Abstract
Mitogen-activated protein kinase cascade plays a very important role in plant signal transduction mechanism. A full length cDNA of 1,514 bp length, corresponding to a mitogen-activated protein kinase gene was cloned from peanut (Arachis hypogaea). Based on its high homology with Arabidopsis AtMPK3, the cDNA was designated as AhMPK3. It carried an open reading frame of 1,113 bp encoding a 371 amino acid polypeptide. AhMPK3 bears TEY motif in its activation loop and belongs to the A1 subgroup of MAPK family. Southern blot analysis revealed that AhMPK3 exists in two copies in peanut genome and its structural organization revealed well-conserved nature of these signaling components across different species. AhMPK3 when transiently expressed in tobacco leaves was found to localize in both nucleus and cytoplasm. Transgenic tobacco plants ectopically expressing AhMPK3 exhibited enhanced resistance to first and second instar larvae of Spodoptera litura and constitutively higher transcript levels of defense response genes like PR1a, PR1b, LOX1, PI–II etc. Apart from this when wounded, transgenic plants accumulated high levels of PI–II and PR1b transcripts rapidly compared to wild type indicating the occurrence of a priming phenomenon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants should respond to the ever-changing environmental conditions in the most befitting manner, in order to survive against the odds. For this purpose, they have evolved a variety of signal transduction mechanisms, which transduce the perceived external signal to the inner cellular components for appropriate response to combat such pressures. Mitogen-activated protein kinase (MAPK) cascade is a conserved transduction mechanism in all eukaryotes involving three functionally related components (Widmann et al. 1999). The upstream MAPKKKs (MAP kinase kinase kinases), which phosphorylate and activate the downstream MAPKKs (MAP kinase kinases), which in turn phosphorylate and activate the MAPKs (MAP kinases). Phosphorylation targets of activated MAP kinases include both nuclear and cytosolic proteins (Morris 2001). Based on the completed Arabidopsis genome sequence, 20 MAPKs were identified and were divided into four groups (A–D). MAPKs belonging to groups A, B, and C all possess a TEY motif in their activation loop, while members of group D harbor a TDY motif (MAPK group 2002).
Mitogen-activated protein kinase (MAPK) pathways in plants have been implicated in signal transduction for a wide variety of stress responses (Jonak et al. 2002; Colcombet and Hirt 2008). In the monocot model plant rice, several MAPKs were characterized to be involved in both biotic and abiotic stress responses (Agrawal et al. 2003; Cheong et al. 2003; Reyna and Yang 2006). AtMPK3, AtMPK6 and their apparent orthologs in other species are present in group A and are found to be activated by many environmental stresses and shown to be involved in non-host resistance (Zhang et al. 1998, 2000), gene for gene signal transduction (Zhang and Klessig 1998; Romeis et al. 1999), hypersensitive response (Liu et al. 2003; Stulemeijer et al. 2007), wounding (Seo et al. 1995), response to elicitors (Zhang et al. 2000; Daxberger et al. 2007), and several abiotic stresses (Jonak et al. 1996; 2004; Samuel et al. 2000; Ahlfors et al. 2004). Recent studies showed their involvement in phytoalexin biosynthesis (Ren et al. 2008), response to herbivores (Wu et al. 2007), key regulators of stomatal development and patterning (Wang et al. 2007), anther (Hord et al. 2008) and ovule (Wang et al. 2008) development.
AtMPK3 and its orthologs NtWIPK (Nicotiana tabacum), LeMPK3 (Lycopersicon esculentum) and MsMMK4 (Medicago sativa) were very well studied and were found to be induced in response to various biotic and abiotic elicitors (Mizoguchi et al. 1996; Jonak et al. 1996; 2004; Bögre et al. 1997; Zhang et al. 2000; Holley et al. 2003; Mayrose et al. 2004; Wan et al. 2004). In tobacco, mechanical wounding induced rapid transcript accumulation and activation of WIPK (wound-induced protein kinase, Seo et al. 1995). Transgenic plants overexpressing NtWIPK showed constitutive PI–II transcript accumulation, WIPK activity, and higher jasmonic acid (JA) levels compared to wild type (Seo et al. 1999). JA quantification in NtWIPK silenced plants demonstrated that NtWIPK is involved in the production of wound induced JA (Seo et al. 2007). Several orthologs of AtMPK3 were shown to play a crucial role in plant defense responses like overexpression of MK1, which encodes the Capsicum ortholog of NtWIPK, display elevated JA levels and resistance to blast fungus in transgenic rice plants (Lee et al. 2004a). Plants overexpressing TIPK (Trichoderma-induced MAPK) an AtMPK3/NtWIPK ortholog from cucumber were more resistant to pathogenic bacterial attack than control plants (Shoresh et al. 2006). And the suppression of NtWIPK or its orthologs led to increased susceptibility against pathogens (Sharma et al. 2003; Shoresh et al. 2006). For example AtMPK3 mutant plants exhibit reduced camalexin accumulation after B. cinerea infection in Arabidopsis (Ren et al. 2008). Although involvement of AtMPK3 and its orthologs was well established in wound and systemin signaling responses (Seo et al. 1995; Holley et al. 2003), their role in plant response to herbivore attack was not well explored till recently. Kandoth et al. (2007) and Wu et al. (2007) demonstrated that LeMPK3 and NaWIPK are involved in regulating the defense response against herbivore attack in L. esculentum and N. attenuata, respectively. Although several AtMPK3/NtWIPK orthologs from various plants were overexpressed and shown to confer resistance to microbial pathogens, there were no reports on the performance of herbivores on such plants.
Though peanut is one of the widely cultivated oilseed crops with economical and nutritional importance, extensive genomic information is not available in public databases pertaining to it. The availability of genetic information would enhance the understanding of mechanisms involved in plant development and stress responses (Guo et al. 2008). This has been accomplished to some extent by the completion of some of the peanut EST projects (Luo et al. 2005; Guo et al. 2008). In the present study, we have cloned a full-length cDNA and its corresponding genomic clone of a mitogen-activated protein kinase gene from Arachis hypogaea. It was designated as AhMPK3 based on its homology with AtMPK3. We have analyzed its genomic and structural organization, transcriptional regulation in response to various stresses and sub-cellular localization of AhMPK3 by translational fusion with GFP. Finally, heterologous expression of AhMPK3 in tobacco plants and resistance against herbivore Spodoptera litura was evaluated. To our knowledge, this is the first report on the characterization of a mitogen-activated protein kinase (MAPK) gene from genus Arachis and the demonstration of its overexpression resulting in enhanced resistance to herbivore attack in a heterologous system, tobacco.
Materials and methods
Plant materials
Detached leaves of peanut (Arachis hypogaea cv JL-24) from 2 to 3 week old plants grown in the greenhouse were used in all experiments. Tobacco (Nicotiana tabacum var Xanthi) seeds were surface sterilized with 4% sodium hypochlorite for 10 min, washed 4–5 times with sterile distilled water and allowed to germinate on Murashige and Skoog (MS) medium (Murashige and Skoog 1962). Individual seedlings were transferred to culture bottles with MS medium and maintained aseptically.
Treatment with chemicals and abiotic stresses
Compound leaves (quadrifoliate) detached from peanut plants were kept in a tray with a moist filter paper saturated with sterile distilled water and covered with a polythene bag to maintain humidity and left overnight to stabilize the wound signal. For various chemical treatments, leaves were floated in the corresponding solution. The treatments given were 500 μM salicylic acid (SA), 100 μM methyl jasmonate (MeJA), 100 μM abscisic acid (ABA), 25 mM hydrogen peroxide (H2O2), 200 mM mannitol, 100 mM sodium chloride (NaCl), 100 μM sodium nitroprusside (SNP) and treatment with water served as control. Wounding was performed by damaging the leaf lamina with a sharp blade and a pointed forceps and cold treatment was given by shifting the leaves to a cold chamber (4°C). Samples were collected at regular intervals, quickly frozen in liquid nitrogen, and stored at −80°C until use. Mannitol, NaCl and H2O2 are obtained from Himedia, India. Rest of the chemicals used for treatments were purchased from Sigma-Aldrich, USA.
DNA and RNA isolation
Leaves of peanut and tobacco were frozen in liquid nitrogen and ground into a fine powder. Total Genomic DNA was then extracted by the cetyl trimethyl ammonium bromide (CTAB) procedure (Murray and Thompson 1980). RNA was isolated from samples harvested at various intervals using TRI reagent (Sigma-Aldrich, USA) following the manufacturer’s instructions. The quality and concentration of RNA and DNA samples were examined by ethidium bromide-stained agarose gel electrophoresis and spectrophotometric analysis.
RT-PCR and amplification of Partial cDNA and cloning of PCR products
Reverse transcription reaction of total RNA and subsequent amplification of partial cDNA clone using degenerate primers IntF and IntR was performed following standard methodologies (see supplementary for detailed methodology followed). Details of all the oligos used for amplifications and subsequent cloning of AhMPK3 were given in Table 1. All the PCR amplified products were electrophoresed, gel eluted (Gel elution kit, Eppendorf, Germany) and cloned into pTZ57R/T (Insta clone T/A cloning kit, Fermentas, Germany).
DNA sequencing and sequence analysis
For all the clones both DNA strands were completely sequenced on an automated DNA sequencer commercially. The sequence similarity search was performed using BLASTn and BLASTp at NCBI website (http://www.ncbi.nlm.nih.gov). Nucleotide translations were performed using (DNA/RNA to protein) Translate tool at ExPASy. (http://www.expasy.ch). Sequence alignments were done using CLUSTALW multiple sequence alignment tool at European Bioinformatics Institute (http://www.ebi.ac.uk). Phylogenetic analysis was performed using CLC Free Workbench (http://www.clcbio.com). Reverse complementation and other sequence formatting was done using BCM search launcher (http://www.searchlauncher.bcm.tmc.edu).
Isolation of full length cDNA and genomic clones of AhMPK3
To obtain the full length sequence of AhMPK3, 5′ and 3′ rapid amplification of cDNA ends (RACE) reactions were performed using 5′/3′ RACE kit (Roche Applied Sciences, Germany) following the manufacturer’s instructions with minor modifications. Based on the available partial cDNA sequence, primers were designed for 5′ and 3′ RACE reactions. Gene specific primers Ah443R and Ah270R were utilized for 5′ RACE. Ah3P1F and Ah3P2F were used for 3′ RACE PCR (see supplementary material for detailed methodology followed). The full length cDNA of AhMPK3 was deduced by aligning 5′ and 3′ RACE product sequences with the partial cDNA fragment cloned. Full length cDNA, including the 5′ and 3′ UTR, was amplified using gene specific primers AhMK31F and AhMK31497R. The PCR reaction was performed in a 50μl containing 2.0 mM MgCl2, 200 μM dNTP (Invitrogen), 1× PCR buffer, and 2.5 units of Taq DNA polymerase (Invitrogen) using cDNA as template. The cycling conditions were 94°C for 3 min, followed by 34 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 2 min. To isolate the genomic clone of AhMPK3, gene specific primers AhMK31F and AhMK31497R were used to PCR amplify the AhMPK3 gene using Arachis hypogaea genomic DNA as template. PCR was carried out using 100 ng of genomic DNA in a 50 μl reaction volume containing 2.0 mM MgCl2, 200 μM dNTP (Invitrogen), 1× PCR buffer, and 2.5 units of Taq DNA polymerase (Invitrogen). The PCR conditions were 94°C for 3 min, followed by 34 cycles of 94°C for 45 s, 55°C for 45 s, 72°C for 3 min and a final extension of 15 min at 72°C.
Genomic Southern blot analysis
Peanut genomic DNA (20 μg) was digested with BclI, EcoRI, EcoRV, HindIII and XbaI (Fermentas, Germany), respectively, fractionated on 0.8% agarose gel and visualized by ethidium bromide staining. The DNA from the gel was transferred onto a Hybond N+ membrane (Amersham Pharmacia, UK) through capillary transfer and the blot was UV-cross-linked. A 281 bp fragment of the 3′ UTR was amplified using primers AhMK31216F and AhMK31497R and labeled with [α-32P] dATP using Primer-a-Gene® Labeling System (Promega, USA) according to the manufacturer’s instructions. The membrane was pre-hybridized for 3–4 h at 65°C and hybridized for 16–18 h at 65°C using α-32P labeled probe. Following hybridization, the membrane was washed with 2X SSC, 0.1%SDS and 1× SSC, 0.1% SDS for 10 min and 0.1× SSC and 0.1% SDS for 5 min each, respectively, at 65°C and then exposed to an X-ray film (Kodak, Japan) using two intensifying screens at −80°C.
Semi-quantitative RT-PCR
Reverse transcription was performed as described earlier in “Materials and methods” except that instead of 4 μg, only 2 μg of RNA was used. The amount of cDNA and number of cycles for linear increase of PCR products was determined (data not shown). Conditions which consistently gave product in linear range were used for all experiments. The expression of AhMPK3 in peanut was studied using specific primers for amplifying the entire coding region, ORF-F and ORF-R (Table 1). Gene specific primers were employed for expression analysis of the defense related genes in transgenic and wild type (WT) tobacco plants (Supplementary Table 2). The house-keeping gene actin amplified using primers Act-F and Act-R served as the internal control. The amplified products were analyzed on 1.2% agarose gel and visualized by staining with ethidium bromide.
Localization of AhMPK3
AhMPK3 cDNA was amplified from reverse-transcribed RNA using Primers ORF-F2 and ORF-R (Table 1) engineered with SmaI and BamHI restriction sites, respectively. The resulting fragment was cloned into pEGAD vector (Cutler et al. 2000) digested with appropriate restriction enzymes to make an in-frame fusion with GFP to obtain pEGAD: AhMPK3. The pEGAD control vector and pEGAD: AhMPK3 constructs were mobilized into A. tumefaciens strain EHA105 by freeze thaw method (Holsters et al. 1978). The resulting strains were utilized in transient transformation of N. tabacum leaves by agroinfiltration as described by Yang et al. (2000). In brief, agrobacterial strains harboring the corresponding clones were grown overnight at 28°C in the presence of appropriate antibiotics, pelleted at 3,000g for 5 min and diluted to an OD600 of 1.0 in 10 mM MES pH 5.6, 10 mM MgCl2, 150 μM acetosyringone and infiltrated into the leaves using a needle less syringe. After 48 h, GFP was visualized with a laser scanning confocal microscope. A total of 10 mM H2O2 was infiltrated into the leaves 60 min before observation to study the dynamic localization of AhMPK3 in response to oxidative stress. Water was used for mock infiltration.
Development of transgenic tobacco plants
The complete open reading frame of AhMPK3 was amplified using primers ORF-F and ORF-R (Table 1) cloned into pTZ57R vector, and confirmed by sequencing. NcoI and BamHI restriction sites were incorporated in the primers at 5′ and 3′ ends to facilitate cloning into plant expression vector pRT100 by digesting it with the same set of enzymes such that the coding region would be flanked by 35S promoter and Poly-A signal in the sense orientation. The entire cassette with AhMPK3 coding region flanked by 35S promoter and Poly-A signal was released from pRT100 (Töpfer et al. 1987) by digesting with HindIII and cloned into the binary vector pRD400 (Datla et al. 1992) digested with the same enzyme. The recombinant binary vector was mobilized into Agrobacterium tumefaciens strain EHA105 using freeze thaw method. Transgenic tobacco plants were generated by standard leaf disc transformation procedure (Horsch et al. 1985). The putative transgenic plants obtained were transferred to soil and acclimatized at 28°C in a growth room and shifted to the greenhouse.
Molecular analysis of transgenic plants
DNA was extracted from 5 to 6 week-old T0 transgenic plants, and around 100 ng of DNA was used for PCR. Putative transgenic plants are confirmed by amplifying the genomic DNA with 35SF (Table 1) as the sense primer designed against the CaMV 35S promoter region and AhMPK3 ORF-R as the antisense primer. Southern analysis for transgenic plants was performed as described earlier in “Materials and methods”, except that the genomic DNA was digested with EcoRI and hybridization was done using [α-32P] dATP labeled nptII fragment obtained by the amplification of neomycin phosphotransferases gene with nptII F and nptII R primers. For Northern analysis of transgenic plants, 20 μg of total RNA was fractionated on a 1.2% agarose–formaldehyde gel. Equal loading and RNA integrity were checked by ethidium bromide staining, and transferred by capillary action overnight to a Hybond-N+ nylon membrane (Amersham Pharmacia, UK) using 20× SSC. The RNA on the membrane was fixed by UV cross-linking. Probe labeling, hybridization, and detection were the same as in the procedure described for Southern blot hybridization. [α-32P] dATP labeled AhMPK3 ORF was used as probe in Northern hybridization.
Herbivore bioassay
Bioassay was performed according to detached leaf method described by Sharma et al. (2005) with minor modifications. In brief, leaves of 2-month-old WT and transgenic plants were cut at their petiole with a sharp blade and immediately planted into 3% agar–agar in a Petri dish. Bioassays were conducted with first, second, and third instar larvae of the generalist herbivore, Spodoptera litura with five larvae per leaf and five replications for each sample. The bioassay plates were maintained in a culture room at 28 ± 1°C and a photoperiod of 16:8 (Light/Dark). The experiments were terminated when >80% of the leaf area was consumed in WT plants, generally 5 days for first instar, 3 days for second instar, and 2 days for third instar larvae, respectively. The area of leaf damage and mass of larvae were recorded after each experiment and mean of five replications was plotted. The data were analyzed by ANOVA and student’s t test.
Preparation of protein extracts
For isolation of the soluble cytoplasmic proteins, leaf material was ground with liquid nitrogen and extracted with Protein extraction buffer (50 mM HEPES pH 7.5, 5 mM EDTA, 5 mM EGTA, 5 mM DTT, 50 mM NaF, 10 mM Na3VO4, 50 mM β-glycerophosphate, 10% Glycerol, 1 mM PMSF, 5 μg/ml Aprotinin, 5 μg/ml Leupeptin). The extracted solution was centrifuged at 13,000 rpm for 20 min at 4°C and supernatant was collected and used for analysis immediately or stored at −80°C. The protein concentration was determined by the method of Bradford (1976) using BSA as standard.
Myelin basic protein (MBP) kinase activity assay
Ten micrograms of total protein extracted from WT and transgenic plants was added to 25 μl of Kinase assay buffer (25 mM Tris, 1 mM EGTA, 1 mM DTT, 5 mM Mgcl2, 1 mM Mncl2, 100 μM Na3VO4, 200 μM ATP, 1 mg/ml MBP and 5 μCi γ–32P-ATP) and incubated at 30°C for 30 min. The reactions were stopped by addition of SDS-gel loading buffer and boiling for 3 min. Reaction products were separated by electrophoresis on a 15% SDS-polyacrylamide gel, and MBP phosphorylation was analyzed by autoradiography. Equal loading was determined by the Coomassie blue staining of the gel.
Results
Isolation of full length cDNA of AhMPK3
In an attempt to clone stress responsive mitogen-activated protein kinases from Arachis hypogaea, degenerate primers were designed from two conserved regions of MAP kinases. The forward primer IntF corresponded to the ATP binding motif (GAYG I/VVC) in subdomain I of protein kinases (Hanks et al. 1988) and the reverse primer IntR corresponded to the region including TEY motif (MTEYVVT) present between subdomain VII and VIII. Using RT-PCR a single fragment of 465 bp was obtained, which might be the product of amplification of several different MAP kinases, because the primers were designed against highly conserved regions. The PCR product was cloned and several clones were sequenced followed by the grouping of identical clones. Among them two clones were identified as diverse, but closely related and considered as different clones. Sequence similarity search using BLASTn and BLASTp showed a high similarity to the existing MAPKs from several plant species. One of the two clones was further extended using 5′ and 3′ RACE to obtain the full length cDNA.
By utilizing the sequence information of the partial cDNA fragment gene specific primers were designed. The same RNA used for amplification of the partial clones was used as a template for reverse transcription using oligo dT-Anchor primer (for 3′ RACE) and degenerate gene specific primer WyrR (for 5′ RACE) followed by the amplification of corresponding 5′ and 3′ cDNA ends with nested gene-specific primers. By using a degenerate primer for reverse transcription, several members of the gene family would get reverse transcribed. Hence, the same cDNA could be used as template for isolating 5′ regions of different genes of the same family using nested gene specific primers in combination with PCR anchor primer. In 5′ RACE, a 750 bp product was obtained with the gene specific primer Ah443R in combination with PCR anchor primer. The product was further confirmed by amplification with the nested primer Ah270R. In 3′ RACE, an 1,100 bp product was obtained with the primer Ah3P1F, which was confirmed with the nested primer Ah3P2F. All the PCR products obtained were cloned and sequenced. The sequences were aligned to obtain the overlapping regions and the full length cDNA of AhMPK3 was deduced. Based on the sequences of RACE products, two gene specific primers AhMK31F and AhMK31497R were designed and the full length cDNA was amplified and sequenced, which was identical to the deduced cDNA. AhMPK3 cDNA was submitted to NCBI Genbank database under the accession number DQ068453.
Nucleotide and protein sequence analysis
The full length AhMPK3 cDNA is 1,514 bp long including the ORF, 5′, 3′ untranslated regions and the poly-A tail. Sequence analysis revealed an open reading frame of 1,113 bp potentially encoding a 371 amino acid polypeptide. The reading frame was the only possible reading frame in the cDNA and had both the translational initiation codon ATG at nucleotide 94 and translational stop codon TAA at nucleotide 1207. A 93 bp of 5′ untranslated region and a 292 bp 3′ untranslated region followed by poly-A tail were present flanking the open reading frame. A potential polyadenylation signal (AATAAA) was found in the 3′ UTR at 1,249 bp. Threonine and tyrosine amino acids of TEY motif were present at 197 and 199 positions, respectively. The 371 amino acids encoded protein had a predicted molecular mass of 42,615.98 Da and a calculated isoelectric point (pI) of 5.52 (Compute pI/MW tool, ExPASy).
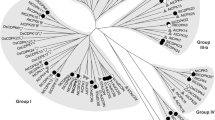
Multiple sequence alignment and phylogenetic analysis
Sequence alignment of the predicted amino acid residues of AhMPK3 with closely related MAP kinases indicated that it contains all the eleven conserved subdomains of protein kinases described previously (Hanks et al. 1988) and possessed a dual phosphorylation activation motif (TEY) located between subdomains VII and VIII (Fig. 1). The phylogenetic analysis showed that AhMPK3 belongs to the A1 subgroup of MAPK family (Fig. 2) (MAPK group 2002). The AhMPK3 protein exhibited 94% sequence identity to GmMPK1 from Glycine max, 91% to MsMMK4 from Medicago sativa, 85% to CsTIPK and PtMPK3-1 of Cucumis sativus and Populus trichocarpa, respectively. Most well characterized A1 subgroup members of MAPK family, AtMPK3 (Arabidopsis thaliana), NtWIPK (Nicotiana tabacum) and LeMPK3 (Lycopersicon esculentum) shared 81% similarity with AhMPK3 protein.
Alignment of deduced amino acid sequences of AhMPK3 with closely related MAPKs from other plant species. The eleven subdomains of protein kinases are marked with roman numerals. Threonine (T) and Tyrosine(Y) residues whose phosphorylation is required for MAPK activation are indicated by Asterisk. At Arabidopsis thaliana, Nt Nicotiana tabacum, Ms Medicago sativa, Cs Cucumis sativus
The phylogenetic relationship of AhMPK3 with other MAPK family members from different plant species. A phylogenetic tree based on the genetic distance of the protein sequences was constructed using ClustalW program and CLC-free workbench 3.1. The MAPK members used for construction of the tree are listed in the GenBank database under the following accession numbers: AhMPK3(DQ068453); AtMPK1(NM_100895); AtMPK2(NM_202320); AtMPK3(NM_114433); AtMPK4(NM_116367); AtMPK5(AK176361); AtMPK6(NM_129941); AtMPK7(NM_127374); AtMPK8(NM_179354); AtMPK9(NM_112686); AtMPK10(NM_115841); AtMPK12(NM_130170); AtMPK13(NM_001035913); AtMPK14(NM_119808); AtMPK15(NM_106026); AtMPK16(NM_121906); AtMPK17(NM_126206); AtMPK18(NM_104229); AtMPK20(NM_129849); CsTIPK(DQ118734); GmMPK1(AF104247); GmMPK2(AF329506); LeMPK1(AY261512); LeMPK3(AY261514); MsMMK2(X82268); MsMMK3(AJ224336); MsMMK4(X82270); MsMSK7(X66469); NtWIPK(D61377); NtSIPK(U94192); OsBWMK1(AF177392); OsMAPK5(AF479883); OsMAPK6(AJ535841); Oswjumk1(AJ512643); OsRMAPK2(AF194416); OsMAPK4(AJ251330); PtMPK3-1(estExt_fgenesh4_pm.C_LG_IX0462); PtMPK6-1(estExt_fgenesh4_pm.C_LG_VII0025); ZmMPK4(AB016801); ZmMPK5(AB016802); Ah Arachis hypogaea, At Arabidopsis thaliana, Cs Cucumis sativus, Gm Glycine max, Le Lycopersicon esculentum, Ms Medicago sativa, Nt Nicotiana tabacum, Os Oryza sativa, Pt Populus trichocarpa, Zm Zea mays
Genomic and structural organization of AhMPK3 gene
The copy number of AhMPK3 gene was analyzed by Southern blot analysis. Arachis hypogaea genomic DNA was digested with restriction enzymes BclI, EcoRI, EcoRV, HindIII, XbaI and subjected to hybridization using 281 bp 3′ UTR region of AhMPK3 as a probe. This 281 bp region did not harbor restriction sites of any of the above enzymes used. Two distinct bands were detected in samples digested with EcoRI, EcoRV and HindIII (Fig. 3). This can be explained by the fact that peanut (Arachis hypogaea) is an amphidiploid, which carries two sets of diploid chromosomes. Hence, one band corresponds to the AhMPK3 gene and the second band in Southern presumably belonged to its ortholog in the second genome of peanut. The sample digested with BclI showed three distinct bands, which could be explained by the possible occurrence of a BclI site in the AhMPK3 ortholog in the second genome of peanut. The single band detected in XbaI digested sample could be due to the near equal size of two bands which co-migrated giving the appearance of a single band. The hybridization pattern suggested that the amphidiploid genome of peanut contains two copies of AhMPK3, with each likely arising from a diploid ancestral parent (Fig. 3).
A genomic clone of AhMPK3 was amplified using gene specific primers designed against 5′ and 3′ termini of full length AhMPK3 transcript using peanut genomic DNA as template. A 3,036 bp fragment was obtained which was cloned and sequenced. The genomic structure of the AhMPK3 gene was established by the alignment with the corresponding cDNA, which revealed that coding region of AhMPK3 contained six exons and five introns (Fig. 4). The size of introns varied from 104 bp (III intron) to 810 bp (II intron). All the 5′ and 3′ splice junctions follow the typical; canonical consensus di-nucleotide sequence GT–AG (Supplementary Table 1). All the introns are A + T-rich; in particular they present an elevated T content, which is a peculiar feature of many plant introns (Ko et al. 1998).
Graphical representation of AhMPK3 gene structure and its comparison with its orthologs from poplar (PtMPK3-1 & 3-2) and Arabidopsis (AtMPK3). Exons are represented by closed boxes and introns by dark lines, the dotted lines represent the 5′ and 3′ UTRs, respectively. The individual exons, introns and UTRs length were given in base pairs. Numbers between brackets correspond to the intron phase. Drawings are not exactly to scale. PtMPK3-1 & 3-2 genomic sequences were retrieved from DOE Joint Genome Institute database (http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html) and AtMPK3 genomic sequence was obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org). NA Not available
AhMPK3 gene structure was compared with its orthologs from Arabidopsis (AtMPK3) and poplar (PtMPK3-1 &3-2) (Fig. 4), where PtMPK3-2 was presumed to be a paralog of PtMPK3-1 (Nicole et al. 2006). Comparative analysis of exon–intron junctions in all the three species indicate that the numbers of exons, and their sizes as well as the intron phases were extremely well conserved. Whereas the intron lengths were varied among the species with poplar and peanut introns were much longer than the corresponding Arabidopsis introns (Fig. 4). AhMPK3 genomic clone can be accessed from NCBI GenBank under the accession number EU182580.
Expression analysis of AhMPK3
To determine the expression pattern of AhMPK3 in response to various stress treatments, a semi-quantitative RT-PCR was carried out using RNA samples harvested at various intervals (Fig. 5). The results showed that a basal level of AhMPK3 is maintained in leaves, which got upregulated upon the incidence of stress. The difference observed in transcript levels at 0 h in various treatments could be due to the plant physiological differences or circadian rhythms. In response to wounding, AhMPK3 transcript expression reached a peak in 15 min, which gradually came down to the basal level by 6 h. Since both the pathogen and wound stress lead to H2O2 accumulation in plants, we studied the effect of H2O2 on the expression pattern of AhMPK3. With H2O2 application, AhMPK3 got upregulated gradually up to 30 min after treatment followed by a sudden decline at 60 min and gradual rebound by 12 h. In response to salicylic acid and methyl jasmonate, which are the signaling molecules for SAR and wound signaling, respectively, the gene expression showed upregulation during the later stages of the treatment. To examine the influence of nitric oxide (NO), which is an emerging essential component of plant defense signaling, SNP treatment was used, which caused steady state increase in the AhMPK3 transcript reaching a peak by 12 h. The analysis of AhMPK3 transcripts in response to mannitol that causes osmotic stress, showed a gradual increase by 30 min, and declined to the basal level by 24 h. ABA, which is the major signaling molecule for abiotic stress responses, induced AhMPK3 transcript accumulation at 30 min and a gradual decline before rebounding at 24 h after treatment. NaCl treatment had no significant impact on the expression pattern of AhMPK3 (data not shown). Treatment with water served as control and it showed a slight increase in AhMPK3 transcripts after 15 min. This suggested that the increase observed at 15 min in various chemical treatments was a combined effect of placing the leaves in an aqueous solution and its corresponding chemical.
Expression analysis of AhMPK3 in response to various treatments using semi-quantitative RT-PCR. A wounding, B hydrogen peroxide (H2O2), C methyl jasmonate (MeJA), D salicylic acid (SA), E Sodium nitroprusside (SNP), F abscisic acid (ABA), G mannitol, H cold and I water (H2O). The semi-quantitative RT-PCR reactions of AhMPK3 are performed as described in “Materials and methods”. cDNA synthesized from RNA samples collected at specific intervals of different treatments were amplified using gene specific primers AhMPK3 (ORF-F and ORF-R). Actin, which served as an internal control, was amplified using Act F and Act R primers
Subcellular localization of AhMPK3
Studies in mammals and yeast have shown that stimulus-induced activation of MAPKs was correlated with dynamic changes in their localization, whereby the proteins often got translocated to, and accumulated in, the nucleus of the cell. This is often required due to the nuclear localization of key MAPK substrates, including transcription factors involved in the control of gene expression (Brunet et al. 1999). Localization of AhMPK3 was analyzed by constructing an N-terminal GFP fusion and transiently expressing in tobacco leaves using agroinfiltration. As previous studies showed that AtMPK6/NtSIPK and AtMPK3/NtWIPK were activated by hydrogen peroxide and superoxide (Kovtun et al. 2000; Samuel et al. 2000; Moon et al. 2003), we studied the dynamic changes in the localization of AhMPK3 in response to H2O2. Under untreated conditions, AhMPK3 localized simultaneously in nucleus and cytoplasm, which upon treatment with H2O2 the staining intensity and frequency of nuclear staining further increased with majority of cells observed, showed predominant nuclear localization (Fig. 6; Supplementary Figure S5). Cells expressing GFP from control vector alone showed free GFP in the entire cell and was unaffected by water or H2O2 treatments.
Subcellular localization of AhMPK3. Control vector pEGAD and pEGAD:AhMPK3 were transiently transformed to Nicotiana tabacum leaves through agroinfiltration. GFP was visualized in epidermal cells using Confocal laser scanning microscope 48 h post agroinfiltration. Water (H2O) or 10 mM hydrogen peroxide (H2O2) were infiltrated 1 h before GFP visualization. a Representative picture of pEGAD control vector shows expression of free GFP throughout the cell without any treatment or when treated with H2O or H2O2. b Representative picture of pEGAD: AhMPK3 shows GFP localized to both cytoplasm and nucleus without any treatment or upon treatment with H2O C. pEGAD: AhMPK3 shows predominant nuclear localization of GFP upon treatment with H2O2. (Bar 20 μm)
Generation of transgenic plants and Herbivore resistance assay
Transgenic tobacco plants with AhMPK3 under CaMV35S promoter were raised using Agrobacterium mediated leaf disc transformation (Horsch et al. 1985). T0 transgenic plants were confirmed by PCR (Supplementary Fig. S1A) and Southern hybridization (data not shown). Northern analysis confirmed the AhMPK3 expression in T0 transgenic plants (Supplementary Fig. S1B). Two single copy, high transgene expression lines (T-5, T-8) and a moderately expressing line (T-9) derived from the corresponding progeny of the primary transgenic plants by selfing were selected for further analysis in T2 generation. T2 seeds were germinated on half strength MS medium supplemented with 100 mg/l kanamycin. The kanamycin tolerant plants were selected and transferred to soil in green house along with WT plants germinated on half strength MS medium.
All the T2 transgenic plants were first confirmed with PCR (data not shown) followed by northern hybridization to analyze the expression of AhMPK3 in T2 transgenic plants (Fig. 7). As we performed all the hybridization and washing steps under high stringency conditions, we did not observe any signal due to cross reactivity in wild type plants using AhMPK3 as probe (Fig. 7, Supplementary Fig. S1B). Herbivore resistance of transgenic plants against the common cut-worm, Spodoptera litura was examined by the level of leaf damage and gain of larval weight upon feeding on leaves of 2-month-old WT and transgenic plants. All the transgenic plants showed a high level of resistance to the first instar larvae, a moderate resistance towards second instar and low resistance towards third instar larvae, respectively (Fig. 8a, Supplementary Fig. S2). Analysis of larval weights after feeding showed that the final biomass of larvae fed on WT plants was significantly higher compared to the larvae fed on high expression transgenic lines (Fig. 8b).
Northern analysis of T2 transgenic plants for AhMPK3 expression. Total RNA was prepared from WT and T2 transgenic plants (T-5, T-8 and T-9). RNA samples (20 μg) were separated by denaturing formaldehyde-agarose gel electrophoresis, blotted, and hybridized with α-32P-labelled AhMPK3 probe. Ethidium bromide stained ribosomal RNA bands are shown as loading controls
a Leaf area consumed (cm2) in WT and transgenic plants with first, second and third instars of S. litura larvae. Data are mean values ± SE and asterisks indicate significant difference between WT and transgenic plants. (*P < 0.05) b Mean mass (±SE) of individual S. litura larvae after feeding on Wild type (WT) and transgenic plants (T-5, T-8, T-9). Asterisks indicate significant difference between WT and transgenic plants. (*P < 0.05)
Transcript levels of defense response genes in transgenic plants
Transgenic plants showing enhanced resistance to Spodoptera litura were analyzed for the levels of various defense related transcripts using semi-quantitative RT-PCR (Fig. 9; Supplementary Fig. S3). They displayed constitutively higher levels of lipoxygenase1 (LOX1), pathogenesis related proteins PR1a, PR1b, acidic β-1,3-glucanase, acidic chitinase, protease inhibitor II (PI–II) and ornithine decarboxylase (ODC) transcripts compared to WT plants (Fig. 9). Transcript levels of isochorismate synthase (ICS), lipoxygenase 3 (LOX3), and 1-aminocyclopropane-1-carboxylic acid synthase (ACS3a), which are the key enzymes in salicylic acid (SA) and jasmonic acid (JA) and ethylene biosynthesis, respectively, were unaffected. Apart from these, other wound or JA responsive gene transcripts like protease inhibitor I (PI–I), allene oxide synthase (AOS), allene oxide cyclase (AOC) were almost similar in both WT and transgenic plants (Fig. 9). Transcript levels of basic PR5 (osmotin) and defensin, which are known to be regulated by ethylene and JA synergistically were also unaffected.
Transcript profile of defense responsive genes in WT and Transgenic plants (T-8). Data for line T-8 alone is provided here. The experiments were performed on all other lines with similar results. Semi-quantitative RT-PCR was performed using total RNA of WT and transgenic plants. AOS allene oxide synthase, AOC allene oxide cyclase, LOX lipoxygenase, PI protease inhibitor, PR pathogenesis related protein, ICS isochorismate synthase, ODC ornithine decarboxylase, ACS 1-aminocyclopropane-1-carboxylic acid synthase
To study the effect of AhMPK3 overexpression on wound induced defense responses, we analyzed the level of PI–II transcripts upon wounding in WT and transgenic plants. Upon wounding, transgenic plants accumulated PI–II rapidly to high levels by 1 h and maintained through out the study (Fig. 10), whereas WT plants exhibited a gradual increase of PI–II transcripts in a time dependent manner. PR1b, which is a wound inducible pathogenesis related protein, also displayed a similar rapid induction in transgenic plants (Fig. 10). LOX3, a key regulator of wound induced JA biosynthesis, was induced rapidly in both WT and transgenic plants reaching peak level by 1 h after wounding. In WT plants, LOX3 transcripts reached the basal level in a time dependent manner, whereas the transgenic plants maintained slightly higher LOX3 transcript levels even in the uninduced state, which got upregulated quickly and the higher levels were maintained constantly up to 24 h after wounding. Neither the constitutive accumulation of defense related transcripts nor the rapid accumulation of PI–II transcripts upon wounding was observed in aged plants of 5 months or older (data not shown).
Time course analysis of wound induced expression of LOX3, PR1b and PI–II in WT and transgenic plants (T-8). Data for line T-8 alone is provided here. The experiments were performed on all other lines with similar results. Semi-quantitative RT-PCR was performed using total RNA extracted from samples collected at the indicated time intervals of WT and transgenic plants after wounding. LOX lipoxygenase, PR pathogenesis related protein, PI protease inhibitor
Total MBP kinase activity in WT and transgenic plants
Transgenic plants exhibiting constitutively higher transcript levels for some of the defense transcripts like LOX1, PI–II and pathogenesis related proteins compared to WT plants were analyzed for total MBP kinase activity levels using myelin basic protein as substrate. A higher MBP kinase activity levels were observed in transgenic plants compared to WT (Supplementary Figure S4).
Discussion
Being sessile, plants have to defend themselves against a wide range of unfavorable conditions for which they have developed elaborate and complex signaling networks to perceive the signal and respond. Mitogen-activated protein kinase (MAPK) cascade is one such signaling network, which is present in all eukaryotic organisms form yeast to mammals and also in plants. The cascade comprises three classes of hierarchically organized protein kinases, namely MAPKKKs, MAPKKs, and MAPKs, which rapidly amplify and transduce extracellular signals into various appropriate intracellular responses (Morris 2001).
A full length cDNA corresponding to a mitogen-activated protein kinase (MAPK) gene from peanut was cloned and based on its high homology with Arabidopsis AtMPK3, the present cDNA was designated as AhMPK3. AhMPK3 contains TEY motif in its activation loop and belongs to the A1 subgroup of MAPK family (MAPK group 2002). AhMPK3 protein shows very high homology with A1 subgroup members from other plants like GmMPK1 (Glycine max), MsMMK4 (Medicago sativa), AtMPK3 (Arabidopsis thaliana), NtWIPK (Nicotiana tabacum). Southern blot analysis revealed that because of the amphidiploid nature of the peanut, two copies of AhMPK3 with each likely arising from a diploid ancestral parent are present in peanut genome. Analyzing the genomic sequence of AhMPK3 showed that it contains six exons and five introns. Structural organization of AhMPK3 when compared with AtMPK3 (Arabidopsis, an herbaceous plant) and PtMPK3 (Poplar, a woody plant) revealed that the number of exons and introns, exon length and intron phases are well conserved, whereas the intron lengths and length of UTRs varied. This highlighted the conservation of these signaling molecules across various species and a strong negative selection for any alteration in protein sequence (Nicole et al. 2006).
AhMPK3 orthologs from other plant species were found to be transcriptionally regulated in response to wounding (Seo et al. 1995; Mayrose et al. 2004), Systemin and UV light (Holley et al. 2003), cold and drought (Jonak et al. 1996). Like other counterparts of A1 subgroup of MAPK family, AhMPK3 transcripts in peanut were also induced in response to various cues. In response to wounding, H2O2, NO, mannitol, ABA, and cold AhMPK3 exhibited distinct expression. SA and MeJA did not induce significant expression of AhMPK3 at an early stage, but an upregulation at later stages of the treatments was observed. Previous reports on TIPK (Shoresh et al. 2006) and LeMPK3 (Mayrose et al. 2004) also suggested that there was no effect of JA on their expression levels.
Identification of subcellular localization of MAPKs would provide an insight into the potential functional roles they harbor in plants. It has long been known that the activation of MAPKs in yeasts and mammals involved their simultaneous transport to the nucleus (Cobb and Goldsmith 2000). The phosphorylated AtMPK3 translocated rapidly to the nucleus upon ozone (O3) exposure (Ahlfors et al. 2004). Elicitation of parsley cell cultures with Pep-13 resulted in the translocation of PcMPK3a/b to the nucleus (Ligterink et al. 1997; Lee et al. 2004b). NtWIPK was also shown to simultaneously locate in nucleus and cytoplasm (Yap et al. 2005). Like its homolog NtWIPK, AhMPK3 was also found to localize in both nucleus and cytoplasm. In our experimental system, we utilized agroinfiltration for transient expression of GFP fusions in tobacco leaves and Agrobacterium itself is known to activate AtMPK3 (Djamei et al. 2007). Although we made observations 48 h after infiltration, we can not completely rule out the possibility of Agrobacterium induced activation and nuclear localization of some portion of AhMPK3 protein. However, AhMPK3 protein predominantly accumulated in the nucleus after H2O2 application, which clearly showed that H2O2 induced activation of AhMPK3 resulted in subsequent translocation to the nucleus. In a recent report, Qiu et al. (2008) elegantly demonstrated that WRKY33 was sequestered with MPK4 and MKS1 in the nucleus under normal conditions. But, challenge with Pseudomonas syringae or flagellin lead to the activation of MPK4 and phosphorylation of MKS1 and subsequent release of WRKY33, which activates camalexin synthesis through regulation of PAD3. This provides a new mechanism by which plant MAPKs could also regulate the gene expression by releasing transcription factors in the nucleus upon activation. A study in yeast also suggests that MAPKs may physically associate with promoters and influence the transcription of certain genes (Pokholok et al. 2006). Hence nuclear localization of AhMPK3 might have significant implications in gene regulation.
Recent evidence demonstrates the involvement of AtMPK3/NtWIPK orthologs and AtMPK6/NtSIPK orthologs in regulating plant defense response against herbivores using VIGS (Wu et al. 2007; Kandoth et al. 2007). Co-silencing LeMPK1and LeMPK2 orthologs of AtMPK6/NtSIPK compromised pro-systemin mediated resistance to Manduca sexta herbivory (Kandoth et al. 2007). There was no direct experimental data available in case of plants with overexpressed or silenced AtMPK3/NtWIPK or its orthologs in terms of their effect in conferring resistance against chewing insects. Hence, the transgenic tobacco plants ectopically expressing AhMPK3 were studied for their resistance against Spodoptera litura. AhMPK3 transgenic plants showed enhanced resistance to the attack by the first instar larvae and moderate resistance against second instar larvae. The decrease in the resistance observed in transgenic plants against the later stages of larvae could be due to the developmentally regulated insect resistance to plant defense protease inhibitors which plays a major role in plant herbivore resistance. For example, in case of southern corn rootworm larvae fed on a diet containing potato multicystatin, high mortality was observed in younger insects, where as the toxic effect was less when later stages of insects were used in the feeding experiment (Orr et al. 1994). It was suggested to be possible through the regulation of numerous protease isoforms often available even at a single developmental stage in the insect larvae varying in their sensitivity to a specific protease inhibitor (Orr et al. 1994; Koiwa et al. 2000).
Analyzing the defense response transcripts in transgenic and WT plants showed higher transcript levels of LOX1, PR1a, PR1b, acidic β-1, 3-glucanase, acidic chitinase and PI–II. The isochorismate synthase (ICS), which is a key enzyme in salicylic acid (SA) biosynthesis (Wildermuth et al. 2001; Catinot et al. 2008) and its transcript levels were similar in WT and transgenic plants indicating a possible SA-independent upregulation of PR genes in AhMPK3 transgenic plants. Except for PI–II and PR1b, whose transcripts were upregulated in transgenic plants, other genes, which are known to be involved in wound or JA responses like AOS, AOC, and PI–I displayed no apparent differences in transcript levels. As PI–II are also known to be regulated by ethylene (Balandin et al. 1995; Kim et al. 2003), we studied the transcript levels 1-aminocyclopropane-1-carboxylic acid synthase (ACS3a) involved in ethylene biosynthesis and other ethylene responsive genes like basic PR5 (osmotin) and defensin in transgenic plants which showed no apparent differences between WT and transgenic plants. The transcript levels of ornithine decarboxylase involved in biosynthesis of nicotine, which is herbivore, wound or JA inducible and ethylene suppressible (Shoji et al. 2000), were higher in transgenic plants compared to WT. This implied a less possible role of ethylene in controlling PI–II levels in transgenic plants.
A time course analysis of PI–II transcripts in response to wounding showed that in WT plants, the accumulation of PI–II transcripts occurred gradually and reached maximum level by 12–24 h, whereas the transgenic plants accumulated high levels of PI–II transcripts within 1 h, which was maintained through out the time of study. A similar expression pattern observed for PR1b suggested that other defense genes were also induced in a similar way. LOX3 is a wound induced lipoxygenase and LOX3-mediated JA signaling accounts for a major part of induced resistance, when plants are damaged by insect herbivores (Rayapuram and Baldwin 2006). Antisense suppression of LOX3 resulted in herbivore susceptibility indicating a crucial role in herbivore tolerance (Halitschke and Baldwin 2003). Sustained transcript levels of LOX3 in transgenic plants after wounding suggested a better wound induced JA or JA responsive gene induction.
Transcript abundance at a given time is an important prerequisite to subsequent production of the corresponding protein required for proper execution of its function. The activation of NtWIPK an ortholog of AhMPK3 was delayed and it requires transcriptional activation and de novo synthesis of a WIPK protein (Zhang et al. 2000). It was postulated that a delayed or lack of activity of WIPK, when treated with phosphatase inhibitors, was likely because of the reduction in upstream kinase activity by the time WIPK accumulated to a significant level (Liu et al. 2003). Like other MAPKs, AhMPK3 also presumably might be activated by its upstream MAPK kinase, which in turn phosphorylate and activate effector proteins that directly or indirectly regulate a spectrum of responses. Hence, by overexpressing AhMPK3, the protein would be available readily to be activated by its upstream kinase upon receiving an appropriate signal. In such a case, plants overexpressing AhMPK3 would be primed to respond rapidly.
The activities of MAPKs in a cell are controlled by the opposing actions of MAPKKs, which phosphorylate and activate them and MAPK phosphatases, which dephosphorylate and inactivate them (Widmann et al. 1999). The observed constitutive upregulation of defense response genes in transgenic plants could be due to the basal level activity of upstream kinase or the level of corresponding phosphatase is not sufficient enough to inactivate the entire pool of protein in a transgenic plant with high expression levels. The higher basal MBP kinase activity levels exhibited by the transgenic plants compared to WT suggest possible reason for higher constitutive transcript levels of some of the defense related genes. It is also possible that more than one MAPKK is involved in the activation of a particular MAPK under different conditions as in the case of yeast and animal systems (Widmann et al. 1999; Davis 2000). The increased transcript levels in transgenic plants can be attributed to the differential regulation of several down stream components. For example AtVIP1 a bZIP type of transcription factor was demonstrated to be regulating AtPR1a expression upon activation of AtMPK3 in Arabidopsis (Djamei et al. 2007). And NaWIPK was found to regulate the transcript levels of MPK4, NaSIPK, WRKY, and several CDPKs (Wu et al. 2007). Our results of higher LOX3 and PI–II transcript levels in AhMPK3 transgenic plants upon wounding are in agreement with the VIGS functional analysis of LeMPK3 and NaWIPK, in which silencing of LeMPK3 in 35S::prosys tomato plants resulted in significant reduction in LoxD transcripts (a homolog of tobacco LOX3) as well as reduced PI–II levels (Kandoth et al. 2007). The silencing of NaWIPK resulted in reduced Trypsin proteinase inhibitor (TPI) activity and reduced LOX3 transcripts with wounding alone and in combination with the oral secretions of Manduca sexta (OS) application in N. attenuata. This shows that PI–II levels are positively regulated by AhMPK3 or its homologs.
In a recent review, Beckers and Conrath (2007) reported some of their unpublished results, that in Arabidopsis priming by the chemical agent benzo (1,2,3) thiadiazole-7-carbothioic acid S-methyl ester (BTH) is based on enhanced accumulation of mitogen-activated protein kinase 3 (AtMPK3) protein with out displaying MPK3 activity. However, upon exposure to biotic or abiotic stresses, MPK3 enzyme activity was induced to enhanced levels in primed plants and associated with boosted defense gene activation and stress resistance. The physiological state, in which plants are able to faster or better activate defense responses, or both, is called the primed state of the plant (Beckers and Conrath 2007). Due to the overexpression of AhMPK3, the transgenic plants presumably are in a primed state, which resulted in rapid induction of PI–II upon wounding. Lack of these enhanced levels of defense response transcripts observed in older AhMPK3 transgenic plants could be imputed to the unavailability of downstream regulatory molecules due to their developmental regulation or the absence of active physiological environment in aged plants. Constitutively higher level of various defense gene transcripts as well as rapid induction of protease inhibitor II (PI–II) transcripts upon wounding, which encodes the antidigestive protein and functions as a direct defense against herbivores, might have collectively resulted in resistance of AhMPK3 transgenic tobacco plants against Spodoptera litura. The observed transcript levels of various genes in WT and transgenic plants indicate that regulation is independent of hormones as all the subset of genes known to be regulated by specific hormone were not affected. Sustained levels of LOX3 in wounded transgenic plants suggested the possible role of AhMPK3 in regulating stress induced hormone levels. This indicates that AhMPK3 probably activates transcription factors with well-defined downstream targets.
Essentially, most of the studies on priming in response to wounding and or herbivore attack were associated with use or involvement of plant derived cues like volatile organic compounds(VOCs) that are emitted in response to herbivory (Frost et al. 2008). In the present investigation, we demonstrated the wound induced priming of defense responses in tobacco plants ectopically expressing AhMPK3 of peanut. The regulatory molecules connecting AhMPK3 and gene expression are being currently investigated. Our results substantiate the function of AtMPK3/NtWIPK orthologs in defense against herbivore attack in plants.
References
Agrawal GK, Iwahashi H, Rakwal R (2003) Rice MAPKs. Biochem Biophys Res Commun 302:171–180
Ahlfors R, Macioszek V, Rudd J, Brosché M, Schlichting R, Scheel D, Kangasjärvi J (2004) Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J 40:512–522
Balandin T, Van Der Does C, Albert JM, Bol JF, Linthorst HJ (1995) Structure and induction pattern of a novel proteinase inhibitor class II gene of tobacco. Plant Mol Biol 27:1197–1204
Beckers GJ, Conrath U (2007) Priming for stress resistance: from the lab to the field. Curr Opin Plant Biol 10:425–431
Bögre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, Hirt H (1997) Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9:75–83
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouysségur J (1999) Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J 18:664–674
Catinot J, Buchala A, Abou-Mansour E, Me’traux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett doi:10.1016/j.febslet.2007.12.039
Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, Yoon HW, Chung WS, Lim CO, Lee SY, Cho MJ (2003) BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 132:1961–1972
Cobb MH, Goldsmith EJ (2000) Dimerization in MAP-kinase signaling. Trends Biochem Sci 25:7–9
Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413:217–226
Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97:3718–3723
Datla RSS, Hammerlindl JK, Panchuk B, Pelcher LE, Keller WA (1992) Modified binary plant transformation vectors with the wild-type gene encoding NPTII. Gene 21:383–384
Davis R (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252
Daxberger A, Nemak A, Mithöfer A, Fliegmann J, Ligterink W, Hirt H, Ebel J (2007) Activation of members of a MAPK module in β-glucan elicitor-mediated non-host resistance of soybean. Planta 225:1559–1571
Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H (2007) Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science 318:453–456
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiology 146:818–824
Guo B, Chen X, Dang P, Scully BT, Liang X, Holbrook CC, Yu J and Culbreath AK (2008) Peanut gene expression profiling in developing seeds at different reproduction stages during Aspergillus parasiticus infection. BMC Developmental Biology 8, art. no. 12. http://www.biomedcentral.com/1471-213X/8/12
Halitschke R, Baldwin IT (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36:794–807
Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family:conserved features and deduced phylogeny of the catalytic domains. Science 24:42–52
Holley SR, Yalamanchili RD, Moura DS, Ryan CA, Stratmann JW (2003) Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiol 132:1728–1738
Holsters M, De Waele D, Depicker A (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Hord CLH, Yu-Jin Sun Y-J, Pillitteri LJ, Torii KU, Wang H, Zhang S, and Ma H (2008) Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol Plant, doi:10.1093/mp/ssn029
Horsch RB, Fry JE, Hofmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:229–1231
Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H (1996) Stress signalling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA 93:11274–11279
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, crosstalk and integration of plant MAP kinase signaling. Curr Opin Plant Biol 5:415–424
Jonak C, Nakagami H, Hirt H (2004) Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136:3276–3283
Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW (2007) Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci USA 104:12205–12210
Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S (2003) Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant cell 15:2707–2718
Ko CH, Brendel V, Taylor RD, Walbot V (1998) U-richness is a defining feature of plant introns and may function as an intron recognition signal in maize. Plant Mol Biol 36:573–583
Koiwa H, Shade RE, Zhu-Salzman K, D’Urzo MP, Murdock LL, Bressan RA, Hasegawa PM (2000) A plant defensive cystatin (soyacystatin) targets cathepsin 1-like digestive cysteine proteinases (DvCALs) in the larval midgut of western corn rootworm (Diabrotica virgifera virgifera). FEBS Lett 471:67–70
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress activated mitogen-activated protein kinase cascades in plants. Proc Natl Acad Sci USA 97:2940–2945
Lee DE, Lee IJ, Han O, Baik MG, Han SS, Back K (2004a) Pathogen resistance of transgenic rice plants expressing mitogen-activated protein kinase 1, MK1, from Capsicum annuum. Mol Cells 17:81–85
Lee J, Rudd JJ, Macioszek VK, Scheel D (2004b) Dynamic changes in the localization of MAP kinase cascade components controlling pathogenesis-related (PR) gene expression during innate immunity in parsley. J Biol Chem 279:22440–22448
Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D (1997) Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276:2054–2057
Liu Y, Jin H, Yang KY, Kim CY, Baker B, Zhang S (2003) Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J 34:149–160
Luo M, Dang P, Guo BZ, He G, Holbrook CC, Bausher MG, Lee RD (2005) Generation of expressed sequence tags (ESTs) for gene discovery and marker development in cultivated peanut. Crop Sci 45:346–353
MAPK group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plants Sci 7:301–308
Mayrose M, Bonshtien A, Sessa G (2004) LeMPK3 is a mitogen-activated protein kinase with dual specificity induced during tomato defense and wounding responses. J Biol Chem 279:14819–14827
Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 93:765–769
Moon H, Lee B, Choi G et al (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 100:358–363
Morris PC (2001) MAP kinase signal transduction pathways in plants. New Phytol 151:67–89
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8:4321–4325
Nicole MC, Hamel LP, Morency MJ, Beaudoin N, Ellis BE, Séguin A (2006) MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinases and MAP kinase kinases. BMC Genomics 7, art. no. 223. http://www.biomedcentral.com/1471-2164/7/223
Orr GL, Strickland JA, Walsh TA (1994) Inhibition of Diabrotica larval growth by a multicystatin from potato-tubers. J Insect Physiol 40:893–900
Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA (2006) Activated signal transduction kinases frequently occupy target genes. Science 313:533–536
Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, Palma K, Petersen M (2008) Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27:2214–2221
Rayapuram C, Baldwin IT (2006) Using nutritional indices to study LOX3-dependent insect resistance. Plant Cell Environ 29:1585–1594
Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105:5638–5643
Reyna NS, Yang Y (2006) Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol Plant Microbe Interact 19:530–540
Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11:273–287
Samuel MA, Miles GP, Ellis BE (2000) Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J 22:367–376
Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathway. Science 270:1988–1992
Seo S, Sano H, Ohashi Y (1999) Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11:289–298
Seo S, Katou S, Seto H, Gomi K, Ohashi Y (2007) The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J 49:899–909
Sharma PC, Ito A, Shimizu T, Terauchi R, Kamoun S, Saitoh H (2003) Virus-induced silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen, but has no effect on the INF1-induced hypersensitive response (HR) in Nicotiana benthamiana. Mol Gen Genomics 269:583–591
Sharma HC, Pampapathy G, Dhillon MK, Ridsdill-Smith JT (2005) Detached leaf assay to screen for host plant resistance to Helicoverpa armigera. J Econ Entomol 98:568–576
Shoji T, Nakajima K, Hashimoto T (2000) Ethylene suppresses Jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol 41:1072–1076
Shoresh M, Gal-On A, Leibman D, Chet I (2006) Characterization of a mitogen-activated protein kinase gene from cucumber required for trichoderma-conferred plant resistance. Plant Physiol 142:1169–1179
Stulemeijer IJE, Stratmann JW, Joosten MHAJ (2007) The tomato MAP kinases LeMPK1, -2 and -3 are activated during the Cf-4/Avr4-induced HR and have distinct phosphorylation specificities. Plant Physiol 144:1481–1494
Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15:5890
Wan J, Zhang S, Stacey G (2004) Activation of a mitogen-activated protein kinase pathway in Arabidopsis by chitin. Mol Plant Pathol 5:125–135
Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19:63–73
Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, Zhang S (2008) Haplo-Insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 20:602–613
Widmann C, Gibson S, Jarpe MB, Johnson GL (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase isrequired to synthesize salicylic acid for plant defence. Nature 414:562–571
Wu J, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuate. Plant Cell 19:1096–1122
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yap YK, Kodama Y, Waller F, Chung KM, Ueda H, Nakamura K, Oldsen M, Yoda H, Yamaguchi Y, Sano H (2005) Activation of a novel transcription factor through phosphorylation by WIPK, a wound-induced mitogen activated protein kinase in tobacco plants. Plant Physiol 139:127–137
Zhang S, Klessig DF (1998) Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc Natl Acad Sci USA 95:7433–7438
Zhang S, Du H, Klessig DF (1998) Activation of the tobacco SIP kinase by both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10:435–450
Zhang S, Liu Y, Klessig DF (2000) Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J 23:339–347
Acknowledgments
KRRK is grateful to the Council of Scientific and Industrial Research, Government of India for financial support in the form of JRF and SRF. This work is funded by Volkswagen Foundation, Germany and by a Research Grant (BT/PR/6853/PBD/16/627/2005) from Department of Biotechnology, Government of India. The authors are thankful to Prof. Heinz Saedler, Max-Planck-Institute for Plant Breeding Research, Cologne, Germany for his interest in the study and also grateful to DST-FIST, UGC-SAP, Government of India, for the facilities provided to the Department of Plant Sciences, University of Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schnittger.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Kumar, K.R.R., Srinivasan, T. & Kirti, P.B. A mitogen-activated protein kinase gene, AhMPK3 of peanut: molecular cloning, genomic organization, and heterologous expression conferring resistance against Spodoptera litura in tobacco. Mol Genet Genomics 282, 65–81 (2009). https://doi.org/10.1007/s00438-009-0446-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-009-0446-6