoxide anion so that the balance of NO and H2O2 is destroyed, AOX plays protective roles in Al-induced Arabidopsis protoplast death (Li and Xing 2011). As a molecular chaperone, mitochondrial HSP70 may be involved in PCD initiation by reducing Δψm in mitochondrial outer membrane (Chen et al. 2009). Through NO/H2O2 cooperation, SOD accelerates O2 − dismutation to H2O2 to minimize the loss of NO by reaction with O2 − and to trigger hypersensitive cell death (Delledonne et al. 2001). Some genes associated with PCD are listed in Table 17.2.
7 Interaction Between NO and Other Signaling Molecules During Plant PCD
There are diverse interactions between NO and other signaling molecules such as H2O2, calcium, ethylene, and SA during PCD. The interaction between NO and H2O2 can be cytotoxic or protective. NO/H2O2 cooperation triggers hypersensitive cell death in soybean cell suspensions (Delledonne et al. 2001). Boosted NO and O .−2 production is required for Cd-induced PCD in lupine roots. Moreover, the NO-dependent Cd-induced PCD in roots of 14-day-old lupine plants was correlated with the enhanced level of the post-stress signals in leaves, including distal NO crosstalk with H2O2 (Arasimowicz-Jelonek et al. 2012). Using biochemical and genetic approaches in the root system, Wang et al. (2010a, b) proposed a pathway for the regulation of NO biosynthesis that involves the modulation of NIA2 by MPK6. With the increase of intracellular H2O2 levels, MPK6 is activated, which in turn leads to the phosphorylation of NIA2 at Ser-627. Phosphorylation of NIA2 by MPK6 dramatically.
Increases the activity of NIA2 and the production of NO and also results in morphological changes. SNP treatment resulted in a rapid [Ca2+]cyt accumulation and the appearance of PCD in tobacco protoplasts. EGTA, LaCl3 or CsA largely prevent NO-induced PCD that is mediated through mitochondrial pathway and regulated by Ca2+ (Wang et al. 2010a, b). Moreover, NO is involved in PCD induction via interacting with the pathways of phytohormones (Wang et al. 2010a, b). NO treatments induce ethylene production in tobacco. NO and ethylene act together to regulate O3-induced AOX expression (Ederli et al. 2006). Transcript profiling indicated a role for NO in attenuation of certain classes of O3 induced genes, many of which were related to SA biosynthesis or SA signaling (Ahlfors et al. 2009a, b).
8 NO Signaling Network in Response to PCD
Based on understanding of related knowledge, we propose NO signaling network in response to PCD in plants (Fig. 17.1). Different signals (developmental, pathogen, invasion, and abiotic stress) trigger NO production. Subsequently, NO promotes the expression of PCD-associated genes (such as VPE, AOX, HSP70, APX) via several pathways. One is cGMP-dependent pathway: NO and cGMP mediate the auxin response during adventitious root formation in cucumber (Pagnussat et al. 2003). Moreover, NO regulates the apoptotic signal cascade through protein S-nitrosylation (Wang et al. 2010a, b). Lin et al. (2011) suggested that S-nitrosylation was involved in light-dependent leaf cell death in noe1 rice. NO targets identified only in noe1 plants included glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and thioredoxin, which have been reported to be involved in S-nitrosylation regulated cell death in animals. The last one is MAPK cascade. Asai et al. (2002) identified a complete plant MAP kinase cascade (MEKK1, MKK4/MKK5, and MPK3/MPK6) and WRKY22/WRKY29 transcription factors that function downstream of the flagellin receptor FLS2. Signaling events initiated by diverse pathogens converge into a conserved MAPK cascade. An MAPK signaling cascade is activated during the adventitious rooting process induced by IAA in a NO-mediated but cGMP-independent pathway (Pagnussat et al. 2004). NO mediated caspase-3-like protease activation under Cd2+ stress conditions. Pretreatment with cPTIO effectively inhibited Cd2+-induced MAPK activation. Cd2+-induced caspase-3-like activity was significantly suppressed in the mpk6 mutant, suggesting that MPK6 was required for caspase-3-like protease activation (Ye et al. 2013). NO contributed caspase-3-like protease activation in Cd2+ induced Arabidopsis thaliana PCD, which was mediated by MPK6 (Ye et al. 2012). NO could also regulate the activity of Ca2+-dependent protein kinase (CDPK) was addressed by Lanteri et al. (2006) who characterized a 50 kDa NO-dependent CDPK in cucumber hypocotyls. These three pathways may work synergistic or solely. In turn, gene expressions provoke some downstream events such as PCD.
9 Control of NO Level in Plant Mitochondrion
Previous studies showed mitochondrion has emerged as modulating center of plant PCD and also important sites in controlling NO levels in plants. Nitrite (the source of NO synthesis) inhibited the respiration of isolated Arabidopsis mitochondria, in competition with oxygen, an effect that was abolished or potentiated when electron flow occurred via AOX or cytochrome c oxidase (COX), respectively. Electron leakage from external NAD(P)H dehydrogenases contributed the most to NO degradation as higher rates of Amplex Red-detected H2O2 production and NO consumption were observed in NAD(P)H-energized mitochondria. Conversely, the NO-insensitive AOX diminished electron leakage from the respiratory chain, allowing the increase of NO half-life without interrupting oxygen consumption. The accumulation of NO derived from nitrite reduction and the superoxide-dependent mechanism of NO degradation in isolated Arabidopsis mitochondria are influenced by the external NAD(P)H dehydrogenases and AOX, revealing a role for these alternative proteins of the mitochondrial respiratory chain in the control of NO levels in plant cells (Wulff et al. 2009). Complex III, COX, and AOX are all involved in nitrite to NO reduction. AOX controls NO generation by directly influencing the rate of electron leakage to nitrite (Cverkovska and Vanlerberghe 2012). Robson and Vanlerberghe (2002) found that knocking down of AOX increases the susceptibility of plants to PCD. There exists a negative feedback loop where NO acts to suppress excess mitochondrial reactive nitrogen species (RNS) and presumably ROS via increased AOX expression to modulate the elicitation of PCD. Three mechanisms of AOX-mediated ROS and RNS homeostasis are suggested. First, AOX can modulate the membrane potential and reduce NO levels. Second, aconitase inhibition leads to increase in citrate which induces AOX to maintain electron flow through the electron transport chain and to lower NO concentrations (Gupta et al. 2012). Third, AOX scavenging of NO might help in decreasing ROS production by preventing over-reduction of ubiquinone pool. However, plant lead to PCD or necrotic cell death in response to stress, because NO and ROS generation from nonmitochondrial sources could swamp any AOX-mediated homeostatic mechanisms.
10 Conclusion and Perspectives
Adverse environmental conditions interferes NO-mediated signal transduction. By direct scavenging of ROS or activating antioxidant enzymes, exogenously applied NO might alleviate metal toxicity in plants. In contrast, NO through S-nitrosylation of PCs or promoting metal uptake via iron transporters contributes or even amplifies metal toxicity. The promoting and suppressing effects of NO on cell death is dependent on a variety of factors, such as cell type, cellular redox status, and the flux and dose of local NO (Wang et al. 2010a, b). Cell signaling dysregulation induced by metal not only leads to the death stimulation pathway, but might be able to activate survival signaling towards tolerance response to heavy metal. Active cell death is required for an enhanced effectiveness of protective responses in neighboring cells (Overmyer et al. 2003). In particular, the relationship between NO, ROS signaling and stress-related hormones might play a key role on the dispute on the expression of gene sets responsible for stress tolerance and in the generation of long-distance sensing from roots to shoots. NO is involved in the generation of systemic signal in systemic acquired resistance to pathogens (Vlot et al. 2008). Xiong et al. (2011) showed that tungstate is not completely a specific NR inhibitor in plant NO research. To investigate the roles of NO in plants, it is necessary to search for more NR-deficient mutants and new specific NR inhibitors. The research on transcriptional factors and NO-regulated genes is the key to understand the mechanism of NO in PCD in higher plants. The recognition of the molecular NO targets will be an exciting challenge for future research.
References
Ahlfors R, Brosche M, Kollist1 H et al (2009a) Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. Plant J 58: 1–12
Ahlfors R, Brosche M, Kangasjarvi J (2009b) Ozone and nitric oxide interaction in Arabidopsis thaliana. Plant Signaling & Behavior 4:878–879
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Deckert J et al (2012) Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiol Biochem 58:124–134
Asai T, Tena G, Plotnikova J et al (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415:977–983
Attia K, Li KG, Wei C et al (2005) Overexpression of OsPDCD5 gene induces programmed cell death in rice. J Integr Plant Biol 47:1115–1122
Beligni MV, Fath A, Bethke PC et al (2002) Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol 129:1642–1650
Brotner CD, Oldenburg NBE, Cidlowski JA (1995) The role of DNA fragmentation in apoptosis. Clinical Cancer Res 11:21–26
Chen X, Wang Y, Li JY et al (2009) Mitochondrial proteome during salt stress-induced programmed cell death in rice. Plant Physiol Biochem 47:407–415
Clarke A, Desikan R, Hurst RD et al (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24:667–677
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
Cverkovska M, Vanlerberghe GC (2012) Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol 195:32–39
Delledonne M, Zeier J, Marocco A et al (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
De Michele R, Vurro E, Rigo C et al (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
De Pinto MC, Tommasi F, Gara LD (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol 130:698–708
Desikan R, Griffiths R, Hancock J et al (2002) A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99:16314–16318
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Ederli L, Morettini R, Borgogni A et al (2006) Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol 142:595–608
Ederli L, Reale L, Madeo L et al (2009) NO release by nitric oxide donors in vitro and in planta. Plant Physiol Biochem 47:42–48
Gabaldon C, Ros Gomez LV, Pedrefio MA et al (2005) Nitric oxide production by the differentiating xylem of Zinnia elegant. New Phytol 165:121–130
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:450–493
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Guo Y, Cai Z, Gan S (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27:521–549
Gupta KJ, Shah JK, Brotman Y et al (2012) Inhibition of aconitase by nitric oxide leads to induction of alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. J Exp Bot 63:1773–1784
Hatsugai N, Kuroyanagi M, Yamada K et al (2004) A plant vacuole protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858
Huckelhoven R (2004) BAX Inhibitor-1, an ancient cell death suppressor in animals and plants with prokaryotic relatives. Apoptosis 9:299–307
Jones A (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci 5:225–230
Kolodziejek I, Koziol-Lipinska J, Waleza M et al (2007) Aspects of programmed cell death during early senescence of barley leaves: possible role of nitric oxide. Protoplasma 232:97–108
Kumar KRR, Kirti PB (2010) A mitogen-activated protein kinase genes, AhMPK6 from peanut localizes to the nucleus and also induces defense responses upon transient expression in tobacco. Plant Physiol Biochem 48:481–486
Kumar KRR, Srinivasan T, Kirti PB (2009) A mitogen-activated protein kinase genes, AhMPK3 of peanut: molecular cloning, genomic organization, and heterologous expression conferring resistance against Spodoptera litura in tobacco. Mol Genet Genomics 282:65–81
Kuthanova A, Opatrny Z, Fischer L (2008) Is internucleosomal DNA fragmentation an indicator of programmed death in plant cells? J Exp Bot 59:2233–2240
Lamattina L, Garcia-Mata C, Graziano M et al (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–139
Lanteri M, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57:1341–1351
Li Z, Xing D (2011) Mechanistic study of mitochondria-dependent programmed cell death induced by aluminum phytotoxicity using fluorescence techniques. J Exp Bot 62:331–343
Li Z, Yue HY, Xing D (2012) MAP kinase 6-mediated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis. New Phytol 195:85–96
Lin AH, Wang YQ, Tang JY (2011) Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide induced leaf cell death in rice. Plant Physiol 158:451–464
Liu HT, Wang YG, Zhang YM (1999) TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1 could enhance apoptosis of some tumor cells induced by growth factor withdraw. Biochem Biophys Res Commun 245:203–210
Lombardi L, Ceccarelli N, Picciarelli P et al (2010) Nitric oxide and hydrogen peroxide involvement during programmed cell death of Sechium edule nucellus. Physiol Plant 140:89–102
Malerba M, Contran N, Tonelli M et al (2008) Role of nitric oxide in actin depolymerization and programmed cell death induced by fusicoccin in sycamore (Acer pseudoplatanus) cultured cells. Physiol Plant 133:449–457
Ma WW, Xu WZ, Xu H et al (2010) Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY-2 cells. Planta 232:325–335
Murgia I, de Pinto MC, Delledonne M et al (2004) Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J Plant Physiol 161:777–783
Neill S (2005) NO way to die – nitric oxide, programmed cell death and xylogenesis. New Phytol 165:1–2
Overmyer K, Brosche M, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8:335–342
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the IAA-induced adventitious rooting process. Plant Physiol 132:1241–1248
Pagnussat GC, Lanteri ML, Lombardo MC et al (2004) Nitric oxide mediats the indole acetic acid induction of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Ren DT, Yang HP, Zhang SQ (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277:559–565
Robson C, Vanlerberghe G (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol 129:1908–1920
Seo S, Okamoto M, Seto H et al (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathway. Science 270:1988–1992
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487
Van Aken O, Giraud E, Clifton R et al (2009) Alternative oxidase: a target and regulator of stress responses. Physiol Plant 137:354–361
Vlot AC, Klessig DF, Park SW (2008) Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol 11:436–442
Wang PC, Du YY, Li Y (2010a) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Wang WZ, Pan JW, Zheng K et al (2009) Ced-9 inhibits Al-induced programmed cell death and promotes Al tolerance in tobacco. Biochem Biophys Res Commun 383:141–145
Wang Y, Lin JS, Wang GX (2010b) Role of calcium in nitric oxide-induced programmed cell death in tobacco protoplasts. Biol Plant 54:471–476
Wang YQ, Chen C, Loake GL et al (2010c) Nitric oxide: promoter or suppressor of programmed cell death? Protein Cell 1:133–142
Wilkins KA, Bancroff J, Bosch M et al (2011) Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of papaver. Plant Physiol 156:404–416
Wulff A, Oliveira HC, Saviani EE et al (2009) Nitrite reduction and superoxide-dependent nitric oxide degradation by Arabidopsis mitochondria: Influence of external NAD(P)H dehydrogenases and alternative oxidase in the control of nitric oxide levels. Nitric Oxide 21:132–139
Xiong J, Fu G, Yang YJ et al (2011) Tungstate: is it really a specific nitrate reductase inhibitor in plant nitric oxide research? J Exp Bot 63:33–41
Ye Y, Li Z, Xing D et al (2012) Sorting out the role of nitric oxide in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Signaling & Behavior 7:1493–1494
Ye Y, Li Z, Xing D (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant, Cell Environ 36:1–15
Zago E, Morsa S, Dat JF (2006) Nitric oxide- and hydrogen peroxide- responsive gene regulation during cell death induction in tobacco. Plant Physiol 141:404–411
Zhan J, He HY, Wang TJ et al (2013) Aluminum-induced programmed cell death promoted by AhSAG, a senescence-associated gene in Arachis hypoganea L. Plant Sci 210:108–117
Zhang C, Czymmek KJ, Shapiro AD (2003) Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the Arabidopsis hypersensitive response. MPMI 16:962–972
Zhao J, Fujita K, Sakai K (2007) Reactive oxygen species, nitric oxide, and their interactions play different roles in Cupressus lusitanica Cell death and phytoalexin biosynthesis. New Phytol 175:215–229
Zheng K, Pan JW, Ye L et al (2007) Programmed cell death-involved aluminum toxicity in yeast alleviated by antiapoptotic members with decreased calcium signals. Plant Physiol 143:38–49
Acknowledgments
This work was supported by the National Science Foundation of China (31260296, 30960181, 30560070).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
He, HY., Gu, MH., He, LF. (2014). The Role of Nitric Oxide in Programmed Cell Death in Higher Plants. In: Khan, M., Mobin, M., Mohammad, F., Corpas, F. (eds) Nitric Oxide in Plants: Metabolism and Role in Stress Physiology. Springer, Cham. https://doi.org/10.1007/978-3-319-06710-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-06710-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06709-4
Online ISBN: 978-3-319-06710-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)