Abstract
Osteosarcoma is a cancer characterized by formation of bone by malignant cells. Routine bone scan imaging with Tc-99m-MDP is done at diagnosis to evaluate primary tumor uptake and check for bone metastases. At time of relapse the Tc-99m-MDP bone scan also provides a specific means to assess formation of bone by malignant osteosarcoma cells and the potential for bone-seeking radiopharmaceuticals to deliver radioactivity directly into osteoblastic osteosarcoma lesions. This chapter will review and compare a bone-seeking radiopharmaceutical that emits beta-particles, samarium-153-EDTMP, with an alpha-particle emitter, radium-223. The charged alpha particles from radium-223 have far more mass and energy than beta particles (electrons) from Sm-153-EDTMP. Because radium-223 has less marrow toxicity and more radiobiological effectiveness, especially if inside the bone forming cancer cell than samarium-153-EDTMP, radium-223 may have greater potential to become widely used against osteosarcoma as a targeted therapy. Radium-223 also has more potential to be used with chemotherapy against osteosarcoma and bone metastases. Because osteosarcoma makes bone and radium-223 acts like calcium, this radiopharmaceutical could possibly become a new targeted means to achieve safe and effective reduction of tumor burden as well as facilitate better surgery and/or radiotherapy for difficult to resect large, or metastatic tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Osteosarcoma
- Internal radiotherapy
- Radium-223
- Samarium-153
- Alpha particle
- Beta particle
- Bone scan for screening
- Double strand DNA breaks
- Resistance is futile
- Radiobiological effectiveness (RBE)
Osteosarcoma Biology Favors Use of Bone-Seeking Radiopharmaceuticals
The pathologic diagnosis of osteosarcoma is characterized by formation of bone [1]. For detection of new bone formation by osteosarcoma, the “routine” 99mTc-MDP bone scan is the best screening test. Because osteoblastic osteosarcoma tumors make new bone, the 99mTc-MDP bone scan is a specific and sensitive test. This should be routinely done at diagnosis and after relapse in patients with osteosarcoma. Avid uptake of the bone-seeking 99mTc-MDP radioactive tracer into osteosarcoma lesions identifies the possibility of using a bone-seeking radiopharmaceutical for targeted therapy. Although currently bone-seeking radiopharmaceuticals are used in the setting of palliative care for patients with bone metastases this chapter will review principles for not only current use, but also future use of internal radiation to control osteosarcoma. Preclinical work and human studies have provided information to understand the advantages and limitations of beta emitters such as samarium-153-EDTMP compared to a new bone-seeking alpha emitter, radium-223 [2].
Radiation for Osteosarcoma Cancer Control
The use of radiation for local control of osteosarcoma has been a controversial topic. Early studies with radiation alone resulted in a high rate of osteosarcoma local relapse and lack of durable local control [3]. Radiotherapy of osteosarcoma can also result in skin toxicity, wound complications, and increased risk of infection [4]. Proton irradiation, carbon ion radiotherapy, and photons using intensity-modulated radiation therapy (IMRT) have been shown to provide some benefit for axial osteosarcoma and metastatic osteosarcoma tumors which are difficult or impossible to resect [5–13]. Stereotactic radiotherapy (i.e., 1–5 large fractions of radiation) has been useful for metastases of brain, spine [14] and in lungs [15, 16]. Because patients with osteosarcoma metastases and/or axial sites have very high rates of relapse and poor prognosis, new and better means of definitive local control are needed [17, 18]. Radiotherapy of osteosarcoma lesions is likely most effective when combined with chemotherapy [13, 19–23].
The Problem of Multiple Bone and/or Metastatic Sites of Osteosarcoma
Osteosarcoma bone metastases at diagnosis are associated with a very poor prognosis [24, 25]. Although the use of ifosfamide was helpful in this group [25], patients with high alkaline phosphatase or metastatic disease in two organs had less than a 5 % survival in the French series [24]. Combined lung and bone metastases and/or relapse at the site of primary tumor sometimes contribute to treatment failure and death from osteosarcoma because of difficulty in local control of multiple sites. Bone-seeking radiopharmaceuticals can offer a potential means to simultaneously treat multiple osseous and osteoblastic non-osseous sites of osteosarcoma (Table 1). This is because lung or other visceral metastases of osteosarcoma can be osteoblastic and thus incorporate bone-seeking radiopharmaceutical. As shared earlier, the bone scan with avid uptake of 99mTc-MDP is the best screening test to identify potential candidates for this approach.
Properties of Samarium-153-EDTMP, a Beta-Emitting Radiopharmaceutical
Samarium-153 manufacture occurs by placing a capsule of samarium-152 oxide into a nuclear reactor. Neutron capture produces the unstable samarium-153 isotope. Decay of samarium-153 to stable europium-153 produces a beta particle (electron) and a photon (gamma ray) which is also useful for gamma camera imaging (Tables 1 and 2) [26, 27]. Samarium-153-EDTMP has been studied since early work by William Goeckeler in 1987 showing that the ethylene diamine tetramethylene phosphonate (EDTMP) chelate was not only one of the most effective chelates to deliver the beta-emitting samarium-153 isotope to the bones, but also was also associated with very little release from bone once it was deposited in the bone mineral hydroxyapatite [28].
Preclinical Studies of Samarium-153-EDTMP in Relation to Osteosarcoma
The potential usefulness of samarium-153-EDTMP for treating osteosarcoma was first described by Lattimer et al. in dogs with spontaneously occurring osteosarcoma primary tumors [29]. Dogs with smaller osteoblastic tumors had more durable responses than dogs with larger tumors; this may be due to more intense and uniform deposition of the samarium-153-EDTMP radiopharmaceutical. Aas et al. showed that a dose of 36–57 MBq/kg (1–1.5 mCi/kg) samarium-153-EDTMP provided approximately 20 Gy to primary osteosarcoma tumors in dogs with reduction in pain as well as delaying the onset of metastatic disease [30]. It is not known whether treatment efficacy was due to rapid reduction of tumor burden or treatment of micro-metastases already in the lungs at the time of presentation.
The biodistribution of samarium-153-EDTMP is almost exclusively skeletal with rapid blood clearance and bone lesion to normal bone ratio of 17:1; unbound radiopharmaceutical is eliminated in the urine [28]. Because growth plates are sites of active deposition of hydroxyapatite, juvenile 8-week-old rabbits were used to investigate potential effects of samarium-153-EDTMP on epiphyses [31]. Clinically significant damage was seen at a dose of 1 mCi/kg when the rabbits were evaluated 8 weeks later (age = 16 weeks). Although no long-term studies of the effects of samarium-153-EDTMP on prepubertal bone growth and repair have been reported, samarium-153-EDTMP can facilitate bone healing of bones involved in older cancer patients indicating potential for healing after damage by internal radiation.
Samarium-153-EDTMP Experience Against Cancer in Humans
This radiopharmaceutical has been available for palliative treatment of bone metastases including osteosarcoma [32] since the mid 1990s. The most extensive use of samarium-153-EDTMP has been in prostate cancer [33–35]. Although the dose limiting toxicity is thrombocytopenia, repeated doses of samarium-153-EDTMP have been safely given to men with prostate cancer [36, 37]. The samarium-153-EDTMP radiopharmaceutical also has been used with docetaxel successfully [38, 39].
Samarium-153-EDTMP has also been used in standard doses (1–1.5 mCi/kg) in osteosarcoma [26, 27, 32, 40, 41]. Because of the heterogeneity of deposition and/or difficulty of standard doses to produce durable responses, samarium-153-EDTMP has also been combined with radiotherapy [42]. A method for dose calculations for combined external beam and internal samarium-153-EDTMP radiotherapy in osteosarcoma tumors has recently been published [43]. Once samarium-153-EDTMP is administered and unbound drug is eliminated in the urine (this occurs within 6 h), then a “bone-specific” radiosensitization chemotherapy drug can be given [22]. The principle is that once the radiopharmaceutical is bound to the target (bone/bone-forming tumor) and unbound 153Sm-EDTMP is eliminated into the urine, then the radiosensitization effects of chemotherapy are localized to regions of bound radiopharmaceutical because visceral organs (e.g., lungs, heart, liver, intestines, brain) have very low amounts of bound samarium-153-EDTMP radiopharmaceutical.
High-Dose Samarium-153-EDTMP
Avid and specific skeletal and bone-forming tumor localization of samarium-153-EDTMP allowed for a 30-fold dose escalation in osteosarcoma [44]. High-dose samarium-153-EDTMP, with or without chemotherapy, requires stem cell support because of the potential for prolonged thrombocytopenia, as shown by Turner et al. [45, 46]. High-dose samarium-153-EDTMP has been used by different investigators to treat osteosarcoma [41, 44, 47–50]. Although increased radiographic responses were seen using gemcitabine radiosensitization 1 day after samarium-153-EDTMP infusion, the durability of response against osteosarcoma metastases was not improved [47]. To summarize, it would appear that samarium-153-EDTMP is useful in the relatively limited osteosarcoma situations: (a) palliation of bone metastases, (b) palliation of metastases of tumors that form bone (i.e., positive on bone scan), and (c) in conjunction with external beam radiotherapy for control of unresectable osteosarcoma.
Advantages of Radium-223, an Alpha Particle Emitting Bone-Seeking Radiopharmaceutical Compared to the Beta Emitter, Samarium-153-EDTMP
Once a radionuclide is deposited in bone and/or in or near a cancer cell or tumor vessel in bone, the rate of rate of radioactive emissions (half-life), range, and energy of particle emissions (MeV) are quite different within the target zone for alpha versus beta emitters [51–54]. Energy, tissue penetration range, gamma camera imaging, and physical characteristics of these bone-seeking radiopharmaceuticals are a summarized in Tables 1, 2, and 3, respectively. Figure 1 depicts mass and energy characteristics of ionizing radiation (gamma rays, electrons or beta particles, protons, and alpha particles) as well as different type of DNA damage from the ionizing radiation particles. Figure 2 illustrates the radioactive decay cascade of radium-223.
Radioactive particle mass, energy, and DNA damage. Top: photons have no mass; protons have ¼ the mass energy of alpha particles. Thus, alpha particles have much greater mass and energy than electrons (beta particles). Bottom: Graphic representation of the high energy of alpha particles causing double strand breaks which are more difficult for cancer cells to repair than single stand breaks
Radium-223 decay cascade. On average, the initial ejection of the high LEt alpha particle takes a relatively long time (t 1/2 11.4 days is almost a million seconds). Subsequent quick decay of unstable isotopes of radon (4 s), polonium (2 ms), lead (2,166 s) bismuth (130 s), and polonium or thallium isotopes (287 s) yields an additional three alpha particles + two beta particles in the same before the stable Pb-207 isotope is finally formed. Alpha particle emissions account for about 94 % of the emitted energy of radium-223. In 1 month (<3 half-lives) ~10 % of radioactivity remains; in 7 weeks (6 half-lives) only about 1/64 (<2 %) of initial radium-223 radioactivity remains
All radium isotopes are unstable and decay to produce radiation. Prior experience with radium for treatment of cancer in the early twentieth century used radium-226 which has long half-life and significant safety problems associated with decay to long-lived radon daughters (i.e., radioactive radon gas) and off-target radiation side effects from radioactive radon (Fig. 3). Hence, the radium-226 isotope is now considered unsuitable for safe internal radiotherapy [55]. However, radium-223 has favorable decay characteristics: radon daughter decay is rapid (4 s), providing much less of a chance for “off target” radon diffusion (Fig. 3).
Safety of Radium-223 compared to other radium isotopes is graphically depicted. Radon (Rn) daughter decay is in red. The very short half-life of Rn daughter for radium-223 (4 s) limits amount of diffusion away from the targeted bone tumor deposition of radium-223. In contrast in the early twentieth century radium-226 was used clinically. This isotope was less safe and is no longer in clinical use because of the radon daughter t 1/2 of 3.8 days resulted in off-target radiation side effects
Preclinical studies of radium-223. Production and characterization of clinical grade radium-223 has been previously described in detail [55, 56]. Because radium-223 is an alkaline earth metal, it acts like calcium. The radium-223 isotope has been shown to specifically deposit alpha particles at sites inside the intended skeletal metastases and/or bone-forming osteosarcoma target lesions [56–60]. Preclinical studies in rodents with radium-223 showed avid skeletal deposition, relative sparing of the bone marrow, and nearly no soft tissue uptake [57, 61]. Extremely high doses of radium-223 in Balb c mice [1,250, 2,500, and 3,750 kBq/kg (25–75× the recommended monthly dose of 50 kBq/kg)] caused some effects on marrow, but the 4-week LD50 was not reached [62]. In this study, the greatest effect was on osteoblasts and osteocytes; it also confirmed marrow sparing and inability of the short-range alpha particles from radium-223 to completely ablate radiation-sensitive hematopoietic stem cells.
Experience with radium-223 in a phase I [59] and a randomized phase II trial in men with metastatic prostate cancer confirmed excellent activity against bone metastases and a low toxicity profile (i.e., a high therapeutic index) [58–60, 63]. Using doses of 5, 25, 50, or 100 kBq/kg, a dose response relationship was seen in pain index at week 2 [60] and the highest dose group also had significantly decreased levels of alkaline phosphatase. Two-year follow-up of the phase II trial shows overall survival benefit of 65 weeks vs 46 weeks comparing radium-223 versus placebo (HR 0.476; cox regression p = 0.017). There were no long-term hematologic toxicities or secondary malignancies reported in this small phase II cohort (N = 33) [63]. Results of a randomized phase III, double-blind, placebo controlled trial of [2, 64] radium-223 in prostate cancer at a dose of 50 kBq/kg monthly × 6 and 2:1 randomization between active and placebo (N = 921) were presented at ASCO 2012 [64] and recently published in the New England Journal of Medicine. This study resulted in the FDA approval of radium-223 in May 2013. Compared to placebo radium-223 was associated with significantly improved overall survival (median, 14.9 months vs. 11.3 months; hazard ratio, 0.70; 95 % CI, 0.58–0.83; P < 0.001) and was also associated with prolonged time to first skeletal-related event (median 15.6 months vs 9.8 months, respectively; HR = 0.658; 95 % CI, 0.522–0.830; p = 0.00037). Hematologic adverse events were uncommon (any grade 3 or 4 neutropenia in 2.2 % and 0.7 % and any grade 3 or 4 thrombocytopenia in 6.3 % and 2 % of the radium-223 and placebo groups, respectively). Although targeting of osteoblastic osteosarcoma tumors would expected to be much more specific than prostate cancer, currently this is an unlabeled use of the radiopharmaceutical.
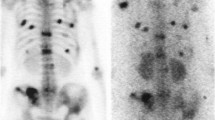
At MD Anderson Cancer Center a single osteosarcoma patient with head, neck, and skull base osteosarcoma with skeletal metastases was provided 2 doses of radium-223 in December 2009 and January 2010 [65]. Decrease in alkaline phosphatase and improvement in pain for approximately 2 months was seen. Bone scan showing the clinical response of this patient is illustrated in Fig. 4. At MD Anderson Cancer Center, a phase I dose trial in osteosarcoma is open to accrual (www.clinicaltrials.gov # NCT01833520). The purpose is to determine safety of escalating doses of radium-223 in osteosarcoma patients with osteoblastic tumors as well as to determine best quantitative imaging to evaluate responses using Tc-99m-MDP Spect-CT, NaF-18 PET, and F-18 deoxyglucose.
Improvement in 3 distant osteosarcoma skeletal metastases after 50 kBq/kg radium-223 × 2 doses 1 month apart. The bone scan shows supine (top) and prone (bottom) views: note the less avid Tc-99m-MDP uptake of T12 spine, right acetabular, and sacral osteosarcoma bone metastases comparing before (right with arrows) to after treatment (left without arrows). This patient also had improved pain at these sites and serum alkaline phosphatase decrease from 964 to 276 in 7 weeks after radium-223 administration
Possible Roles of Bone-Seeking Radiopharmaceuticals in Osteosarcoma Therapy
Palliation of painful bone metastases can be accomplished in a number of ways: medical treatment (opiates), or using local control measures including surgery, radiofrequency ablation, and/or radiotherapy. Thus, the use of external beam radiotherapy for treatment of painful osseous metastases is a widely accepted medical practice. Techniques are improving and stereotactic radiotherapy for spine metastases has become a frontline strategy [14, 66]. Larger single fractions seem to be more effective; this has been reviewed in meta-analyses of more than 25 clinical trials [67–71]. Because of internal lesion deposition and low marrow toxicity the usefulness of radium-223 and external beam radiotherapy for control of osteoblastic osteosarcoma remains to be determined, but is a strategy that may yield more durable control, particularly if combined with chemotherapy after localization of the bone-seeking isotope to the target lesion(s).
Experience with combined use of radiopharmaceuticals with chemotherapy: Combining 153Sm-EDTMP with docetaxel has been reported to have synergy in prostate cancer [39], and with bortezomib in myeloma [72]. Unfortunately, because of delayed thrombocytopenia (usually ~3–6 weeks after a dose), the combination of 153Sm-EDTMP in routine osteosarcoma is probably not feasible in many patients.
Would Low Marrow Toxicity of Radium-223 Allow Concurrent Use with Osteosarcoma Chemotherapy?
Radium-223 should be suitable for use in combination with chemotherapy, but additional work needs to be done. If the experience with humans is the same as the experience of dogs with osteosarcoma treated with samarium-153-EDTMP who had a delayed development of lung metastases [30], it is possible that early treatment with radium-223 could affect control in lung metastases. Thus far, the evidence suggests that radium-223 should have a higher therapeutic index (low marrow toxicity, more effect on malignant bone-forming cells that take up the radiopharmaceutical) than samarium-153-EDTMP. Because of current poor survival, patients likely to benefit are those with bone metastases [24] or axial tumors [17, 18]. Benefit in these very high-risk groups could then provide the rationale for randomized clinical trials and wider application of this targeted radiopharmaceutical against osteosarcoma.
Conclusion
Samarium-153-EDTMP has modest efficacy in the setting of palliative treatment of osteosarcoma metastases, but it is sometimes difficult to use repeated doses or with chemotherapy. The path length (range) of radium-223 is shorter, and thus, there is less hematologic toxicity because fewer marrow stem cells are “innocent bystanders.” It is the author’s view that radium-223 has the potential to significantly improve effectiveness of osteosarcoma chemotherapy as well as external beam radiation of unresectable tumors. Radium-223 may also possibly provide rapid control of initial pain and could possibly contribute to increased necrosis of osteoblastic tumors. Furthermore, because radium-223 has the potential to reduce viability of lung osteosarcoma micro-metastases, it also has potential to impact survival and reduce the incidence of relapses in the lungs as well as in the bones.
References
Raymond AK, Jaffe N (2009) Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat Res 152:63–84
Parker C et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369(3):213–223
Beck JC et al (1976) The role of radiation therapy in the treatment of osteosarcoma. Radiology 120(1):163–165
Trapeznikov, N.N., et al., [Treatment of limb osteosarcoma at the turn of the century (half century of experience in research)]. Vestn Ross Akad Med Nauk, 2001(9): p. 46-9.
Ciernik IF et al (2011) Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 117(19):4522–4530
DeLaney TF et al (2009) Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 74(3):732–739
DeLaney TF et al (2005) Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 61(2):492–498
Mahajan A et al (2008) Multimodality treatment of osteosarcoma: radiation in a high-risk cohort. Pediatr Blood Cancer 50(5):976–982
Matsunobu A et al (2012) Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 118(18):4555–4563
Imai R et al (2006) Cervical spine osteosarcoma treated with carbon-ion radiotherapy. Lancet Oncol 7(12):1034–1035
Schwarz R et al (2009) The role of radiotherapy in oseosarcoma. Cancer Treat Res 152:147–164
Wagner TD et al (2009) Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys 73(1):259–266
Hernberg MM et al (2011) Chemoradiotherapy in the treatment of inoperable high-grade osteosarcoma. Med Oncol 28(4):1475–1480
Wang XS et al (2012) Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol 13(4):395–402
Ricardi U et al (2012) Stereotactic body radiation therapy for lung metastases. Lung Cancer 75(1):77–81
Dhakal S et al (2012) Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82(2):940–945
Ozaki T et al (2003) Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol 21(2):334–341
Ozaki T et al (2002) Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer 94(4):1069–1077
Machak GN et al (2003) Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clin Proc 78(2):147–155
Anderson P, Salazar-Abshire M (2006) Improving outcomes in difficult bone cancers using multimodality therapy, including radiation: physician and nursing perspectives. Curr Oncol Rep 8(6):415–422
Anderson PM (2003) Effectiveness of radiotherapy for osteosarcoma that responds to chemotherapy. Mayo Clin Proc 78(2):145–146
Anderson P et al (2008) Outpatient chemotherapy plus radiotherapy in sarcomas: improving cancer control with radiosensitizing agents. Cancer Control 15(1):38–46
Dincbas FO et al (2005) The role of preoperative radiotherapy in nonmetastatic high-grade osteosarcoma of the extremities for limb-sparing surgery. Int J Radiat Oncol Biol Phys 62(3):820–828
Mialou V et al (2005) Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome – the French pediatric experience. Cancer 104(5):1100–1109
Goorin AM et al (2002) Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol 20(2):426–433
Anderson P, Nunez R (2007) Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther 7(11):1517–1527
Anderson P (2006) Samarium for osteoblastic bone metastases and osteosarcoma. Expert Opin Pharmacother 7(11):1475–1486
Goeckeler WF et al (1987) Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med 28(4):495–504
Lattimer JC et al (1990) Clinical and clinicopathologic response of canine bone tumor patients to treatment with samarium-153-EDTMP. J Nucl Med 31(8):1316–1325
Aas, M., et al., Internal radionuclide therapy of primary osteosarcoma in dogs, using 153Sm-ethylene-diamino-tetramethylene-phosphonate (EDTMP). Clin Cancer Res, 1999. 5(10 Suppl): p. 3148 s-3152s.
Essman SC et al (2003) Effects of 153Sm-ethylenediaminetetramethylene phosphonate on physeal and articular cartilage in juvenile rabbits. J Nucl Med 44(9):1510–1515
Bruland OS et al (1996) Targeted radiotherapy of osteosarcoma using 153 Sm-EDTMP. A new promising approach. Acta Oncol 35(3):381–384
Sandeman TF, Budd RS, Martin JJ (1992) Samarium-153-labelled EDTMP for bone metastases from cancer of the prostate. Clin Oncol (R Coll Radiol) 4(3):160–164
Sartor O (2004) Overview of samarium sm 153 lexidronam in the treatment of painful metastatic bone disease. Rev Urol 6(Suppl 10):S3–S12
Sartor O et al (2004) Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 63(5):940–945
Higano CS et al (2008) Safety analysis of repeated high doses of samarium-153 lexidronam in men with hormone-naive prostate cancer metastatic to bone. Clin Genitourin Cancer 6(1):40–45
Menda Y et al (2000) Efficacy and safety of repeated samarium-153 lexidronam treatment in a patient with prostate cancer and metastatic bone pain. Clin Nucl Med 25(9):698–700
Morris MJ et al (2009) Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol 27(15):2436–2442
Tu SM et al (2009) Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol 27(20):3319–3324
Loeb DM et al (2009) Dose-finding study of 153Sm-EDTMP in patients with poor-prognosis osteosarcoma. Cancer 115(11):2514–2522
Loeb DM et al (2010) Tandem dosing of samarium-153 ethylenediamine tetramethylene phosphoric acid with stem cell support for patients with high-risk osteosarcoma. Cancer 116(23):5470–5478
Hobbs RF et al (2011) A treatment planning method for sequentially combining radiopharmaceutical therapy and external radiation therapy. Int J Radiat Oncol Biol Phys 80(4):1256–1262
Senthamizhchelvan S et al (2012) Tumor dosimetry and response for 153Sm-ethylenediamine tetramethylene phosphonic acid therapy of high-risk osteosarcoma. J Nucl Med 53(2):215–224
Anderson PM et al (2002) High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol 20(1):189–196
Turner JH et al (1992) 153Sm-EDTMP and melphalan chemoradiotherapy regimen for bone marrow ablation prior to marrow transplantation: an experimental model in the rat. Nucl Med Commun 13(5):321–329
Turner JH et al (1993) Radiopharmaceutical therapy of 5 T33 murine myeloma by sequential treatment with samarium-153 ethylenediaminetetramethylene phosphonate, melphalan, and bone marrow transplantation. J Natl Cancer Inst 85(18):1508–1513
Anderson PM et al (2005) Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res 11(19 Pt 1):6895–6900
Franzius C et al (2001) High-activity samarium-153-EDTMP therapy followed by autologous peripheral blood stem cell support in unresectable osteosarcoma. Nuklearmedizin 40(6):215–220
Franzius C et al (1999) High-activity samarium-153-EDTMP therapy in unresectable osteosarcoma. Nuklearmedizin 38(8):337–340
Franzius C, Schuck A, Bielack SS (2002) High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol 20(7):1953–1954
Kassis AI (2008) Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med 38(5):358–366
Zhu X et al (2010) Solid-tumor radionuclide therapy dosimetry: new paradigms in view of tumor microenvironment and angiogenesis. Med Phys 37(6):2974–2984
Huang CY et al (2012) Microdosimetry for targeted alpha therapy of cancer. Comput Math Methods Med 2012:153212
Baidoo KE, Yong K, Brechbiel MW (2013) Molecular pathways: targeted alpha-particle radiation therapy. Clin Cancer Res 19(3):530–537
Bruland OS et al (2008) Radium-223: from Radiochemical Development to Clinical Applications in Targeted Cancer Therapy. Curr Radoipharmaceut 1(1):203–208
Bruland, O.S., et al., High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res, 2006. 12(20 Pt 2): p. 6250 s-6257s.
Henriksen G et al (2003) Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 44(2):252–259
Nilsson S et al (2007) Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 8(7):587–594
Nilsson S et al (2005) First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 11(12):4451–4459
Nilsson S et al (2012) A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 48(5):678–686
Henriksen G et al (2002) Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 62(11):3120–3125
Larsen RH et al (2006) Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo 20(3):325–331
Nilsson S et al (2013) Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer 11(1):20–26
Parker, C., et al., Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). J Clin Oncol, 2012. 30(suppl; abstr LBA4512).
Anderson P (2011) Osteosarcoma: an opportunity for targeted radiotherapy. In: Speer TW (ed) Targeted radionuclide therapy. Lippincott Williams & Wilkins, Wolters Kluwer Health, Philadelphia, PA, pp 473–477
Garg AK et al (2012) Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 118(20):5069–5077
Sze WM et al (2004) Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Cochrane Database Syst Rev (2): CD004721
Sze WM et al (2003) Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy – a systematic review of randomised trials. Clin Oncol (R Coll Radiol) 15(6):345–352
Wu JS et al (2004) Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases - an evidence-based practice guideline. BMC Cancer 4:71
Wu JS et al (2003) Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 55(3):594–605
Chow E et al (2007) Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 25(11):1423–1436
Goel A et al (2006) Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood 107(10):4063–4070
Acknowledgements
Peter M. Anderson acknowledges Greg Wiseman and Oyvind Bruland for their advice and sharing ideas during in the development of bone-seeking radiopharmaceuticals for osteosarcoma and Norman Jaffe for his mentorship when working with metastatic osteosarcoma patients. Research has been supported by the Shannon Wilkes Osteosarcoma fund, and Lauren Edwards Behr sarcoma research fund, Sarah’s Garden of Hope. The University of Texas MD Anderson Cancer Center is supported by Cancer Center Support Grant No. CA 016672. Dr. Anderson was supported by the Curtis Distinguished Professorship and is currently partially supported by Levine Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Anderson, P.M., Subbiah, V., Rohren, E. (2014). Bone-Seeking Radiopharmaceuticals as Targeted Agents of Osteosarcoma: Samarium-153-EDTMP and Radium-223. In: Kleinerman, M.D., E. (eds) Current Advances in Osteosarcoma. Advances in Experimental Medicine and Biology, vol 804. Springer, Cham. https://doi.org/10.1007/978-3-319-04843-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-04843-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-04842-0
Online ISBN: 978-3-319-04843-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)