Abstract
The monitoring of dissolved oxygen (DO) in water is of great interest in order to provide information concerning the presence of pollutants or more generally about the health state of aquatic ecosystems. The majority of existing dissolved oxygen analyzers use electrochemical sensors which are accurate, reliable, stable, and require low or zero maintenance. However, these commercial sensors are not very compact and relatively expensive, since the fabrication process is complicated and noble metals are normally used as electrode materials. Herein, we present an electrochemical sensor for the determination of dissolved oxygen, by using reduced graphene oxide (RGO) as the oxygen sensitive probe. We prepared RGO by a chemical reduction method from graphene oxide (GO). A simple sensor was fabricated by printing RGO dispersed in Nafion, on the working electrode surface of a flexible commercial device. The surface and morphological properties of the sensing layer were investigated by XPS and TEM analysis. The electrochemical characteristics of the RGO-modified electrode were investigated by cyclic voltammetry (CV). Amperometric measurements were also performed in order to evaluate the sensor performance. Preliminary tests show good sensitivity and reproducibility. Tests of dissolved oxygen monitoring on environmental samples show the possibility of use in real applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Oxygen is one of the most important chemical species present in the environment and it is the basis of life of most living species. Oxygen is also involved in several chemical and biochemical processes that are part of the ecosystem. Its concentration level in the gaseous atmosphere as well as in water is then extremely important.
In this work, the attention has been focused on monitoring of dissolved oxygen (DO) in water. The determination of DO has been a subject of considerable interest in the last decades, due to its importance in environmental monitoring, industrial safety, energy conversion, waste management, medical applications, aquaculture, and many other biochemical processes [1]. Classical DO measurement was performed by Winkler titration method. Recently, the development of chemical sensors has replaced this technique. The two most common technologies available for dissolved oxygen sensing are the optical and electrochemical ones [2]. Clark-type sensor has been the first electrochemical device employed to monitor DO, and variations of this one are still widely used [1]. Commercial Clark sensors have high accuracy and sensitivity; however, they are often not very compact and relatively expensive due to the complexity of manufacture and the use of noble metals as electrodes. Actually, many of these problems can be overcome by using screen-printing techniques and more performance electrocatalytic materials [3]. Carbon nanostructures such as CNTs and graphene have attracted considerable interest in the field of electrochemical sensors [4, 5]. Due to their larger surface area, fast electron transfer and excellent support to metal catalyst and protein immobilization, carbon nanostructures promise electrocatalytic ability when used as electrodes modifier in electrochemical reactions.
In order to fabricate a simple DO sensor, in this work RGO has been used to modify the electrode surface of a commercial screen-printed device. So, no noble metal has been used in the formulation of the sensing layer with obvious advantages in saving costs.
2 Experimental
2.1 Graphene Synthesis
RGO was prepared by microwave-assisted chemical reduction method starting from graphene oxide (GO) according to what was already reported in a previous paper [6]. GO was prepared from graphite powder (<20 μm, synthetic, Aldrich) by a modified Hummers method. GO was added in benzyl alcohol and sonicated until the homogenous dispersion in solvent. The suspension was heated under microwave irradiation at 185 °C for 10 min, giving the RGO sample.
2.2 Sensor Fabrication
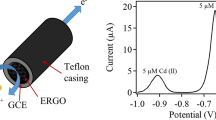
Sensor was fabricated by using a commercial screen-printed electrode device. It consists of a flexible plastic support above which are deposited two silver electrical contacts. The two electrodes, with function of working and counter/reference electrodes, are made of carbon and Ag/AgCl, respectively. Their surfaces are 1.8 mm2 and 6.2 mm2, respectively. The working electrode was modified by depositing above it a suspension of RGO dispersed in 5 % Nafion solution and dried at room temperature till the solvent evaporation.
2.3 Characterization
Transmission electron microscopy (TEM) images were obtained on a JEOL EM-2010 at an accelerating voltage of 200 kV. The XPS experiments were performed in an UHV multipurpose surface analysis system (Sigma Probe, ThermoFisher Scientific, UK).
Electrochemical measurements were carried out in a two-electrode mode using a homemade, portable, USB power electronic potentiostat. A dedicated software allows to operate in both chronoamperometric and voltammetric methods. The DO measurements were carried out in 0.1 M KCl solution. DO level was controlled saturating the electrolyte solution by bubbling N2/O2 gas mixtures.
CV was performed in the potential range 0 to −2 V at scan rate of 100 mV/s in both nitrogen- and oxygen-saturated solution.
Chronoamperometry experiments were carried out at working electrode potential of −1.35 V. Sensor signal was registered during the bubbling of oxygen into solution. To obtain a homogeneous oxygen concentration, solution was kept under magnetic stirring.
3 Results and Discussion
3.1 Graphene Characterization
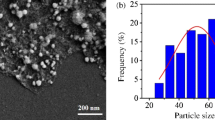
XPS and TEM analyses of materials investigated were performed in order to evaluate the nature of the surface functional groups and the morphological characteristics. Figure 1a shows the XPS analysis of the as-prepared RGO and GO precursor. XPS spectra indicate a high contribution due to C–O bond for the GO, while O/C ratio decreases notably in RGO sample. This means that benzyl alcohol under microwave treatment acts as a reducing agent leading to the partial reduction of GO to reduced graphene oxide. Figure 1b reports a TEM micrograph showing the morphological characteristic of exfoliated RGO synthesized.
3.2 Electrochemical Characterization
CV was used to investigate the electrochemical behavior of RGO-modified screen-printed commercial sensor. Figure 2 shows the CV cycles carried out in both deaerated and oxygen-saturated 0.1 M KCl solution, in the potential range 0 to −2 V and at scan rate of 100 mV/s. Preliminarily, it has been observed that no significant electrochemical process occurred when the sensor with unmodified electrodes was tested under these conditions. Instead, an intense reduction peak has been registered by the RGO-modified electrode at −1.35 V, with onset potential higher than −0.7 V, when oxygen was present in solution.
Figure 3a, b shows the dynamic response of the sensor to increasing concentrations of DO in water and the response reproducibility, respectively. The sensor follows quickly the diffusion of oxygen in solution and completely recovers the baseline signal when the solution is deaerated. A high reproducibility of the response has been also observed. Calibration curves obtained by plotting the measured current values to different molar concentration of DO, for both RGO-modified electrode and unmodified sensors, are shown in the inset of Fig. 3a. The average sensitivity was calculated to be 6.3 μA/mM with resolution of about 2 mg/l. Instead, much lower sensitivity was shown by the sensor with unmodified electrodes.
In order to assess the possibility to use the fabricated sensor in real applications, it was tested in measuring of the DO level in a seawater sample. The currents obtained by the sensor operated at −1.35 V in 0.1 M KCl solution and in seawater, in the presence of the same molar concentration of DO, are shown in Table 1. Sensor response variation was lower than 5 %. Furthermore, it has demonstrated that the high concentrations of ions present in the seawater do not interfere with the measurement.
4 Conclusion
RGO, prepared by a chemical reduction method from graphene oxide, was used as material for the electrocatalytic reduction of oxygen. Such material was then used for the fabrication of a simple DO electrochemical sensor modifying the working electrode surface of a commercial screen-printed device. The sensor showed excellent sensitivity and reproducibility. Furthermore, tests carried out in seawater samples have shown the possibility of use in real applications.
References
M. L. Hichman. The Measurement of Dissolved Oxygen. (John Wiley, New York, 1978), 255
The Dissolved Oxygen Handbook. (YSI Incorporated, 2009). www.ysi.com/weknowdo
I. Shitanda, S. Mori, M. Itagaki. Screen-printed Dissolved Oxygen Sensor Based on Cerium Oxide-Supported Silver Catalyst and Polydimethylsiloxane Film. Anal. Sci. 27 (10), 1049–1052 (2011)
A. Ahammad, J. Lee, M. Rahman. Electrochemical Sensors Based on Carbon Nanotubes. Sensors 9 (4), 2289–2319 (2009)
Y. Shao, J. Wang, H. Wu, J. Liu, Ilhan A. Aksay, Y. Lin. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 22, 1027–1036 (2010)
P. A. Russo, N. Donato, S. G. Leonardi, S. Baek, D. E. Conte, G. Neri, N. Pinna. Room-Temperature Hydrogen Sensing with Heteronanostructures Based on Reduced Graphene Oxide and Tin Oxide. Angew. Chem. Int. Ed. 51, 11053–11057 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this paper
Cite this paper
Leonardi, S.G., Aloisio, D., Latino, M., Donato, N., Neri, G. (2014). Dissolved Oxygen Sensor Based on Reduced Graphene Oxide. In: Di Natale, C., Ferrari, V., Ponzoni, A., Sberveglieri, G., Ferrari, M. (eds) Sensors and Microsystems. Lecture Notes in Electrical Engineering, vol 268. Springer, Cham. https://doi.org/10.1007/978-3-319-00684-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-00684-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00683-3
Online ISBN: 978-3-319-00684-0
eBook Packages: EngineeringEngineering (R0)