Abstract
Leukemia is a complex disease that is associated with several causes, one of which is viral infection. Human T-lymphotropic virus (HTLV) is the most studied virus and is associated with human leukemia. The epidemiology of HTLV-1 has been under investigation in several countries and is now well known. In this context Latin America has shown a high prevalence. Virus family proteins such as Tax and HBZ modulate several signaling pathways that modulate the biological activities of the cell, including cell growth and proliferation, which affect the physiology and immunology of the cell. In this chapter we analyze the most frequent mechanisms induced by HTLV-1 that affect cell proliferation and the immune response to viral infection. The effects on these processes can lead to cell transformation and avoidance of immune recognition of the virus in affected cells. The epidemiological, molecular, and immunological characteristics of HTLV-1 virus involved in leukemia in humans are reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Several physical, chemical, and biological agents can trigger the mechanisms leading to the development of leukemia. Viral infection is one important cause (Graves 2006). This chapter outlines the role of viral infection, its epidemiology, and the mechanisms associated with the proteins of human T-lymphotropic virus (HTLV-1) that can lead to leukemia.
General Characteristics of HTLV
The discovery of HTLV-1 was published in 1980. The first report described how T cells from patients with T-cell leukemia were cultured and analyzed by reverse transcription. Viral particles were identified by electronic microscopy, and the presence of antibodies in infected patients and the ability of the virus to integrate into DNA were reported (Poiesz et al. 1980). There are four types of HTLV, but only HTLV-1 is associated with leukemia. This virus belongs to the Retroviridae family, Orthoretrovirinae subfamily, genus Deltaretrovirus. As species, HTLVs are classified as lymphotropic viruses (Poiesz et al. 1980).
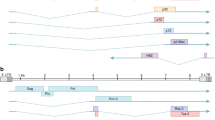
HTLV-1 is an enveloped virus with a single-stranded RNA. Its genome is reverse transcribed, and subsequent alternative splicing gives rise to at least nine different messenger RNAs, all of which encode the viral structural and functional proteins. One of the proteins that take part in the induction of leukemia is encoded in open reading frame (ORF) IV. This protein is called Tax (transactivator of the region X) (Fig. 3.1). HBZ (basic leucine zipper), or b-zipper protein (b-ZIP), is encoded in one antisense RNA (Poiesz et al. 1980).
The possibility of viral infections causing leukemia was first proposed in the nineteenth century. However, this was not confirmed until 1908 when Ellerman and Bang demonstrated that Jaagsiekte sheep retrovirus could induce erythroleukemia in chickens. Subsequently, the discovery of new infectious agents with the ability to induce leukemia in animals rekindled the debate about the roles of viral infections as causes of leukemia (Greaves 2006). The first evidence of a link between leukemia and viral infections in primates was reported in the 1970s, when Kawakami et al. discovered the gibbon ape leukemia virus and demonstrated its association with myeloid leukemia. Subsequently, Gallo et al. identified a variant of this virus that caused leukemia in gibbon T cells. In 1972, Sarngadharan et al. measured the activity of viral reverse transcriptase in patients with lymphoblastic acute leukemia, but the virus could not be isolated. The identification of HTLV was not possible until 1980, when stabilized T-cell cultures were obtained from patients with T-cell leukemia that exhibited reverse transcription, and the viral particles were detected by electron microscopy. The association was demonstrated according to Koch’s postulates, and specific antibodies were detected in infected patients (Poiesz et al. 1980).
Four types of HTLV have been identified, but only HTLV-1 is associated with leukemia. HTLVs belong to the family Retroviridae, subfamily Orthoretroviridae, genus Deltaretrovirus (International Committee on Taxonomy of Viruses 2012).
HTLV-1 is an enveloped virus with a single linear RNA genome; it comprises one coding region with four ORFs flanked by a large terminal repeat region and a terminal region called pX (Lairmore and Franchini 2007). The proteins are encoded as follows (from 5′ to 3′): GAG, Pro-Pol precursors, ENV protein, functional proteins, expression regulatory proteins, accessory proteins, and others with unknown functions (Francesconi do Valle et al. 2001) (Fig. 3.1).
Epidemiology
HTLV-1 was the first human retrovirus to be isolated, and its association with leukemia has been clearly demonstrated. HTLV-1 is distributed throughout the world and its epidemiology has been well characterized in some countries. In China, a large cross-sectional study of 5,417 individuals detected HTLV-1 in 0.13 % of samples obtained from donors with hematological malignancies, where a high-risk group included patients who were positive for human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus (HCV), or Treponema pallidum. Most of the high-risk patients were positive for HTLV-1, and it was suggested that HTLV-1 infections may occur via coinfection. In addition, it was suggested that HTLV-1 is not endemic to China (Ma et al. 2013). In Israel, another study involving a cohort of patients who donated blood over a period of 14 years showed that 0.005 % were HTLV-1 carriers, i.e., 90 who were positive for HTLV-1, including 6 who were diagnosed with a malignancy, 3 of whom developed leukemia. Thus, according to that study, only in 0.37 % of HTLV-1 was involved with leukemia. The authors suggested that their results were high compared with previous studies because they employed a very long follow-up period (Stienlauf et al. 2013). A study in Japan based on a cohort of 272,043 blood samples obtained from a regional blood bank also detected a high prevalence of HTLV-1, where the seroprevalence was higher in females than in males (2.05 % and 1.80 %, respectively). Furthermore, the seroprevalence was higher in older patients in comparison with either males or females. The role of age in the transmission of HTLV-1 has been analyzed in the context of sexual activity and pregnancy, where it has been shown that the prevalence of HTLV-1 infection increased with the age of pregnancy, and the risk of vertical transfer from the mother to newborns also increased with age (Eshimaa et al. 2009). Intrafamilial transmission and the factors involved in the acquisition of HTLV-1 infection in pregnant women were studied in Brazil, where the prevalence was found to be 1.05 % in a group of 2,766 pregnant women. An analysis of families within this group indicated that 32.6 % showed reactivity, but there were low associations with the level of education, age, or ethnic group (Gomes Mello et al. 2014). In Spain, a study of 6,460 subjects detected a prevalence of 0.06 %, but the authors suggested that the seroprevalence is actually lower in Spain because most of the HTLV-1-positive patients came from Latin America and Africa (Treviño et al. 2012). Some studies performed in Latin America have reported high prevalence rates. For example, a study in Peru comprising 638 subjects from 27 indigenous communities detected an HTLV-1 prevalence of 1.9 %, although the prevalence was 4.1 % in one community, thereby demonstrating its high prevalence in some indigenous populations in Latin America (Alva et al. 2012). In addition, there is a frequent association between HTLV-1 and coinfection with other viruses in drug users, e.g., coinfection with HTLV-1/-2, HIV, and HCV, although the triple coinfection rate was low (0.8 %) (Prasetyo et al. 2013). This suggests that some lifestyles, such as drug use, are risk factors for the acquisition of HTLV-1 infection.
HTLV-1 and Leukemia
Despite the oncogenic activity of retrovirus being observed previously in several animal species (Gallo and Todaro 1976), the correlation between HTLV-1 and oncogenicity was not cleared in humans until 1980, when viral particles were proved in HUT-102 and CTCL-3 cell lines derived from the lymph node and in fresh peripheral blood lymphocytes of one patient T-cell lymphoma (Reitz et al. 1981). Recent studies show that adult T-cell leukemia/lymphoma occurs in ~5 % of HTLV-1-infected individuals (Cook et al. 2014; Akinbami et al. 2014). Although such a correlation appears low, more than frequency of emergence, some molecular mechanisms which involve host and viral interaction appear to be more closely associated. For example, clonality has been more associated with other diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), although in leukemia such a relation is still not clear, and further research is necessary to discover the role of such mechanism in the development of leukemia induced by HTLV-1 (Bangham et al. 2014). In addition, adult T-cell leukemia has been more frequent in geographical regions where HTLV-1 is more frequent, and age appears to be an important factor because leukemia associated with HTLV-1 is frequent in adults but not in children. Only a very small number of isolated cases have been identified in children, e.g., a 15-year-old adolescent in Brazil who developed a lymphoma of T cells after which HTLV-1 viral infection was confirmed (Francesconi do Valle et al. 2001).
Some of the molecular mechanisms by which HTLV is able to induce leukemia are known, but other mechanisms underlying the role of viral infection are not completely clear. Of the viruses known to cause leukemia, HTLV is perhaps the most representative and the virus that is most known. Genotype 1 exhibits several viral proteins that are involved in the mechanisms that lead to cell transformation (Jun-ichirou and Matsuoka 2007). Two of these proteins, Tax and HBZ, are now discussed further.
Tax
This protein is well recognized as an oncoprotein. Its role lies in the transactivation of viral transcription through its interaction with the 5′ long terminal repeat of HTLV-1 (Felber et al. 1985), although it can also transactivate transcription. Tax interacts with transcription factors such as cAMP response element-binding protein (CREB) to produce a ternary complex, which regulates the cell cycle machinery (Tie et al. 1996). Once the virus has control of the cell, several mechanisms induced by Tax lead to cell immortalization (Fig. 3.2). These processes are described as follows:
-
(a)
p53 function is silenced through a mechanism that is independent of nuclear factor (NF)-κB (Jeang et al. 1990).
-
(b)
Antiapoptotic proteins such as Bfl-1, a member of the BCL2 protein family, are expressed. Such proteins have been shown to contribute to the survival of HTLV-1-infected cells (Ressler et al. 1997).
-
(c)
Tax-2 causes permanent arrest of the cell in G1, leading to failure of the G1 checkpoint, which can contribute to a nucleotide excision repair deficiency, leading to genomic instability (Tie et al. 1996).
-
(d)
Genomic stability is reduced. Some studies have identified an association between Tax and genomic stability and have provided evidence that Tax reduces genomic stability by downregulating human polymerase β, which is involved in DNA repair and blocks the repair of cellular damage (Ressler et al. 1997).
-
(e)
Tax can transactivate the proliferating cell nuclear antigen (PCNA) promoter and transform infected HTLV-1 cells, leading to changes in the expression of PCNA protein, which is involved in the regulation of cell proliferation and DNA replication and repair (Ressler et al. 1997). Tax also reduces the level of histones by uncoupling replication-dependent histone gene expression and DNA replication. Histones can also be acetylated by Tax recruitment of the cellular coactivator CBP/p300 (Nyborg et al. 2010).
HBZ
In an in vitro model using T lymphocytes, HBZ was shown to support cell proliferation. The mechanism involves p65, a member of the NF-κB protein family. HBZ alters p65 activity by decreasing its affinity for DNA. HBZ also increases the expression of PDLIM2, which encodes a cell ubiquitin that is responsible for the degradation of p65 (Takashi et al. 2007; Tiejun et al. 2009; Turvey and Broide 2010).
Immunological Mechanisms Involved in Viral Leukemia
The innate immune response is the first line of host defense against viral infection. Once activated, the innate immune response serves two functions: (1) the production of effector molecules, which restrict the viral infection, and (2) the initiation of the acquired immune response, which leads to the complete elimination of the pathogen from the infected cells (Turvey and Broide 2010). One aspect of the innate immune response is the family of molecular receptors known as pattern-recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs). The interaction of PRRs with PAMPs is essential for triggering the effector mechanisms of the innate immune response (Kumar et al. 2011). During viral infection, the innate immune system recognizes viral nucleic acids (DNA or RNA, either single-stranded or double-stranded) as PAMPs and viral glycoproteins (Lester and Li 2014).
Three important classes of PRRs have been identified recently: the toll-like receptors (TLRs), the cytoplasmic proteins (NRLs) (Kawai and Akira 2011), and the retinoic-inducible gene 1-like receptors (RLRs) (Journo and Mahieux 2011). These molecules participate in different aspects of signaling that lead to the activation of transcription factors such as NF-κB. Such factors are important for the synthesis of proinflammatory cytokines, chemokines, and effector molecules such as type 1 interferons (IFNs), which contribute to the elimination of viral components and apoptosis of infected cells (Colisson et al. 2010). RLRs activate the inflammasome complex, which plays an essential role in the antiviral response (Colisson et al. 2010). Several families of viruses are associated with PRR activation, including HTLV-1, the human retrovirus associated with leukemia of T cells.
The first data on direct binding between HTLV-1 and PRRs from the innate immune system were reported in an in vitro model of infected plasmacytoid dendritic cells (pDCs) (Fig. 3.3). A strong response was observed for the production of IFN-α, which was dependent on the TLR7 receptor (Kane et al. 2011). The addition of an inhibitor of TLR7 (oligonucleotide A151) and acidification using chloroquine contributed to the proposed binding of HTLV-1 to TLR7 (Kane et al. 2011). Other viruses that cause tumors are also related to TLR7, such as the mouse mammary tumor virus (Kane et al. 2011).
Recognition of HTLV-1 by innate immune response. A critical component of the immune response to the viral infection involves the detection of viral PAMPS (RNAs) by PRRs, which are activated and induce activation of interferons. TLR7 localized endosomally recognizes nucleic acid and begins the way of interferon production in this mechanism take part IRF7 (interferon regulatory factor 7) and TLR3 independent way of MyD88
In addition, the immune response acquired involved in the late phase includes both humoral and cellular mechanisms. The effector molecules of the humoral response (antibodies) prevent the viral dissemination from the infected cells toward the cells of adjacent tissues, whereas the cytotoxic cells (CTLs) remove the infected cells by induction of apoptosis. The antibody response to the protein Tax of HTLV-1 was reported in 2002 (Levin et al. 2002). Such viral antigens induce a cross-linking of the heterogeneous ribonucleoprotein. It has been suggested that such a mechanism is involved as a form of molecular mimicry in HTLV-1 infection. In disorders such as HAM/TSP, it has been proposed that the anti-Tax produced can have an important role in the inflammation mechanisms in lesions and tissues even up to the blood-brain barrier, as well as the releasing of autoantigens. HAM/TSP disease shows a high number of immunoglobulin M antibodies with dominant reactivity to four immunodominant epitopes of the Tax protein. Other antibodies detected include envelope proteins with the ability to neutralize viral activity (Tanaka et al. 1994). CTLs with specific activity to HTLV-1 have also been reported (Bangham 2000). It has been proposed that high avidity of antibodies and the lytic efficiency of these cells might correlate with the viral load and be crucial in the outcome of the HTLV-1 infection (Kattan et al. 2009). In addition, the common antigen of HTLV-1 is recognized for CD4+ cells specific to HTLV-1 (Sakaguchi et al. 2008).
Immune Evasion
HTLV-1 uses several strategies to evade the immune response (Fig. 3.4), all of which involve blocking of cell signaling. One strategy is to interfere with the signaling pathway leading to IFN-1 production even during a strong immune response (Olière et al. 2011; Saha et al. 2010). Some studies suggest that Tax protein is taking part in the immune evasion by obstruction of the signal of transduction of IFN-γ. Other proteins of HTLV-1 also take part; for example, the HBS protein inhibits the effector activity of CD4 affecting the cytokine production of TH1, leading to an immunosuppressive effect. Other immune mechanisms that contribute to HTLV-1 pathogenesis are cell immortality and viral persistence, which allow the virus to remain within a patient for a long time, often without producing symptoms. The proviral genome expresses several proteins, of which Tax and HBZ are considered the most important to viral pathogenesis and persistence (Peloponese et al. 2006; Nyborg et al. 2010). Tax is a nuclear protein of 40 kDa that is encoded in ORF X-IV. This protein is an activator of transcription that exerts pleiotropic effects on the interactions of several signaling pathways (Jaworski et al. 2014). Tax is detectable primarily in the nucleus but can be detected in the cytoplasm and is one of the main oncogenic determinants of HTLV-1. This protein upregulates the transcription of NF-κB, which leads to deregulation of important genes controlling cell growth and signal transduction such as cytokines, growth factors, cytokine receptors, proto-oncogenes, and antiapoptotic proteins involved in the kinase signaling cascades. Tax also downregulates the tumor-suppressor proteins p53 and Rb (Kastan et al. 1992).
Downregulation of pathways involved in immortalization of cells infected by oncogenic viruses. The infection of oncogenic virus leads to the immortalization of infected cells by deregulation of different pathways involved in cellular homeostasis, including immune escape by the synthesis of oncogenic proteins
HBZ, another important protein involved in the regulation of viral transcription, acts by inhibiting and activating cellular genes (Kastan et al. 1992; Stoppa et al. 2012; Tomita et al. 2007). This protein interacts with p65 and can degrade or sequestrate c-Jun and disrupt IFN-β (Table 3.1). The dual functions of Tax and HBZ can modulate the direct or indirect signals of the PRRs, which limit the production of HTLV-1 ligands such as viral proteins and nucleic acids. The manipulation of the immune mechanisms associated with HTLV-1 is attributed to p30 and p12 proteins. These proteins are essential for the productive infection of monocyte-derived dendritic cells. The role of p12 protein in the viral cycle is not clear, although some in vitro studies have suggested that this protein participates in the maintenance of viral infection. For HTLV-1, la p12 binds to MHC class I and prevents its expression and maturation, leading to the infected cell escaping recognition (Table 3.1) (Satou and Matsuoka 2012). The viral protein p30 can also modulate innate immunity. Research using the THP-1 macrophage line has shown that p30 disturbs the signaling of TLR4. This pathway is critical to the innate immune system’s response to bacterial infection, and p30 inhibits the production of cytokines normally secreted under TLR4 stimulation (Chan et al. 2013). Such disturbance of TLR4 induced by the p30 protein is mediated by the dependent interaction of inhibition of the transcription factor PU.1 (Table 3.1). p30 protein can also inhibit the proinflammatory cytokines by causing an increase in the release of interleukin-10, thereby interfering in the balance between the pro- and anti-inflammatory cytokine responses to bacterial infection. This may explain why some patients with adult T-cell leukemia show immunodeficiency and susceptibility to bacterial infections and suggests that p30 may be a therapeutic target (Fenizia et al. 2014).
HTLV-1 infection can also affect the acquired immune response. It has been reported that p12 CD4+ can induce protection again the cytotoxicity of natural killer cells (Datta et al. 2006). HTLV-1 primary infection of CD4+ cells can induce the downregulation of MHC-1, thus affecting effector T, memory, and regulatory cells (Fig. 3.4). Several studies have reported that HBZ induces the expression of Foxp3 by modulating transforming growth factor β signaling, which increases the expression of factors that can change the population phenotype of CD4+ cells, compromises cellular immunity, and suppresses the release of Th1 cytokines (Johnson et al. 2001).
In addition, the regulatory complex involved in the generation and migration of regulatory T cells that express Foxp3 protein has been associated with modifications of the reprogramming system of these CD4+, CD25+, and CCR4+ cells. This can lead to reduced expression of Foxp3, which is required to suppress inflammation, suggesting that HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells (Figs. 3.4 and 3.5) (Miyazato and Matsuoka 2014; Sugata et al. 2012; Toulza et al. 2010). The flexibility of differentiation in the programming of CD4+ T cells as part of the adaptive immune response has been recently associated with the pathogenesis of inflammatory diseases (Araya et al. 2014; Ishida and Ueda 2011; Miyazato and Matsuoka 2014; Murphy and Stockinger 2010; Sugata et al. 2012; Toulza et al. 2010). Moreover, the propagation of and pathological damage caused by HTLV-1 involve both the innate and adaptive immune systems. This may explain the long persistence and immune evasion by this virus.
Regulation of the immune response acquired by HTLV-1. The cellular tropism of HTLV-1 involves several types of cells. The lymphocyte CD4+ is the target of HTLV-1. HTLV-1 downregulates the acquired immune response by changing the expression of the protein FOX P3, which involves to the protein HBZ and transforming growth factor TGF-ß. For leading to immunosuppression
Conclusion
The epidemiology of HTLV-1 has become clearer in the preceding years. The knowledge of geographical distribution, risk factors involved in acquiring the viral infection, and its role in human viral leukemia are important tools in the prevention and treatment of HTLV-1 viral infection and its clinical implications. In addition, it is now accepted that viral infection, specifically HTLV-1, is a cause of human leukemia. Several mechanisms triggered during the viral replication are associated with proteins such as Tax and HBZ, which can affect cell functions to maintain the survival of the infected cell. However, such effects induce molecular disorders that alter the cell cycle, apoptosis, or immune responses and thus can lead to leukemia. Future research to extend our knowledge about the biology of these proteins is needed to determine whether they are also potential therapeutic targets.
References
Akinbami A, Durojaiye I, Dosunmu A, et al. Seroprevalence of human T-lymphotropic virus antibodies among patients with lymphoid malignancies at a tertiary center in Lagos, Nigeria. J Blood Med. 2014;5:169–74.
Alva I, Orellana R, Blas M, et al. HTLV-1 and -2 infections among 10 indigenous groups in the Peruvian Amazon. Am J Trop Med Hyg. 2012;87(5):954–6.
Araya N, Sato T, Ando H, et al. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J Clin Invest. 2014;124(8):3431–42.
Bangham C. The immune response to HTLV-I. Curr Opin Immunol. 2000;12(4):397–402.
Bangham C, Cook L, Melamed A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin Cancer Biol. 2014;26:89–98.
Boxus M, Willems L. Mechanisms of HTLV-1 persistence and transformation. Br J Cancer. 2009;101(9):1497–501.
Chan C, Siu Y, Kok K, et al. p21-activated kinases facilitate Tax-mediated transcriptional activation of the human T-cell leukemia virus type 1 long terminal repeats. Retrovirology. 2013;26:10–47.
Colisson R, Barblu L, Gras C, et al. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115(11):2177–85.
Cook LB, Melamed A, Niederer H, et al. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123(25):3925–31.
Datta A, Sinha-Datta U, Dhillon N, et al. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J Biol Chem. 2006;281(33):23414–24.
Eshimaa N, Iwata O, Iwata S, et al. Age and gender specific prevalence of HTLV-1. J Clin Virol. 2009;45:135–8.
Felber B, Paskalis H, Kleinman-Ewing C. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–9.
Fenizia C, Fiocchi M, Jones K, et al. Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells. J Virol. 2014;88(1):393–402.
Francesconi do Valle A, Gutierrez Galhardo, Celestino Leite A. Adult T-cell leukemia/lymphoma associated with HTLV-1 infection in a Brazilian adolescent. Rev Inst Med Trop S Paulo. 2001;43(5):283–6.
Gallo R, Todaro G. Oncogenic RNA viruses. Semin Oncol. 1976;8–95.
Gomes Mello M, Ferreira da Conceição A, Bispo Sousa S, et al. HTLV-1 in pregnant women from the Southern Bahia, Brazil: a neglected condition despite the high prevalence. Virol J. 2014;11:28.
Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6(3):193–203.
International Committee on Taxonomy of Viruses (ICTV). Virus taxonomy: classification and nomenclature of viruses. [Online]. 2012 [cited 2014 Nov 5]. Available from http://www.ictvonline.org/.
Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci. 2011;102(1):44–50.
Jaworski E, Narayanan A, Van Duyne R. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem. 2014;289(32):22284–305.
Jeang K, Widen G, Semmes J, et al. HTLV trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;1990(247):1082–4.
Johnson J, Nicot C, Fullen J, et al. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J Virol. 2001;75(13):6086–94.
Journo C, Mahieux R. HTLV-1 and innate immunity. Viruses. 2011;3(8):1374–94.
Jun-ichirou Y, Matsuoka M. Human T-cell leukemia virus type I induces adult T-cell leukemia: from clinical aspects to molecular mechanisms. Cancer Control. 2007;14(2):133–40.
Kane M, Case L, Wang C, et al. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35(1):135–45.
Kastan M, Zhan Q, el-Deiry W, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–97.
Kattan T, MacNamara A, Rowan A, et al. The avidity and lytic efficiency of the CTL response to HTLV-1. J Immunol. 2009;182(9):5723–9.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50.
Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34.
Lairmore MD, Franchini G. Chapter 56: Human T-cell leukemia virus types 1 and 2. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 2072–105.
Lester S, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426(6):1246–64.
Levin M, Lee S, Kalume F, Stuart JM, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8(5):509–13.
Ma Y, Zheng S, Wang N, et al. Epidemiological analysis of HTLV-1 and HTLV-2 infection among different populations in central China. PLoS One. 2013;8(6):e66795.
Miyazato P, Matsuoka M. Human T-cell leukemia virus type 1 and Foxp3 expression: viral strategy in vivo. Int Immunol. 2014;26(8):419–25.
Murphy K, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11(8):674–80.
Nyborg J, Egan D, Sharma N. The HTLV-1 Tax protein: revealing mechanisms of transcriptional activation through histone acetylation and nucleosome disassembly. Biochim Biophys Acta. 2010;1799:266–74.
Olière S, Douville R, Sze A, et al. Modulation of innate immune responses during human T-cell leukemia virus (HTLV-1) pathogenesis. Cytokine Growth Factor Rev. 2011;22(4):197–210.
Peloponese J, Yeung M, Jeang K. Modulation of nuclear factor-kappa B by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol Res. 2006;34(1):1–12.
Poiesz B, Ruscetti F, Gazdar A, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77(12):7415–9.
Prasetyo A, Dirgahayu P, Sari Y, et al. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J Infect Dev Ctries. 2013;7(6):453–67.
Reitz Jr MS, Poiesz BJ, Ruscetti FW, Gallo R. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981;78(3):1887–91.
Ressler S, Morris G, Marriott J. Human T-cell leukemia virus type 1 Tax transactivates the human. J Virol. 1997;71:1181–90.
Saha A, Kaul R, Murakami M, et al. Tumor viruses and cancer biology: modulating signaling pathways for therapeutic intervention. Cancer Biol Ther. 2010;10(10):961–78.
Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87.
Satou Y, Matsuoka M. Molecular and cellular mechanism of leukemogenesis of ATL: emergent evidence of a significant role for HBZ in HTLV-1-induced pathogenesis. Leuk Res Treat. 2012;2012:213653.
Stienlauf S, Yahalom V, Shinar E, et al. Malignant diseases and mortality in blood donors infected with human T-lymphotropic virus type 1 in Israel. Int J Infect Dis. 2013;17(11):e1022–4.
Stoppa G, Rumiato E, Saggioro D. Ras signaling contributes to survival of human T-cell leukemia/lymphoma virus type 1 (HTLV-1) Tax-positive T-cells. Apoptosis. 2012;17(3):219–28.
Sugata K, Satou Y, et al. HTLV-1 bZIP factor impairs cell-mediated immunity by suppressing production of Th1 cytokines. Blood. 2012;119(2):434–44.
Takashi T, Michael J, Tsuneyasu K. PDLIM2-mediated termination of transcription factor NF-κB activation by intranuclear sequestration and degradation of the p65 subunit. Nat Immunol. 2007;8:584–91.
Tanaka Y, Tanaka R, Terada E, et al. Induction of antibody responses that neutralize human T-cell leukemia virus type I infection in vitro and in vivo by peptide immunization. J Virol. 1994;68(10):6323–31.
Tie F, Adya N, Greene G, et al. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70(12):8368–74.
Tiejun Z, Jun-ichirou Y, Yorifumi S, et al. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κ. Blood. 2009;113(12):2755–64.
Tomita M, Semenza G, Michiels C, et al. Activation of hypoxia-inducible factor 1 in human T-cell leukaemia virus type 1-infected cell lines and primary adult T-cell leukaemia cells. Biochem J. 2007;406(2):317–23.
Toulza F, Nosaka K, Tanaka Y, et al. Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3+ regulatory T cells. J Immunol. 2010;185(1):183–9.
Treviño A, Aguilera A, Caballero E, HTLV Spanish Study Group, et al. Trends in the prevalence and distribution of HTLV-1 and HTLV-2 infections in Spain. Virol J. 2012;9:71.
Turvey SE, Broide DH. Innate immunity. J Allergy Clin Immunol. 2010;125 Suppl 2:S24–32.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Arellano-Galindo, J. et al. (2016). HTLV-1 as a Model for Identifying the Causes of Human Leukemia. In: Mejía-Aranguré, J. (eds) Etiology of Acute Leukemias in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-05798-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-05798-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05797-2
Online ISBN: 978-3-319-05798-9
eBook Packages: MedicineMedicine (R0)