Abstract
Multitasking capability of nitric oxide (NO) makes it a highly investigating signaling molecule in plant biology. In plants including inflection of hormonal levels, fruit ripening, wound suppression and defensive responses, and regulation of programmed cell death, much progress in NO signaling cascades has been achieved. Additionally, growing evidences suggest the interactive behavior of NO with auxin, salicylic acid, abscisic acid, jasmonic acid and thus regulates their signaling pathways. Parallel to this, reactive oxygen species (ROS) along with NO are supposed to accomplish various developmental and stress responses. Under biotic stress, signaling initiated by NO was found to be mediated by two specific protein i.e. pathogenesis-related 1 (PR-1) and phenylalanine ammonia lyase. The above mentioned genes were also promoted by second messengers like cyclic GMP (cGMP) and cyclic ADP-ribose (cADPR), which further initiate and regulate NO signaling. In plants, important mechanism is programmed cell death regulating various growth and developmental aspects by acting as a damage control. Under stress condition the infected cells are removed by involving signaling agents i.e. NO and ROS which is a matter of crosstalk in recent years. Keeping above facts into consideration, present work mainly deals with NO signaling under adverse conditions as well as its interaction with different phytohormones and ROS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
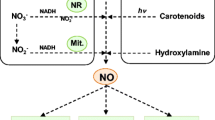
Nitric oxide (NO), an effective biological factor involved in signaling cascade and a gaseous reactive nitrogen species (RNS), is mostly known for its multitasking role in plants (Domingos et al. 2015). A number of reports have recognized the NO generation endogenously in both higher plants as well as in algae, lichens and also in some non-flowering plants including ferns and gymnosperms (Domingos et al. 2015; Chakraborty and Acharya 2017; Tiwari et al. 2019). Among many vital components, NO is considered as a lipophilic, ubiquitous biologically active signaling molecule which is now recognized as a possible intercessor for regulating responses of plants against stress (Asgher et al. 2017; Begara-Morales et al. 2018). NO is an elementary molecule which governs signaling and controls metabolic processes of plants and makes them able to combat with stressful situations (Arasimowicz et al. 2009; Baudouin 2011; Asgher et al. 2017; Singh et al. 2017; Begara-Morales et al. 2018; Corpas and Palma 2018). Previously, NO was considered as a significant messenger involved in signaling process during pathogens attack (Huang et al. 2004). However in the recent years, NO has attracted more attention due to its omnipresence and the diversity of physiological functions. Figure 1 depicts the role of NO in plant development, gene expression, defense responses (expression of antioxidant enzymes) and stomatal movement under stress condition. Figure 2 illustrates the crosstalk of NO with other signaling molecules and involvement of NO in plant protein modifications through nitration and S-nitrosylation process. Under abiotic stress, NO reacts with superoxide radical (O ·−2 ) and gets converted into peroxinitrite (ONOO−) which is a powerful oxidant RNS molecule which further involved in nitration process for post translational modifications. In nitration reaction addition of nitro group (–NO2) to the proteins, fatty acids or nucleic acids takes place. Nitro-tyrosine is a most studied modification found in case of protein modification (Corpas et al. 2009; Kolbert et al. 2017). Peroxinitrite is toxic to animals but in plant cells its toxic effect may depend on its concentration. Several researchers have reported its role in defense, apoptosis and gene expression in plants (Groß et al. 2013; Qiao et al. 2014). On the other hand, the covalent attachment of NO with thiol (-SH) group in the side chain of cysteine (Cys) is depicted as S-nitrosylation process (Fig. 2) (Fares et al. 2011; Broniowska and Hogg 2012). Fröhlich and Durner (2011) demonstrated that l-arginine is as a foremost source of endogenous NO production in animals. But in case of higher plants, more investigations are required to know the occurrence of such enzymes similar to mammalian nitric oxide synthase (NOS). There are several studies are available to detect the precise characteristic feature of a gene like mammalian NOS. For instance, nitric oxide associated-1 gene (s) was activated by Ca2+ in plants (Galatro and Puntarulo 2014). On the other hand, Chakraborty and Acharya (2017) reported two ways for NO production (i.e. reductive and oxidative) in plants (Fig. 1) and also revealed the signaling role of NO in plants. The multitasking role of NO has been proven during few decades because of its multidimensional role along with interactive behavior with biomolecules and phytohormones (Domingos et al. 2015). Under drought conditions, higher plants are required to check the water loss. In this unfavorable condition, guard cells are known to balance the opening and closing of stomata to equilibrate the gaseous interchange for active photosynthesis. Nitric oxide governs stomatal movement induced by abscisic acid (ABA), a water-stress hormone (Freschi 2013). Thus, another important role of NO is to make plants more capable against drought condition (García-Mata and Lamattina 2007; Shabbir et al. 2016). According to Wendehenne et al. (2004), programmed cell death (PCD) has been observed under synchronised increase of NO and H2O2 in tobacco cells; however in soybean cells, PCD does not successfully occur in lack of reactive oxygen species (ROS). This indicates that only increased level of NO is not much enough to drive end of cell life. In recent years, several reports showed that NO and its related RNS have the capacity to govern every single step of plant developmental process (Table 1) by balancing antioxidants and ROS under stress conditions as described in Fig. 4 (Begara-Morales et al. 2018). Though there are many studies carried out to understand the nature and signaling of NO that triggers the gene expression to modulate plant metabolic processes, but the facts are still remained unclear. In spite of many evidences which show the behavior and role of NO as a plant growth regulator under stress, the current information regarding NO synthesis and signaling pathways in plants is still limited. In this review, we have tried to appraise the plant signaling governed by NO and its cross talks with ROS and different phytohormones, and other second messengers under stress conditions.
Synthesis of NO in plants
Production of NO is not only limited via enzymatic action but can also be formed as a by-product of various processes like respiration, nitrogen fixation and denitrification (Domingos et al. 2015). Chakraborty and Acharya (2017) demonstrated two pathways of NO synthesis i.e. oxidative and reductive. The former one is arginine or hydroxylamine- dependent pathway and latter one is nitrate dependent pathway (Fig. 1). Previous studies show that NO primarily produced through the family of NOS enzymes in mammalian cells. Each NOS enzyme catalyzes the production of NO from l-arginine i.e. NADPH-dependent simultaneously producing L-citrulline (Li and Poulos 2005; Domingos et al. 2015). There are fewer evidences for NOS existence or similar enzymes in plant system but Guo et al. (2003) described some possibility and identification of AtNOS1 gene in Arabidopsis thaliana. Further, AtNOS1 has been renamed to AtNOA1 as nitric oxide associated- 1 gene (Crawford et al. 2006). Zemojtel et al. (2006) have reported the involvement of GTPase implied AtNOA1-gene in NO formation from l-arginine in mitochondrial biogenesis. However, according to the latest available literature, there is a possible existence of AtNOA1 that encrypts cGTPase which is localized in the chloroplast, possibly engaged in process of mRNA translation resulting in protein formation (Flores-Pérez et al. 2008; Moreau et al. 2008). The more recent evidences regarding the presence of NOS like gene sequence in land plants as well as in algal system have been shown by Jeandroz et al. (2016). On the basis of comparative gene sequencing of mammalian NOS with algal and plant NOS they found more compatibility with algal system. Overall findings still not clearly prove the presence of NOS enzyme in higher plants and there are much contradictions about its major role in synthesis of NO. Several reports have noticed that nitrite found as a foremost substrate for sequential process of NO synthesis in higher plants (Santolini et al. 2017). There are two ways of NO productions through nitrite involving enzymatic along with non-enzymatic pathways (Fig. 1). Nitrate reductase (NR) is mainly known to reduce nitrate and converts it into nitrite by utilizing NADPH as a reducing agent, but it also produces NO from nitrite by both in vitro as well as in vivo processes (Yamasaki and Sakihama 2000; Rockel et al. 2002; Santolini et al. 2017). Mohn et al. (2019) observed two isoforms (NIA1 and NIA2) of NR in A. thaliana. They also found that NIA1 mainly involved in NO production whereas nitrate reduction is the main function of NIA2. Some biochemical and genetic-based approaches show that during ABA signaling and hypoxia, NR is the enzyme which chiefly acts as a mediator for NO synthesis (Desikan et al. 2002; Dordas et al. 2004; Bright et al. 2006). Another aspect of non-enzymatic conversion of nitrite to NO in the apoplast has been found at low pH having reductant ascorbic acid (Bethke et al. 2004). Still controversy exists regarding the co-involvement of l-arginine and NR dependent pathways in NO production in plants (Rasul et al. 2012).
Some recent studies describe that polyamines also participate in synthesis of NO in some cases (Fig. 1). In Arabidopsis seedlings, Tun et al. (2006) described spermidine and spermine, two polyamines which are known to induce quick generation of NO in root tip (in elongation zone) and also in primary leaves (in the trichomes and veins).
Cross talk of NO with other second messengers (Ca2+, cGMP and cADPR)
In animals, it has been reported that guanylate cyclase (sGC) in soluble state is involved in NO signaling. Mechanism includes the binding of NO with sGC heme triggering the enzymatic action and thereby, increase in the level of ubiquitous cGMP as a second messenger was noticed which is for a short time activates various respective goals (Besson-Barda et al. 2008). Further, it has also been reported that similar mechanism runs in Tobacco (Durner et al. 1998) and cucumber (Pagnussat et al. 2003) hence, attests the fact that cGMP amount increased after the exogenous application of NO. Besides this, Wendehenne et al. (2004) demonstrated in tobacco plant that sGC inhibitors chunk the behavioral role of gene phenylalanine ammonia lyase (PAL) and its enzymatic action, as well as root development in cucumber which is dependent on NO and auxin interactions (Pagnussat et al. 2003). However, several major constituents of the NO/cGMP pathway and its role are still to be identified.
Additionally, cGMP by inducing cyclic ADP-ribose (cADPR) and Ca2+ mobilization, NO may pose its functions in both plants and animals. Another second messenger i.e. cADPR, stimulates the release of Ca2+ through intracellular channel permeable to Ca2+ i.e. RYR (ryanodine receptor channels) (Wendehenne et al. 2001). In animals, cGMP dependent pathway mediates cADPR synthesis which directly activates NO (Fig. 3) by acting as downstream messenger for NO and also by up-regulating PAL and PR-1 genes and ultimately Ca2+ mobilization is enhanced which directly governs the response against signaling (Wendehenne et al. 2001). NO is known to regulate signaling cascade i.e. cADPR-dependent action governed by PAL and PR-1 genes is simultaneously sensitive to RYR inhibitors (Wendehenne et al. 2004). Whereas 8- Br-cADPR plays opponent role against cADPR and suppresses the induction caused by PR-1 and NO (Klessig et al. 2000). In guard cells of Vicia faba, it has been described that NO promots the levels of cytosolic Ca2+ (Garcia-Mata et al. 2003). As in above mentioned evidences, NO regulates its function through cGMP (Beligni and Lamattina 2001) and cADPR through activated intracellular Ca2+-permeable channels and liberating free cytosolic Ca2+ as reported earlier in cells of tobacco that are reacting against cryptogein (Lamotte et al. 2004). Hossain et al. (2014) showed positive interaction of cADPR with NO and ROS in methyl jasmonate mediated guard cells movement. In addition, NO shows its importance against fight with drought condition by mediating ABA mediated opening and closing of stomatal guard cells along with cGMP and cADPR (Garcia-Mata and Lamattina, 2001). With regards to the role of NO in stomatal closure in Vicia faba, c-PTIO affect the Ca2+-dependent ABA induced processes i.e. c-PTIO inactivates the inward- movement mediated by K+ channel while activates the outward-movement mediated by Cl− channel (Garcia-Mata et al. 2003). Hence, it is cleared that NO involved in the regulatory process of plants but their regulatory pathway(s) have yet not been demarcated. The study concludes that NO regulates the genes that are involved in maintaining the level of intrinsic Ca2+ through its channels and also via the modulators of cGMP and cADPR which are NO/redox-sensitive processes (Fig. 3).
NO combines with sCG and activates the enzymatic activity resulting in production of secondary messenger i.e. cGMP which further induces cADPR activity and is also directly involved in the process of NO synthesis. Simultaneously, sCG inhibitor deactivates PAL activity as well as auxin mediated root development by inhibiting the role of NO. cGMP with cADPR increases the Ca+ release intracellularly and further signaling cascade regulates the cell response. Mobilization of Ca+ through RYR channel is sensitive to its inhibitor which also blocks the PR-1 gene mediated PAL activity directed by cGMP and cADPR messengers induced by NO
Crosstalk of NO and phytohormones
Nitric oxide, being a signaling bioactive molecule, shows its interaction with phytohormones and with its related reactive nitrogen species (RNS: ONOO−, N2O3, NO2) regulates metabolic processes against pathogenic attack and environmental stresses in plants. Its mechanism of targeting in overall metabolic process in order to counter stress responses is still not properly known. Over the last few decades some findings have enlighten the rapid induction and potential role of NO in plant growth regulation as described in Table 1. The intrinsic level of NO increased during stress condition and simultaneously involved in plant growth and developmental processes (Delledonne 2005). Hao and Zhang (2010) suggested a governing factor “ABA–H2O2–NO–MAPK-antioxidant existence sequence” that indicates the ABA performed ameliorative functions during water stress. Nitric oxide has been shown to act as a dynamic signaling intermediate resulting in vital phenomena i.e. closure of stomata to moderate the dehydration along with triggering the antioxidant defense system to respond to damage caused by oxidative species (Shabbir et al. 2016). In another study the interactive role of NO has been implicated in regulating metabolism in CAM plants by up-regulating the cytosolic Ca2+ (Freschi et al. 2010; Mioto and Mercier 2013). Seed dormancy during germination is mainly decided by the stability between ABA and gibbrellins (Table 1), hence acts as signals triggered by environmental factors to further proceed the physiological and metabolic functions (Arc et al. 2013). The studies demonstrated that exogenous NO application is also capable of breaking the seed dormancy in Arabidopsis and barley, and its role was further confirmed by the use of NO scavenger (c-PTIO) (Bethke et al. 2004, 2006a, b; Libourel et al. 2006). Evidences showed that exogenous ABA application promotes NO production endogenously in tobacco and Arabidopsis (Guo et al. 2003; Bright et al. 2006; Liu et al. 2009), and similar phenomena was also noticed in the aleurone layer specifically in apoplast cell throughout the process of germination in barley (Bethke et al. 2004). Likewise, various responses mediated by auxin, for instance- development of root (Pagnussat et al. 2003; Correa-Aragunde et al. 2004), root movement towards gravity (Hu et al. 2005), process of root re-differentiation (Correa-Aragunde et al. 2004; Pagnussat et al. 2004; Lanteri et al. 2006), formation of nodules in root (Pii et al. 2007) are also modulated by ABA and NO (Table 1). Several other responses such as development of root under deficiency of iron (Chen et al. 2010), cell division, embryonic development (Ötvös et al. 2005) and induction of NR activity (Du et al. 2008) are also synergistically regulated by auxin and NO. In Arabidopsis, exogenously applied NO reduced the PIN1-dependent acropetal auxin transport in root apical meristem thereby inhibits the polar transport of auxin indicating NO acts downstream regulator of auxin (Fernández et al. 2011; Fernández-Marcos et al. 2012).
Previous reports underlined that NO inhibits the hypocotyl elongation (Table 1) in Arabidopsis and lettuce grown in the absence of light (Beligni and Lamattina 2000). Furthermore, Tonón et al. (2010) have noticed that in etiolated A. thaliana seedlings, NO together with superoxide and ATP regulates hypocotyl elongation. Gibberellic acid (GA) has been shown to regulate NO mediated signaling pathway by a possible mechanism in which PIF and DELLA proteins are also actively involved. Hence, it was concluded that increase in DELLA proteins and growth-promoting PIF gene products are also coordinated by NO (Lozano-Juste and León 2011). In recent past, accumulating evidences suggest a complex interaction between NO and CKs. For example zeatin (a kind of CK) has been shown to induce NO production in Arabidopsis seedlings (Tun et al. 2008). However, other evidences point out either unchanged response or quite low level of NO production after CK treatment/in mutant or transgenic plants with increased level of CK (Xiao-Ping and Xi-Gui 2006; Romanov et al. 2008; Liu et al. 2013). The synergistic role of CKs with NO has been clearly demonstrated in the plants treated with NO scavengers/NOS inhibitors or in mutant plants deprived of NO production demolish the expression of CKs induced gene CYCD3;1 (transcriptional gene for cell cycle activation). Furthermore, Freschi (2013) has also pointed out the antagonistic effect of CKs with NO in epidermal strips of Vicia faba where SNP as a donor of NO reduced the generation of NO inside the guard cell.
In the last decade, several observations focused on a probable role of NO and it is considered as a crucial game changer against hypersensitivity like wounding/cutting or other power-driven stresses (Pedroso et al. 2000; Garcês et al. 2001). Work of Liu et al. (2016) on tomato reflected the role of NO as wound healer. Nitric oxide acts as a downstream mediator of jasmonic acid (JA) synthesis, by hindering the H2O2 production and inhibiting proteinase gene expression. Now, it is clear that during pathogenesis, the role of those genes which express themselves when wound occurs was down-regulated by NO. The inhibition of this defense gene was not dependent on salicylic acid (SA), which is an antagonistic phenomenon against JA synthesis and/or its activity (Alavi et al. 2014). Though, systemic acquired resistance (SAR) induced by SA has been weaken by the application of inhibitors of NOS enzyme and scavengers of NO and thereby, these results revealed that both SA and NO are dependent on each other. In this phenomenon nitric oxide required for the action and biosynthesis of SA and NO requires SA for its functions (Ji et al. 2016). After detailed observations based on these studies it can be pretended that NO is a key component to regulate phytohormones homeostasis. Moreover, some researchers have considered that NO itself as an “artificial plant hormone” for its extraordinary performance (Qian et al. 2009).
NO regulates gene expression to maintain hormonal balance in plants
Neill et al. (2002) have reported interactive relation between ROS and NO and came to a conclusion that NO has the capability of modifying the way of working of many genes in plant cells. Like in soybean and tobacco plants, NO triggers the expression of several proteins such as PR-1 which is related to pathogenesis while PAL and GST show a vibrant role in defensive routes inside the cell (Delledonne et al. 1998; Durner et al. 1998). del Rio et al. (2003) described that expression of genes related to diverse form of peroxidases, ferritin, and biosynthesis of crucial enzymes of jasmonic acid are induced by NO. In Arabidopsis, it has been reported that NO induces expression of genes encoding several enzymes like GST, CHS (chalcone synthase), GPX (glutathione peroxidase), and AOX1a (alternative oxidase) which detoxify stress biomarkers and also down regulate ascorbate peroxidase (tAPX), present in thylakoid region (Huang et al. 2002). The SnRK2 kinase regulated by abscisic acid (ABA) and SRK2C/SnRK2.8 in Arabidopsis notified as a drought resistant factor in plants by up-regulating DREB1A/CBF3 which encodes transcription factor regulating those genes which are responsive under stress condition (Umezawa et al. 2004). In addition, to activate AREB1 and TRAB1 they are activated by phosphorylation of SnRK2 kinases in both Arabidopsis as well as rice plant, respectively (Kobayashi et al. 2005; Furihata et al. 2008) and regulate ABA-responsive genes. Beside this, osmotic stress facing cells might use NO as an initial signaling factor to promote the defensive pathway guided by SnRK2 (Courtois et al. 2008). Guo et al. (2003) have also observed that stomatal opening guided by lower light and resistivity towards drought in Arabidopsis was due to mutant nitrate transporter Chl1 gene. Similarly, Meyer et al. (2005) revealed the role of AtNOS1 which is dually involved in NO production and in ABA responsive closure of stomata in Arabidopsis. Groß et al. (2013) have also noticed that in wild type Arabidopsis plant, higher accumulation of SA, JA and ethylene occurred during pathogen attack. As a result of this, the endogenous accumulation of NO inside the cell ultimately suppresses the Hb1-coding gene GLB1 in order to resist plants against pathogen attack (Table 1).
NO and ROS crosstalk: nitric oxide-induced programmed cell death (PCD)
Reactive oxygen species (ROS) are mainly consequential result of a sequential processes involved in cellular metabolism (Halliwell and Gutteridge 2007). Sharma et al. (2012) considered their excessive accumulation as necessary evil and in excess caused deleterious effect on plants and animals. As Fig. 4 shows that plants possess an effective endogenous defensive mechanism including a wide network of antioxidant machinery which defends them from harm caused by ROS, and hence harmonizing their internal ratio (Wrzaczek et al. 2013). Figure 4 depicts a group of oxidative molecules/ions: (1) superoxide anion, (2) hydroxyl free radical, (3) hydrogen peroxide and (4) oxygen species having single electron are considered as ubiquitous ROS in cell (Gechev et al. 2006; Sharma et al. 2012; Singh et al. 2016). In plant cells, the major sites of oxidative biomarkers production are chloroplasts, mitochondria, and peroxisomes (Sharma et al. 2012; Kong et al. 2013; Sandalio et al. 2013; Singh et al. 2016). Previous studies have considered ROS as signaling molecules in many cellular processes (Mittler et al. 2011; Wrzaczek et al. 2013). Equilibrium of ROS is a crucial phenomenon i.e. production of ROS and their scavenging decide their fate as damaging or signaling molecules (Fig. 4). ROS level in cell triggers different signaling networks which depend on multiple aspects such as: (1) chemical individuality of ROS, (2) concentration of ROS, (3) signal intensity, (4) site of ROS production, (5) plant developing stage, and (6) crosstalk among ROS, hormones, and NO (Chaudhuri et al. 2013; Mor et al. 2014; Singh et al. 2016). Klepper (1979) had first time reported generation of NO within cell. Thereafter, Delledonne et al. (1998) reported NO as a key factor in Arabidopsis and tobacco which is involved in defense response. Although, NO may pause destructing effect of ROS depending on its rate and site of production (Beligni and Lamattina 1999). Crosstalk among NO and ROS decides the fate of cells under stress conditions and also determined the physiological phenomena inside the cell (Rodríguez-Serrano et al. 2009). In fact, targets of S-nitrosylation process i.e. attachment of NO to cysteine thiol within a protein to form an S-nitrosothiol (SNO) (Fig. 2) which operates the signaling for the action of antioxidant enzymes against ROS suggesting a perfect balance between NO and ROS (de Pinto et al. 2013; Romero-Puertas et al. 2013). NO has a short life time and considered as a free radical which limits its effect on surrounding environment. Apart from NO, S-nitrosylated glutathione (GSNO) is relatively a stable reservoir and transportable form of NO (Kovacs et al. 2016). GSNO level inside cell is either guided by its production or by enzymatic action catalyzed by GSNO reductase (GSNOR). Mutation in GSNOR gene caused deteriorated plant growth (Lee et al. 2008; Xu et al. 2013). The gsnor-ko plants contain increased level of S-nitrothiols (SNO) and nitroso species reflecting the controlled activity of GSNOR for both GSNO and protein-SNOs (Lee et al. 2008).
An active interaction between NO and ROS affects antioxidant defense system under abiotic stress. Under low stress condition, moderately produced ROS act as signals for NO synthesis that enhances antioxidant defense system to provide protection to stressed plants. Whereas under heavy stress situation, excessively produced ROS promotes production of RNS that tends to create imbalance in AOX and ROS. This imbalance causes cell damage or cell death
Programmed cell death (PCD) is an active plus genetically controlled phenomenon promoting the cell death in plants which includes cellular metabolic processes that occurs throughout plant life and considered as an essential process for normal development against biotic and abiotic stresses (van Doorn 2005; Gechev et al. 2006; Bozhkov and Lam 2011; Singh et al. 2016). Every cell completes its life cycle by a sequential event PCD which includes several peculiar proceedings: (1) transformed morphology of nucleus, (2) inflammation of vacuoles along with mitochondria, plus ER, (3) protoplast contraction, and (4) cytoskeleton reformation (Serrano et al. 2015). Additional demarcations are fragmentation of DNA, caspase mediated activity, and an increase of ROS and RNS (Xu and Huang 2017). According to de Pinto et al. (2012), ROS and NO are considered as the important component for PCD in plants. Plant develops a multifaceted response i.e. innate and immune which is collectively called as hypersensitivity (HR). This is mainly evolved to defend themselves against insects and microbial pathogen which can uniquely end in systemic acquired resistance (SAR) (Domingos et al. 2015). This was mainly regulated by increase in the Ca2+ level inside the cell through CNGC (cyclic nucleotide-gated ion channels) (Ali et al. 2007). CNGC is cGMP-mediated channel which is mainly regulated by peptide signaling molecule, AtPeps, and their receptor, AtPepR1 (Qi et al. 2010). Further, the increase in endogenous Ca2+ triggers salicylic acid (SA) (Yun et al. 2016), NO and ROS generation leads to PCD in the area of infection, in that way limits the pathogen growth (Domingos et al. 2015). Nitric oxide and H2O2 play crucial role in governing hypersensitivity (Grant and Loake 2000; Kovacs et al. 2016) (Table 1). Delledonne et al. (2001) mentioned in their work that cell death phenomena are determined by the ratio of NO to H2O2. Interestingly, tropospheric ozone (O3) induces ROS production and enhances the HR programme in the apoplast and thereby, considered as an ideal for regulation of end of life of cell by the initiating and proliferating the death signals (Overmyer et al. 2003). In animal cell, infection (also considered as HR) results in PCD phenomena and similar to the plants, NO and H2O2 are considered as responsible molecules for the same (Wang et al. 2013). Either of them could be responsible for functioning of cytochrome c and also for the regulation of signaling cascade guided by caspase leading to the hypersensitivity as coated by Mur et al. (2006) and Tan et al. (2013). Besides these, certain other significant machineries are also involved in defense signaling pathway i.e. activation of protein kinases (MAPKs) and phosphatases which are also influenced by ROS and NO and therefore, cumulative role of NO and H2O2 enhanced by the stimulation of central MAPK cascade against pathogen infection (Domingos et al. 2015). In plants, leaf senescence is also a consequence of PCD, which is considered as the ultimate leaf developmental phase which is controlled by age of organ and environmental conditions as conveyed by Jing et al. (2005). The work of Cui et al. (2013) verified that at senescence stage, the amount of H2O2 increases leading to cell death in Arabidopsis. Along with oxidizing properties, H2O2 also transduces the signals and regulates the gene expression involved in the senescence process. With this context, several senescence-associated genes (SAG) are characterized from Arabidopsis which is ROS-induced expressions of these genes as reported by Navabpour et al. (2003) clarifying the role of ROS in senescence. Apart from ROS, NO can provoke as well as hinder senescence process which dependents upon its amount and place of act (Wang et al. 2013). It may increase the ROS toxicity resulting in leaf senescence (Niu and Guo 2012). Kong et al. (2013) observed that H2O2 was implicated in NO-mediated PCD in maize.
Conclusions and future perspectives
To maintain all the primary and basic functions of the cell, NO manages an internal environment that pretends to change gene expression patterns and thus modulates chemical homeostasis of the cell. By this way, NO successfully makes a cell capable of alleviating impact of different stress. Nitric oxide suppresses Hb1 coded GLB1 genes during hypoxia and pathogen attack in Arabidopsis that clear its role in defense gene regulation and protein modifications which provide a base for further study in this area. Involvement of NO signaling in several post translational modifications also provide a significance to its crucial role in defense machinery. Further in defense mechanism, the NPR1/TGA interaction is critical for regulating responses by salicylic acid action (Astier and Lindermayr 2012). Few reports proposed the efficient role of NO in defense signaling, over the modulation of PCD mediated by ROS and some new assessments regarding organic and bio molecular information intended for this crucial functional intermediary component are also explained in Fig. 4. Some recent discoveries described plant NOSs that have little sequential similarities to their mammalian counterparts. Intrinsic NO production by pathways involved in different biosynthesis processes in varied cellular sections including different organelles, involving physiological, biochemical and molecular events in response to specific ecological stimulus is still having an area of curiosity. Although NO, is known for its extraordinary faith for all around growth and developmental processes, however, it is not only limited to plants but also applied for animals as well as various organisms like algae, bacteria, lichen, fungi etc. Therefore, multidimensional studies will be helpful for further researches regarding role of NO in plant biology. Future research involving investigation on intricate NO signaling network in plants will certainly witness exciting outcomes.
References
Alavi SM, Arvin MJ, Manoochehri KK (2014) Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J Plant Interact 9:683–688
Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19:1081–1095
Arasimowicz M, Floryszak-Wieczorek J, Milczarek G, Jelonek T (2009) Nitric oxide, induced by wounding, mediates redox regulation in pelargonium leaves. Plant Biol 11:650–663
Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A (2013) ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4:63
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:2273–2285
Astier J, Lindermayr C (2012) Nitric oxide-dependent posttranslational modification in plants: an update. Int J Mol Sci 13:15193–15208
Baudouin E (2011) The language of nitric oxide signalling. Plant Biol 13:233–242
Begara-Morales JC, Chaki M, Valderrama R, Sánchez-Calvo B, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB (2018) Nitric oxide buffering and conditional nitric oxide release in stress response. J Exp Bot 69:3425–3438
Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Beligni MV, Lamattina L (2001) Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci 6:508–509
Besson-Barda A, Courtoisa C, Gauthiera A, Dahana J, Dobrowolskab G, Jeandrozc S, Pugina A, Wendehenne D (2008) Nitric oxide in plants: production and cross-talk with Ca2 + signaling. Mol Plant 1:218–228
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Bethke PC, Libourel IGL, Jones RL (2006a) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57:517–526
Bethke PC, Libourel IGL, Reinöhl V, Jones RL (2006b) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223:805–812
Bozhkov PV, Lam E (2011) Green death: revealing programmed cell death in plants. Cell Death Differ 18:1239–1240
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Broniowska KA, Hogg N (2012) The chemical biology of S-nitrosothiols. Antioxid Redox Signal 17:669–680
Chakraborty N, Acharya K (2017) “NO way”! Says the plant to abiotic stress. Plant Gene 11:99–105
Chaudhuri A, Singh KL, Kar RK (2013) Interaction of hormones with reactive oxygen species in regulating seed germination of Vigna radiata (L.) Wilczek. J Plant Biochem Physiol 1:103
Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H, Näsholm T (2010) Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H(+)-ATPase and Na(+)/H(+) antiporter under high salinity. Tree Physiol 30:1570–1585
Corpas FJ, Palma JM (2018) Nitric oxide on/off in fruit ripening. Plant Biol 20:805–807
Corpas FJ, Chaki M, Leterrier M, Barroso JB (2009) Protein tyrosine nitration: a new challenge in plants. Plant Signal Behav 4:920–923
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D (2008) Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. J Exp Bot 59:155–163
Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A (2006) Response to Zemojtel et al.: plant nitric oxide synthase: back to square one. Trends Plant Sci 11:526–527
Cui MH, Ok SH, Yoo KS, Jung KW, Yoo SD, Shin JS (2013) An Arabidopsis cell growth defect factor-related protein, CRS, promotes plant senescence by increasing the production of hydrogen peroxide. Plant Cell Physiol 54:155–167
de Pinto MC, Locato V, De Gara L (2012) Redox regulation in plant programmed cell death. Plant, Cell Environ 35:234–244
de Pinto MC, Locato V, Sgobba A, Romero-Puertas MC, Gadaleta C, Delledonne M, De Gara L (2013) S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol 163:1766–1775
del Rio LA, Corpas FJ, Sandalio LM, Palma JM, Barroso JB (2003) Plant peroxisomes, reactive oxygen metabolism and nitric oxide. IUBMB Life 55:71–81
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci 98:13454–13459
Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99:16314–16318
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55:205–212
Domingos P, Prado AM, Wong A, Gehring C, Feijo JA (2015) Nitric oxide: a multitasked signaling gas in plants. Mol Plant 8:506–520
Dordas C, Hasinoff BB, Rivoal J, Hill RD (2004) Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell suspension cultures. Planta 219:66–72
Du ST, Zhang YS, Lin XY, Wang Y, Tang CX (2008) Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant, Cell Environ 31:195–204
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Fares A, Rossignol M, Peltier JB (2011) Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem Biophys Res Commun 416:331–336
Fernández M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)- dependent acropetal auxin transport. Proc Natl Acad Sci USA 108:18506–18511
Fernández-Marcos M, Sanz L, Lorenzo O (2012) Nitric oxide: an emerging regulator of cell elongation during primary root growth. Plant Signal Behav 7:196–200
Flores-Pérez Ú, Sauret-Güeto S, Gas E, Jarvis P, Rodríguez-Concepción M (2008) A mutant impaired in the production of plastome-encoded proteins uncovers a mechanism for the homeostasis of isoprenoid biosynthetic enzymes in Arabidopsis plastids. Plant Cell 20:1303–1315
Freschi L (2013) Nitric oxide and phytohormones interactions: current status and perspectives. Front Plant Sci 4:198. https://doi.org/10.3389/fpls.2013.00398
Freschi L, Rodrigues MA, Domingues DS, Purgatto E, Van Sluys MA, Magalhaes JR, Kaiser WM, Mercier H (2010) Nitric oxide mediates the hormonal control of Crassulacean acid metabolism expression in young pineapple plants. Plant Physiol 152:1971–1985
Fröhlich A, Durner J (2011) The hunt for plant nitric oxide synthase (NOS): is one really needed? Plant Sci 181:401–404
Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozak K (2008) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103:1988–1993
Galatro A, Puntarulo S (2014) An update to the understanding of nitric oxide metabolism in plants. In: Khan MN, Mohammad MMF, Corpas FJ (eds) Nitric oxide in plants: metabolism and role in stress physiology. Springer, Switzerland, pp 3–16
Garcês H, Durzan D, Pedroso MC (2001) Mechanical stress elicits nitric oxide formation and DNA fragmentation in Arabidopsis thaliana. Ann Bot 87:553–707
Garcia-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128:790–792
García-Mata C, Lamattina L (2007) Abscisic acid (ABA) inhibits light induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide 17:143–151
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100:11116–11121
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124:21–29
Groß F, Durner J, Gaupels F (2013) Nitric oxide, antioxidants and prooxidants in plant defense responses. Front Plant Sci 7:419. https://doi.org/10.3389/fpls.2013.00419
Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302:100–103
Halliwell B, Gutteridge J (2007) What is an antioxidant? Freeradic Biol Med 4:79–186
Hao GP, Zhang JH (2010) The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In: Hayat S, Mori M, Pichtel J, Ahmad A (eds) Nitric oxide in plant physiology. Wiley-VCH Verlag, Weinheim, pp 115–138
Hossain MA, Ye W, Munemasa S, Nakamura Y, Mori IC, Murata Y (2014) Cyclic adenosine 5′-diphosphoribose (cADPR) cyclic guanosine 3′,5′-monophosphate positively function in Ca2 + elevation in methyl jasmonate-induced stomatal closure, cADPR is required for methyl jasmonate-induced ROS accumulation NO production in guard cells. Plant Biol 16:1140–1144
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Huang X, von Rad U, Durner J (2002) Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215:914–923
Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J (2004) Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 218:938–946
Jeandroz S, Wipf D, Stuehr DJ, Lamattina L, Melkonian M, Tian Z, Zhu Y, Carpenter EJ, Wong GK, Wendehenne D (2016) Occurrence, structure, and evolution of nitric oxide synthase-like proteins in the plant kingdom. Plant Biol 9:417
Ji Y, Liu J, Xing D (2016) Low concentrations of salicylic acid delay methyl jasmonate-induced leaf senescence by up-regulating nitric oxide synthase activity. J Exp Bot 67:280
Jing HC, Schippers JH, Hille J, Dijkwel PP (2005) Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J Exp Bot 56:2915–2923. https://doi.org/10.1093/jxb/eri287
Klepper L (1979) Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmos Environ 13:537–542
Klessig D, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y, Pontier D, Lam E, Silva H (2000) Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA 97:8849–8855
Kobayashi Y, Murata M, Minami H, Yamamoto S, KagayaY Hobo T, Yamamoto A, Hattori T (2005) Abscisic acid-activated SNRK2 protein kinases function in the generegulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J44:939–949
Kolbert Z, Feigl G, Bordé Á, Molnár Á, Erdei L (2017) Protein tyrosine nitration in plants: present knowledge, computational prediction and future perspectives. Plant Physiol Biochem 113:56–63
Kong X, Zhang D, Pan J, Zhou Y, Li D (2013) Hydrogen peroxide is involved in nitric oxide-induced cell death in maize leaves. Plant Biol 15:53–59
Kovacs I, Holzmeister C, Wirtz M, Geerlof A, Fröhlich T, Römling G, Kuruthukulangarakoola GT, Linster E, Hell R, Arnold GJ, Durner J, Lindermayr C (2016) ROS-mediated inhibition of s-nitrosoglutathione reductase contributes to the activation of anti-oxidative mechanisms. Front Plant Sci 7:1669
Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebun-Garcia A, Durner J, Pugin A, Wendehenne D (2004) Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol 135:516–529
Lanteri ML, Pagnussat GC, Lamattina L (2006) Calciumand calcium-dependent protein kinases are involved in nitric oxide and auxin-induced adventitious root formation in cucumber. J Exp Bot 57:1341–1351
Lee U, Wie C, Fernandez BO, Feelisch M, Vierling E (2008) Modulation of nitrosative stress by S-nitroso glutathione reductase is critical for thermo tolerance and plant growth in Arabidopsis. Plant Cell 20:786–802
Leshem Y, Pinchasov Y (2000) Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Duch.) and avocados Persea americana (Mill.). J Exp Bot 51:1471–1473
Li H, Poulos TL (2005) Structure–function studies on nitric oxide synthases. JInorg Biochem 99:293–305
Libourel IGL, Bethke PC, De Michele R, Jones RL (2006) Nitric oxide gas stimulates germination of dormant Arabidopsis seeds: use of a flow through apparatus for delivery of nitric oxide. Planta 223:813–820
Liu F, Guo F (2013) Nitric oxide deficiency accelerates chlorophyll breakdown and stability loss of thylakoid membranes during dark-induced leaf senescence in Arabidopsis. PLoS ONE 8(2):e56345. https://doi.org/10.1371/journal.pone.0056345
Liu YG, Shi L, Ye NH, Liu R, Jia WS, Zhang JH (2009) Nitric oxide induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 183:1030–1042
Liu WZ, Kong DD, Gu XX, Gao HB, Wang JZ, Xia M (2013) Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proc Natl Acad Sci USA 110:1548–1553. https://doi.org/10.1073/pnas.1213235110
Liu Y, Yang X, Zhu S, Wang Y (2016) Postharvest application of MeJA and NO reduced chilling injury in cucumber (Cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol Technol 119:77–83
Lombardo MC, Graziano M, Polacco JC, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33
Lozano-Juste J, León J (2011) Nitric oxide regulates DELLA content and PIF expression to promote photo-morphogenesis in Arabidopsis. Plant Physiol 156:1410–1423
Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J (2010) Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol 51:1612–1626
Meyer C, Lea US, Provan F, Kaiser WM, Lillo C (2005) Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res 83:181–189
Mioto PT, Mercier H (2013) Abscisic acid and nitric oxide signaling in two different portions of detached leaves of Guzmania monostachia with CAM up-regulated by drought. J Plant Physiol 170:996–1002
Mishina TE, Lamb C, Zeier J (2007) Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant, Cell Environ 30:39–52
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mohn MA, Thaqi B, Fischer-Schrader K (2019) Isoform-specific NO synthesis by Arabidopsis thaliana nitrate reductase. Plants 8:67
Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R (2014) Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol 165:249–261
Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem 283:32957–32967
Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140:249–262
Navabpour S, Morris K, Allen R, Harrison E, Mackerness SAH, Buchanan-Wollaston V (2003) Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot 54:2285–2292
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
Niu YH, Guo FQ (2012) Nitric oxide regulates dark-induced leaf senescence through EIN2 in Arabidopsis. J Integr Plant Biol 54:516–525
Ötvös K, Pasternak TP, Miskolczi P, Domoki M, Dorjgotov D, Szucs A, Bottka, Dudits D, Fehér A (2005) Nitric oxide is required for and promotes auxin-mediated activation of cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J 43:849–860
Overmyer K, Brosché M, Kangasjärvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8:335–342
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Pedroso MC, Magalhaes JR, Durzan D (2000) A nitric oxide burst precedes apoptosis in angiosperm and gymnosperm callus cells and foliar tissues. J Exp Bot 51:1027–1036
Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T (2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7:21
Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. PNAS 107:21193–21198
Qian H, Chen W, Li J, Wang J, Zhou Z, Liu W, Fu Z (2009) The effect of exogenous nitric oxide on alleviating herbicide damage in Chlorella vulgaris. Aquat Toxicol 92:250–257
Qiao W, Li C, Fan L (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93
Rasul S, Wendehenne D, Jeandroz S (2012) Study of oligogalacturonides-triggered nitric oxide (NO) production provokes new questioning about the origin of NO biosynthesis in plants. Plant Signal Behav 7:1031–1033
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Rodríguez-Serrano M, Romero-Puertas MC, Sparkes I, Hawes C, del Río LA, Sandalio LM (2009) Peroxisome dynamics in Arabidopsis plants under oxidative stress induced by cadmium. Free Radic Biol Med 47:1632–1639
Romanov GA, Lomin SN, Rakova NY, Heyl A, Schmülling T (2008) Does NO play a role in cytokinin signal transduction?. FEBS Lett 582:874–880
Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM (2013) Protein S-nitrosylation in plants under abiotic stress: an overview. Front Plant Sci 4:373
Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA (2013) Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Subcell Biochem 69:231–255
Santolini J, Andre F, Jeandroz S, Wendehenne D (2017) Nitric oxide synthase in plants: where do we stand? Nitric Oxide 63:30–38
Serrano I, Romero-Puertas MC, Sandalio LM, Olmedilla A (2015) The role of reactive oxygen species and nitric oxide in programmed cell death associated with self in-compatibility. J Exp Bot 66:2869–2876
Shabbir RN, Waraich EA, Ali H, Nawaz F, Ashraf MY, Ahmad R, Awan MI, Ahmad S, Irfan M, Hussain S, Ahmad Z (2016) Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.). Environ Sci Pollut Res 23:2651–2662
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM (2016) Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Front Plant Sci 7:1299. https://doi.org/10.3389/fpls.2016.01299
Singh R, Parihar P, Singh S, Singh MPVVB, Singh VP, Prasad SM (2017) MicroRNAs and nitric oxide cross talk in stress tolerance in plants. Plant Growth Regul 83:199–205
Tan J, Zhuo C, Guo Z (2013) Nitric oxide mediates cold and dehydration-induced expression of an ovel MfHyPRP that confers tolerance to abiotic stress. Physiol Planta. https://doi.org/10.1111/ppl.12032
Tiwari S, Verma N, Singh VP, Prasad SM (2019) Nitric oxide ameliorates aluminium toxicity in Anabaena PCC7120: regulation of aluminium accumulation, exopolysaccharides secretion, photosynthesis and oxidative stress markers. Environ Exp Bot 161:218–227
Tonón C, Terrile CM, Iglesias MJ, Lamattina L, Casalongué C (2010) Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyls growth in etiolated Arabidopsis seedlings. J Plant Physiol 167:540–546
Tossi V, Lamattina L, Jenkins GI, Cassia RO (2014) Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol 164:2220–2230
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EI, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Tun NN, Livaja M, Kieber JJ, Scherer GF (2008) Zeatin-induced nitric oxide (NO) biosynthesis in Arabidopsis thaliana mutants of NO biosynthesis and of two-component signaling genes. New Phytol 178:515–531
Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K (2004) SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proc Natl Acad Sci USA 101:17306–17311
Van Doorn WG (2005) Plant programmed cell death and the point of no return. Trends Plant Sci 10:478–483
Wang Y, Loake G, Chu C (2013) Cross-talk of nitric oxide and reactive oxygen species in plant programmed cell death. Front Plant Sci 4:314
Wendehenne D, Pugin A, Klessig D, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6:177–183
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signaling and defence responses. Curr Opin Plant Biol 7:449–455
Wrzaczek M, Brosché M, Kangasjärvi J (2013) ROS signaling loops: production, perception, regulation. Curr Opin Plant Biol 16:575–582
Xiao-Ping S, Xi-Gui S (2006) Cytokinin and auxin induced stomatal opening is related to the change of nitric oxide levels in guard cells in broad bean. Physiol Plant 128:569–579. https://doi.org/10.1111/j.1399-3054.2006.00782
Xu W, Huang W (2017) Calcium-dependent protein kinases in phytohormone signaling pathways. Int J Mol Sci. https://doi.org/10.3390/ijms18112436
Xu S, Guerra D, Lee U, Vierling E (2013) S-nitroso glutathione reductases are low-copy number, cysteine-rich proteins in plants that control multiple developmental and defense responses in Arabidopsis. Front Plant Sci 4:430
Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468:89–92
Yun BW, Skelly MJ, Yin M, Yu M, Mun BG, Lee SU, Hussain A, Spoel SH, Loake G (2016) Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol 21:516–526
Zemojtel T, Fröhlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, Durner J (2006) Plant nitric oxide synthase: a never-ending story? Trends Plant Sci 11:524–525
Acknowledgements
Nidhi Verma and Santwana Tiwari are grateful to the University Grants Commission, New Delhi for granting D.Phil. Scholarship. Dr. Vijay Pratap Singh is obliged to the Department of Biotechnology, New Delhi (BT/PR12980/BPA/118/80/2015) for providing financial assistance. Professor Sheo Mohan Prasad is pleased to the SERB-DST, New Delhi (EMR/2016/004745) for providing financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, N., Tiwari, S., Singh, V.P. et al. Nitric oxide in plants: an ancient molecule with new tasks. Plant Growth Regul 90, 1–13 (2020). https://doi.org/10.1007/s10725-019-00543-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00543-w