Abstract
Although trace amounts of radioactivity are routinely used to detect osteosarcoma, the use of larger therapeutic amounts of radiation is often an unrecognized opportunity to treat metastatic osteosarcoma. This chapter will review a number of approaches to use ionizing radiation in the form of injectable radiopharmaceuticals. Since bone metastases are a common pattern of metastatic spread of cancer in general, a number of bone-seeking radiopharmaceuticals have been developed and FDA approved for treatment of bone metastases. Although osteosarcoma, a bone-forming cancer, would seem ideally suited to be treated with bone seekers, patterns of relapse involving non-ossifying metastases remain a major problem to be overcome. Thus, this review will not only describe experience using a number of bone-seeking radiopharmaceuticals such as 153-samarium-EDTMP, 153-samarium-DOTMP, and 223-radium against osteosarcoma, but also approaches to identify patients who may benefit as well as some means to the improve overall efficacy including combination therapy with routine agents and using nuclear imaging to develop best strategy for use. These include imaging with not only 99mTc-MDP standard bone scans, but also 99mTc-MDP bone scans with SPECT CT, bone-specific sodium fluoride PET-CT (Na18F), and 18FDG-PET-CT. Accurate knowledge of oligometastatic active disease can facilitate more effective use of combination therapy, including radiosensitizers and local control measures, for example, stereotactic body radiotherapy (SBRT) and/or cryoablation to reduce disease burden as well as manage and prevent micrometastatic disease from growing and metastasizing. Finally, a new tumor-specific radiopharmaceutical, CLR 131, may also provide another radiopharmaceutical to treat both osteoblastic and non-ossifying areas of osteosarcoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Strontium
- Samarium
- Radium
- Radiosensitizer
- Sodium fluoride PET
- SPECT CT
- Ifosfamide
- Doxorubicin liposomes
- Denosumab
- Zolendronate
- Pazopanib
- Gemcitabine
- Beta emitter
- Alpha emitter
- Stereotactic body radiotherapy (SBRT)
- Cryoablation
- Lipid raft-seeking radiopharmaceutical
Introduction
Osteosarcoma is a bone-forming tumor; alkaline phosphatase is a tumor marker associated with high osteoblastic activity. Metastatic osteosarcoma at diagnosis with high alkaline phosphatase in more than two organs (e.g., bone and lung) was associated with significantly inferior survival [1]. The initial bone-seeking radiopharmaceuticals, 89SrCl and 32P, were limited by a long half-life (50 days and 14 days, respectively) and nonspecific uptake of 32P in other tissues. These were generally used for one and done palliation of bone pain [2]. The next era of radiopharmaceuticals with bone-seeking specificity used metal chelates to deliver a radioactive payload which tightly binds bone matrix (Table 4.1). 133-Ho-DOTMP development was halted because of renal toxicity which occurred when radiopharmaceutical that did not bind bone passed through the kidneys into the urine. 186-Re-HEDP and 188-HEDP were used for skeletal metastases in 1997–2007 [3,4,5,6] but are not currently available in North America.
Samarium
Goeckeler tested a number of chelates and ethylene diamine tetramethylene phosphonate (EDTMP) was shown to not only have very high bone specificity, but also very high retention in bone [7, 8]. Canine osteosarcoma studies with 153-Sm-EDTMP showed activity excellent against osteoblastic osteosarcoma [9]. 153-Sm-EDMP that does not bind bone is excreted into the urine unchanged [10]. Thus, when Anderson et al. dose escalated 153-Sm-EDTMP with stem cell rescue, the protocol used saline hydration, furosemide to increase urine output, and instructions to void frequently for 6 hours to reduce renal and bladder exposure to unbound radiopharmaceutical [11]. In this study, hypocalcemia from carrier EDTMP was found to be the dose-limiting toxicity when 153-Sm-EDTMP was escalated 30-fold from a standard dose of 1 mCi/kg to 30 mCi/kg. Others have successfully used high-dose samarium for osteosarcoma [12,13,14]. Loeb et al. also showed tandem dosing was possible in osteosarcoma [15].
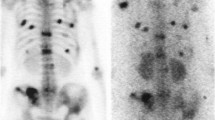
Use of gemcitabine as a radiosensitizer after the highly bone-specific binding of high-dose 153-Sm-EDTMP resulted in improved imaging responses [16]. Total body measurements after 153-Sm-EDTMP then gemcitabine were 1.08+/−0.4 mCi (<3.6 mCi for safe infusion of stem cells) after 6–7 half-lives (12–14 days) [16] and all patients recovered hematologic function within 2 weeks after getting the stem cells (Fig. 4.1).
The “Double Tap” for increased tumor-specific lethality. After elimination of unbound agent (e.g., unbound 153-Sm-EDTMP or 153-Sm-DOTMP is eliminated in the urine within 3- 6 hours), only bone bound agent remains when a radiosensitizer (e.g., gemcitabine, ifosfamide, or doxorubicin liposomes) is given later. Specifically bound radiopharmaceutical then decays; this leaves ½ the amount of radioactivity in the tumor after each half-life. Thus, after 7 half-lives 1/128th of the initial radiation is present
Standard dose 153-Sm-ETMP usefulness in osteosarcoma has been reviewed previously [17, 18]; the dose-limiting toxicity of 153-Sm-EDTP is delayed thrombocytopenia. This generally occurs 3–4 weeks after administration and resolves within 4–6 weeks. To date there are no reports of use of TPO agonists such as eltrombopag or romiplostim after 153-Sm-EDTMP to limit duration and/or severity of this side effect. Although 153-Sm decays to stable 153-Eu by beta decay, trace quantities of 154-Eu are produces during synthesis of 153-Sm via neutron capture. Thus, although not associated with any clinical effects, patients need a letter about prior 153-Sm therapy when traveling because of the extremely sensitive radiation detectors in airports will detect emissions form 145-Eu [18]. Loeb also described detection of 152-Eu in treated patients, too [19].
One approach to the saturation effect and excess EDTMP at high doses of 153-Sm-EDTMP is to synthesize a different chelate with higher purity and specific activity such as 153-Sm-DOTMP [20, 21]. This preparation has been termed “CycloSam”. With high doses it may be avoid hyopcalcemia and 153-Sm-DOTA may become useful for both osteosarcoma and total skeletal irradiation.
Even high-dose samarium patients seem to have only temporary benefit. Isolated limb perfusion (ILP) of 153-Sm-EDTMP of dogs with osteosarcoma at provided some insights about potential reasons for osteosarcoma relapses after bone-seeking radiopharmaceutical administration. Autoradiography showed heterogeneous bone tumor distribution despite achieving a high dose for a short time using ILP. Lung metastases are often another pattern of osteosarcoma relapse or progression after bone-seeking radiopharmaceuticals because some lung metastases have very low amount of bone formation compared to bone metastases. Finally, the mass energy of a beta emitter is much less than alpha emitters which readily cause double-strand breaks.
Radium
Alpha-emitting radiopharmaceuticals have some advantages compared to beta-emitting agents. These include not only very high linear energy transfer (LET) because of high mass (an alpha particle has 2 protons and 2 neutrons), but also safer handling and lower radiation exposure of nontarget tissues [22, 23]. 226-radium was used >100 years ago but the major naturally occurring 226-radium isotope has not only an extremely long half-life but also long-lived radon daughters and thus was abandoned because of safety concerns [22]. Larsen, Henriksen, Nilsson, and Bruland were responsible for early development of 223-radium as a safe and effective agent for bone metastases [23,24,25,26,27,28]. Preclinical and early clinical trials work established an extremely favorable safety profile including low marrow toxicity and few side effects [27, 28]. Phase 2 studies showed safety, improved pain, and better survival in prostate cancer patients [27,28,29,30]. A subsequent randomized, placebo, double-blind phase 3 clinical trial showed improved pain and was stopped early because of a significantly improved survival benefit; this resulted in FDA and EMEA approval [31, 32]. Since prostate cancer causes osteoblastic reactive bone around the neoplastic cells, the 223-Ra may act to kill and contain the viable rim of a bone metastasis.
223-radium was first used for recurrent, progressive, metastatic osteosarcoma using the FDA compassionate access IND mechanism. In these patients not only pain but also the tumor marker, alkaline phosphatase, improved [22, 33]. Subsequently 223-radium has become part of the NCCN guidelines for relapsed osteosarcoma. Subbiah et al. showed safety of 1.5–3.0 microCi/kg [34] and blood-brain barrier penetration of 223-radium in osteosarcoma [35]. This group also demonstrated usefulness of Na18F PET for screening and monitoring of response [36]. The next step was combination therapy using radiotherapy (RT) and stereotactic body radiotherapy (SBRT) with other agents as detailed in Table 4.2.
Denosumab is an agent useful in the treatment of giant cell tumor and osteosarcoma [37], reducing osteopenia, and preventing complications of skeletal metastases. Since I have observed that denosumab also causes increased ossification of osteoblastic osteosarcoma tumors, the agent can be used improve the therapeutic index of 223-radium by facilitating increase 223-radium uptake. At Cleveland Clinic, 14 of 15 recent patients have also had denosumab as part of the 223-radium treatment regimen. It is possible that zolendronate may also be active in this respect and if osteosarcoma cells are like giant cell tumor zolendronate may also have an antiapoptotic effect [38]. Since zolendronate is now generic and has become inexpensive future use would be expected to increase in the treatment of osteosarcoma skeletal metastases, especially in combination with 223-radium. Figure 4.2 shows activity of combined use of continuous infusion 14-day ifosfamide/mesna and 223-radium.
Ifosfamide +223-radium combination therapy. Heterogeneous osteoblastic activity of an osteosarcoma lung metastasis using 99mTc-MDP bone scan/SPECT CT. This patient had an excellent response to the combination of denosumab+14-day continuous infusion ifosfamide/mesna and monthly 223-radium after two cycles. This allowed thoracic surgery to be done to remove the large mass
Choice of cytotoxic agents to combine with 223-radium was driven by agents and combinations with low marrow toxicity so as not to delay monthly 223-radium infusions. For example, oral cyclophosphamide can be adjusted to keep ANC > 1000–1500, and anemia and thrombocytopenia are rarely problematic. Although high-dose ifosfamide has high activity against relapsed osteosarcoma [39] including bone metastases [40], the 5-day regimen results in pancytopenia and would not be suitable for use with 223-radium. However, high-dose ifosfamide/mesna (14 gm/cycle but given as a continuous at 1 gm/m2/day) has very low potential to cause thrombocytopenia; neutropenia can be overcome using PEG-GCSF [41,42,43,44].
Another means to attempt to overcome the problem of heterogeneous biodistribution of 223-radium is to use additional external beam radiation as either SBRT or RT if normal structures (e.g., trachea, carina, heart, mediastinum, stomach) do not permit SBRT to be safely given. In 15 patients treated with 223-radium treated at Cleveland Clinic >50 sites of osteosarcoma metastases have had SBRT or RT to improve both pain and/or durability of responses. Figure 4.3 shows an example to SBRT to the sacrum.
Scan images and SBRT plans of osteosarcoma involving sacrum treated with denosumab, pazopanib, and 223-radium. Top: PET-CT showing 18FDG activity; middle: SBRT plan (8 Gy × 5 = 40Gy; bottom: CT, planar 99mTc-MDP bone scan, and SPECT CT of lesion. This patient had a durable response in this location to the combination therapy and was able to participate fully in activities including climbing again and attending college
Other means of improving 223-radium efficacy have included use of TKI agents such as pazopanib, sorafenib, and regorafenib to provide radiosensitization and antiproliferative effects [45,46,47]. Although pazopanib, sorafenib, and regorafenib have activity against metastatic osteosarcoma [47,48,49,50,51], side effect profile for each is different. Since pazopanib seems to have fewer problems with rash and GI toxicity, this has been used in more of our 223-radium patients than other TKI agents at our institution. Finally, doxorubicin liposomes have been used with 223-radium because this agent is outpatient and well tolerated (Table 4.2). The anthracycline liposomal formulation, unlike the parent drug, has very low hematologic and heart toxicity [52] and may also have an effect on sarcoma stem cells in combination with mTOR inhibition [53, 54]. Nevertheless, relapse of metastatic osteosarcoma after 223-radium in non-osseous sites is common. In our series of patients with osteosarcoma osteoblastic metastases, 6/15 alive after 1 year and 3/15 > 2 years.
Another Radiopharmaceutical for Osteosarcoma: CLR 131
A new radiopharmaceutical with other tumor-specific properties is CLR 131 . This agent has specificity for tumors via [36] lipid rafts which are highly expressed on tumor cells but not normal tissues [55].Thus, CLR 131 can deliver a nuclear payload containing iodine to osteosarcoma tumor deposits, even when these do not make bone. Preclinical models also show synergy with external bean radiation in vivo [56]. Preclinical work with pediatric cancers including neuroblastoma, rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma demonstrated in vivo concentration ~6× in tumors as well as antitumor efficacy [57, 58]. The University of Wisconsin has a clinical trial testing this agent in children and college-aged young adults with solid tumors including osteosarcoma (NCT03478462). Escalation using stem cells (like MIBG) and/or gemcitabine radiosensitization should also be possible with the CLR 131 agent.
Patient Selection for Radiopharmaceuticals for Osteosarcoma: Practical Considerations
Table 4.3 reviews some aspects of how specific nuclear medicine scans can help make plans and/or decide on suitability (or not) as well as following response(s).
Although planar 99mTc-MDP bone scan can give a yes or no about lesion being osteoblastic (avid) and 223-radium suitability, combining this imaging with SPECT CT can help one know more about location and heterogeneity of uptake as well and to develop plans for other local control measures (e.g., brachytherapy, RT, SBRT, or cryoablation) [59,60,61]. Sodium fluoride PET is perhaps the most sensitive means to follow osteoblastic lesions after 223-radium [36].
Table 4.4 shows an example of multiple osteoblastic lesions responding using Na18F PET-CT as a means to show improvement. 18FDG is the best means to follow non-osteoblastic bone or visceral lesions since these may not change much in size and/or be detected by the bone-specific 99mTc-MDP or Na18F bone scans. Sometimes CT done with PET scans is not of diagnostic quality and a dedicated chest CT with and without contrast is the most specific and sensitive means to follow lung metastases. Instead of relying on tumor specificity of radiopharmaceuticals, treatment of oligometastatic disease using SBRT or cryoablation using CT guidance [59,60,61], may offer additional modalities to reduce osteosarcoma disease burden.
Summary and Obtaining Access to Radiopharmaceuticals for Osteosarcoma
Bone-seeking radiopharmaceuticals 153-Sm-EDTMP and 223-radium may improve pain and provide an underutilized means to treat osteoblastic metastases of osteosarcoma. Although dose escalation of 153-Sm-EDTMP and 153-Sm-DOTA is possible, osteoblastic heterogeneity may limit long-term effectiveness (cannot hit the target if there is no uptake). Because of low marrow toxicity and ease of administration, 223-radium can be used in combination with other agents. Nevertheless, other control strategies (e.g., SBRT, cryoablation) then immune therapy such as Cincinnati Children’s trial of pembrolizumab, decitabine, and SBRT (NCT 03445858) or CLR 131 at the University of Wisconsin (NCT03478462) may be other options to consider.
Radiopharmaceuticals can provide benefit to osteosarcoma patients. This is an evolving field. The author uses virtual visits to help patients and caregivers understand what options are not only feasible but with a likelihood of benefit and also how to get access to these remarkable agents [62].
References
Mialou V et al (2005) Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome–the French pediatric experience. Cancer 104(5):1100–1109
Giammarile F et al (1999) Bone pain palliation with 85Sr therapy. J Nucl Med 40(4):585–590
Lin WY et al (1997) Rhenium-188 hydroxyethylidene diphosphonate: a new generator-produced radiotherapeutic drug of potential value for the treatment of bone metastases. Eur J Nucl Med 24(6):590–595
Liepe K et al (2000) Rhenium-188-HEDP in the palliative treatment of bone metastases. Cancer Biother Radiopharm 15(3):261–265
Liepe K et al (2003) Dosimetry of 188Re-hydroxyethylidene diphosphonate in human prostate cancer skeletal metastases. J Nucl Med 44(6):953–960
Lam MG, de Klerk JM, van Rijk PP (2004) 186Re-HEDP for metastatic bone pain in breast cancer patients. Eur J Nucl Med Mol Imaging 31(Suppl 1):S162–S170
Goeckeler WF et al (1986) 153Sm radiotherapeutic bone agents. Int J Rad Appl Instrum B 13(4):479–482
Goeckeler WF et al (1987) Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med 28(4):495–504
Lattimer JC et al (1990) Clinical and clinicopathologic response of canine bone tumor patients to treatment with samarium-153-EDTMP. J Nucl Med 31(8):1316–1325
Goeckeler WF et al (1993) Analysis of urine samples from metastatic bone cancer patients administered 153Sm-EDTMP. Nucl Med Biol 20(5):657–661
Anderson PM et al (2002) High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol 20(1):189–196
Franzius C et al (1999) High-activity samarium-153-EDTMP therapy in unresectable osteosarcoma. Nuklearmedizin 38(8):337–340
Franzius C, Schuck A, Bielack SS (2002) High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol 20(7):1953–1954
Loeb DM et al (2009) Dose-finding study of 153Sm-EDTMP in patients with poor-prognosis osteosarcoma. Cancer 115(11):2514–2522
Loeb DM et al (2010) Tandem dosing of samarium-153 ethylenediamine tetramethylene phosphoric acid with stem cell support for patients with high-risk osteosarcoma. Cancer 116(23):5470–5478
Anderson PM et al (2005) Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res 11(19 Pt 1):6895–6900
Anderson P (2006) Samarium for osteoblastic bone metastases and osteosarcoma. Expert Opin Pharmacother 7(11):1475–1486
Anderson P, Nunez R (2007) Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther 7(11):1517–1527
Loebe T, Hettwig B, Fischer HW (2014) Detection of long-lived europium-152 in samarium-153-lexidronam. Appl Radiat Isot 94:40–43
Chakraborty S et al (2004) Preparation and biological evaluation of 153Sm-DOTMP as a potential agent for bone pain palliation. Nucl Med Commun 25(12):1169–1176
Simon J et al (2012) A preclinical investigation of the saturation and dosimetry of 153Sm-DOTMP as a bone-seeking radiopharmaceutical. Nucl Med Biol 39(6):770–776
Anderson PM, Subbiah V, Rohren E (2014) Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: samarium-153-EDTMP and radium-223. Adv Exp Med Biol 804:291–304
Bruland OS et al (2006) High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 12(20 Pt 2):6250s–6257s
Bruland OS et al (2008) Radium-223: from radiochemical development to clinical applications in targeted cancer therapy. Curr Radoipharm 1(1):203–208
Larsen RH et al (2006) Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo 20(3):325–331
Henriksen G et al (2003) Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 44(2):252–259
Nilsson S et al (2007) Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 8(7):587–594
Nilsson S et al (2005) First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 11(12):4451–4459
Nilsson S et al (2013) Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer 11(1):20–26
Nilsson S et al (2012) A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 48(5):678–686
Parker C et al (2012) Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). J Clin Oncol 30(suppl); Late Breaking Abstract 4512
Parker CC et al (2013) A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 63(2):189–197
Anderson P (2011) Osteosarcoma: an opportunity for targeted radiotherapy. In: Speer TW (ed) Targeted radionucleide therapy. Lippincott Williams &Wilkins, Wolters Kluwer Health, Philadelphia, pp 473–477
Subbiah V et al (2019) Alpha particle radium 223 dichloride in high-risk osteosarcoma: a phase I dose escalation trial. Clin Cancer Res 25(13):3802–3810
Subbiah V, Anderson P, Rohren E (2015) Alpha emitter radium 223 in high-risk osteosarcoma: first clinical evidence of response and blood-brain barrier penetration. JAMA Oncol 1(2):253–255
Kairemo K et al (2019) Development of sodium fluoride PET response criteria for solid tumours (NAFCIST) in a clinical trial of radium-223 in osteosarcoma: from RECIST to PERCIST to NAFCIST. ESMO Open 4(1):e000439
Cathomas R et al (2015) RANK ligand blockade with denosumab in combination with sorafenib in chemorefractory osteosarcoma: a possible step forward? Oncology 88(4):257–260
Lau CP et al (2013) Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res 54(6):439–449
Harris MB et al (1995) Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study. Med Pediatr Oncol 24(2):87–92
Goorin AM et al (2002) Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol 20(2):426–433
Meazza C et al (2010) Prolonged 14-day continuous infusion of high-dose ifosfamide with an external portable pump: feasibility and efficacy in refractory pediatric sarcoma. Pediatr Blood Cancer 55(4):617–620
Anderson P (2010) Continuously improving ifosfamide/mesna: a winning combination. Pediatr Blood Cancer 55(4):599–600
Zhang Y et al (2014) Physical and chemical stability of high-dose ifosfamide and mesna for prolonged 14-day continuous infusion. J Oncol Pharm Pract 20(1):51–57
Martin-Liberal J et al (2013) Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma 2013:868973
Wang F et al (2018) Pazopanib radio-sensitization of human sarcoma tumors. Oncotarget 9(10):9311–9324
Canter RJ et al (2014) Anti-proliferative but not anti-angiogenic tyrosine kinase inhibitors enrich for cancer stem cells in soft tissue sarcoma. BMC Cancer 14:756
Daudigeos-Dubus E et al (2015) Regorafenib: antitumor activity upon mono and combination therapy in preclinical pediatric malignancy models. PLoS One 10(11):e0142612
Safwat A et al (2014) Pazopanib in metastatic osteosarcoma: significant clinical response in three consecutive patients. Acta Oncol 53(10):1451–1454
Grignani G et al (2012) A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group Study. Ann Oncol 23(2):508–516
Grignani G et al (2015) Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol 16(1):98–107
Duffaud F et al (2019) Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 20(1):120–133
Blank N et al (2017) Absence of cardiotoxicity with prolonged treatment and large accumulating doses of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol 80(4):737–743
Thornton KA et al (2013) A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int J Cancer 133(4):997–1005
Trucco MM et al (2018) A phase II study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcomas. Clin Sarcoma Res 8:21
Weichert JP et al (2014) Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med 6(240):240ra75
Morris ZS et al (2015) Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems. Radiother Oncol 116(3):504–509
Baiu DC et al (2018) Targeted molecular radiotherapy of pediatric solid tumors using a radioiodinated alkyl-phospholipid ether analog. J Nucl Med 59(2):244–250
Marsh IR et al (2019) Preclinical pharmacokinetics and dosimetry studies of [(124)I/(131)I]-CLR1404 for treatment of pediatric solid tumors in murine xenograft models. J Nucl Med 60(10):1414–1420
Brown LC et al (2014) Stereotactic body radiotherapy for metastatic and recurrent Ewing sarcoma and osteosarcoma. Sarcoma 2014:418270
Luigi Cazzato R et al (2018) Percutaneous image-guided ablation of bone metastases: local tumor control in oligometastatic patients. Int J Hyperth 35(1):493–499
Weichselbaum RR (2018) The 46th David A. Karnofsky memorial award lecture: oligometastasis-from conception to treatment. J Clin Oncol:JCO1800847
Anderson PM, Hanna R (2019) Defining moments: making time for virtual visits and catalyzing better cancer care. Health Communication, https://doi.org/10.1080/10410236.2019.1587695:1–5
Acknowledgments
The author is extremely grateful for the efforts of Doretha Pendleton and Samantha Garzone for facilitating virtual visits, Taylor Buss and Shauna Sartoski for being helping hands to make things happen such as travel, appointments, and pre-authorization in kind and friendly ways, and Dr. Sankaran Shrikanthan, Andy Miller, Nick Jordan, Kanak Amin, and Rick Full for Nuclear Medicine studies and facilitating 223-radium ordering, 153-Sm-DOTMP dosimetry and administration appointments. Finally, it has been a pleasure to learn more about a new radiopharmaceutical, CLR 131, from Jarrod Longcor and Kate Oliver at Cellectar Biosciences and Mario Otto at the University of Wisconsin.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Anderson, P.M. (2020). Radiopharmaceuticals for Treatment of Osteosarcoma. In: Kleinerman, E.S., Gorlick, R. (eds) Current Advances in Osteosarcoma . Advances in Experimental Medicine and Biology, vol 1257. Springer, Cham. https://doi.org/10.1007/978-3-030-43032-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-43032-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43031-3
Online ISBN: 978-3-030-43032-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)