Abstract
Camptothecin, a well-known monoterpenoid indole alkaloid originally identified in the extracts of the Chinese tree Camptotheca acuminata (Nyssaceae), exhibits antitumor activity due to its ability to kill cancer cells via topoisomerase I poisoning. Other plant species have since been shown to produce camptothecin and related compounds. In particular, Ophiorrhiza species (Rubiaceae) are important resources for the production of various alkaloids, including camptothecin. This chapter describes the production of camptothecin-related alkaloids and the elucidation of the mechanisms of camptothecin biosynthesis using plant cell and tissue cultures. In particular, aseptically grown plants, callus cultures, and hairy root cultures were established for several species, O. liukiuensis, O. kuroiwai, and O. pumila, which were then evaluated for production of camptothecin and related alkaloids. The metabolite profiles differed between the species, and between tissues of the same species; for example, profiles from hairy roots were not identical to those of aseptic plants. The complementary DNAs (cDNAs) for strictosidine synthase, tryptophan decarboxylase, and cytochrome P450 reductase were cloned from O. pumila and evaluated for involvement in production of camptothecin in this species. RNA interference (RNAi)-mediated knockdown of gene expression indicated that the production of camptothecin, strictosidine, and camptothecin-related alkaloids was suppressed in a TDC expression-dependent manner in RNAi hairy roots.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Camptothecin
- Monoterpenoid indole alkaloid
- Camptotheca acuminata
- Topoisomerase I
- Ophiorrhiza
- Biosynthesis
- Cell and tissue culture
- Hairy root
- Strictosidine synthase
- Tryptophan decarboxylase
3.1 Introduction
Alkaloids are nitrogen-containing basic compounds known from about 20 % of all plant species. Many alkaloids are pharmacologically active and have been used traditionally in the form of medicinal plant extracts as treatments for various diseases [1]. A few dozen pharmacologically active alkaloids, including camptothecin, are widely used in modern medicine, and worldwide sales of alkaloid-containing drugs were projected to exceed US$ 4 billion in 2002 [2].
Camptothecin (1) is a well-known monoterpenoid indole alkaloid and was originally identified in the extracts of the Chinese tree Camptotheca acuminata (Nyssaceae) [3]. Camptothecin exhibits antitumor activity , which is due to its ability to kill cancer cells via topoisomerase I poisoning [4]. At present, the semi-synthetic water-soluble camptothecin derivatives, topotecan (2) and irinotecan (3), are used worldwide as clinical antitumor agents against cancers of the lung, cervix, ovaries, colon [5], and other organs [6] (Fig. 3.1). In addition, a number of reports are available announcing the therapeutic values of camptothecin derivatives against acquired immunodeficiency syndrome (AIDS) [7] and falciparum malaria [8]. Consequently, the demand for camptothecin will continue to increase in the future.
Camptothecin (1) and its clinically used derivatives, topotecan (2) and irinotecan (3). (With permission from Ref. [38])
Despite the rapid growth of the pharmaceutical market for this compound, camptothecin is still supplied exclusively from intact plants, mainly C. acuminata and Nothapodytes foetida [9]. However, the extraction of this compound from intact plants is problematic because of the shortage of natural resources and the resultant environmental concerns. Thus, the production of secondary metabolites by genetically engineered plant cell cultures, particularly for compounds such as camptothecin , has become a keen issue [10].
Camptothecin-related alkaloids have been reported to be produced in a relatively wide array of plant species, besides C. acuminata and N. foetida [11]. For instance, Merrilliodendron megacarpum [12], Pyrenacantha klaineana (Icacinaceae) [13], Ervatamia heyneana (Apocynaceae) [14], Mostuea brunonis (Loganiaceae) [15], Ophiorrhiza mungos [16], and O. filistipula (Rubiaceae) [17] have been reported to produce camptothecin-related compounds. Moreover, the results of phytochemical studies of the genus Ophiorrhiza have shown that camptothecin also accumulates in some Ophiorrhiza species (e.g., O. pumila) distributed in Japan [18, 19].
The genus Ophiorrhiza is widely distributed around tropical and subtropical Asia and comprises about 150 species [20]. Moreover, some of these species produce indole alkaloids [21]. With regard to the chemical constituents of Ophiorrhiza species distributed in Japan, O. pumila accumulated camptothecin and related alkaloids [18, 22] and O. japonica accumulated β-carboline-type alkaloids, such as lyalosidic acid and harman [23, 24]. Meanwhile, O. liukiuensis [25] and O. kuroiwai [26], which was shown to be an interspecies hybrid of O. pumila and O. liukiuensis, accumulated both camptothecin-related alkaloids and β-carboline-type alkaloids (Fig. 3.2). Therefore, these Ophiorrhiza species are important as resources for the production of various alkaloids, including camptothecin.
In this chapter, we describe the production of camptothecin-related alkaloids and the elucidation of the mechanisms of camptothecin biosynthesis by use of plant cell and tissue cultures.
3.2 In Vitro Cultures of Camptothecin-Producing Plants
3.2.1 Establishment of In Vitro Cultures
Cell and tissue cultures of several camptothecin-producing plants have been investigated as alternative sources for camptothecin production [27]. Sakato et al. [28] reported the first establishment of a rapidly growing cell suspension culture of C. acuminata, although the camptothecin productivity was insufficient (0.002 mg g−1 dry weight) for practical use. Callus cultures of C. acuminata established by Wiedenfeld et al. [29] produced comparatively adequate amounts of camptothecin (2 mg g−1 dry weight). These callus cultures were also reported to contain 10-hydroxycamptothecin, from trace amounts up to 0.08–0.1 mg g−1 dry weight [29]. Callus cultures of N. foetida were found to accumulate small amounts of camptothecin and 9-methoxycamptothecin [30–32], but the level of alkaloid production was 100- to 1000-fold lower than that from soil-grown plants. Callus cultures of O. pumila produced no camptothecin-related alkaloids but accumulated only anthraquinones [33, 34].
Since alkaloid biosynthesis and accumulation are under the strict control of cell developmental and environmental factors [35], establishing cultures of cell types suitable for the production of the camptothecin is important. Accordingly, aseptic plants and hairy roots of Ophiorrhiza species have been established as an effective means of producing camptothecin (Fig. 3.3) [36–38].
Established tissue cultures of Ophiorrhiza liukiuensis, O. kuroiwai, and O. pumila. a Aseptic plants cultured for 5 weeks on 1/2 MS medium containing 1 % sucrose and 0.2 % gellan gum in test tubes. b Hairy roots cultured for 4 weeks in B5 liquid medium containing 2 % sucrose. (With permission from Ref. [38])
3.2.2 Camptothecin Production and Metabolite Profiles in Tissue Cultures of Ophiorrhiza Species
In shoots and roots of established aseptic plants of Ophiorrhiza species, camptothecin production per tissue weight was the highest in the roots of O. pumila. On the other hand, the production per tube was the highest in O. kuroiwai because it showed the higher growth rate of the two species. The concentration and total amount of camptothecin in O. liukiuensis were lower than those of O. kuroiwai and O. pumila.
Camptothecin accumulated to higher levels in hairy root lines of O. pumila than in those of O. liukiuensis and O. kuroiwai [38]. Camptothecin accumulation and increased growth rate of O. pumila hairy roots have the best results in the reports of camptothecin production by in vitro tissue cultures [37, 39] .
The patterns of secondary metabolite production in the aseptic plants and hairy roots of Ophiorrhiza species were profiled by high-performance liquid chromatography–diode array detection–electrospray ion trap tandem mass spectrometry (Fig. 3.4 and Table 3.1) [38]. The metabolite profiles of O. liukiuensis and O. kuroiwai were highly similar in the shoot and root. 10-Methoxycamptothecin (5) and lyalosidic acid (6) were detected in the roots and shoots, respectively, of both O. liukiuensis and O. kuroiwai but not in those of O. pumila. Moreover, 3(S)- and 3(R)-deoxypumilosides (9, 10) were detected only in O. pumila. Camptothecin (1), 9-methoxycamptothecin (4), strictosamide (7), pumiloside (8), strictosidinic acid (11), and 3-O-caffeoylquinic acid (13) were detected in all three species. The metabolite profiles of the hairy roots were not identical to those of aseptic plants .
Chemical structures of secondary metabolites detected in tissue cultures of Ophiorrhiza species. (With permission from Ref. [38])
3.3 Biosynthesis of Camptothecin
3.3.1 Camptothecin Biosynthetic Genes
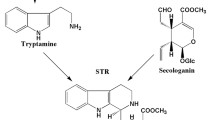
Monoterpenoid indole alkaloids , including camptothecin, are derived from strictosidine, which is a common intermediate formed by condensation of the indole tryptamine with the iridoid glucoside secologanin by the enzyme strictosidine synthase (STR) [40–42] (Fig. 3.5) . The intramolecular cyclization of strictosidine results in strictosamide, which is an intermediate peculiar to camptothecin biosynthesis, as proven by the incorporation of radiolabeled precursor [43]. The remaining details between strictosamide and camptothecin are not completely defined. However, camptothecin has been postulated to be formed potentially from strictosamide by three transformations: (1) oxidation–recyclization of the B- and C-rings, (2) oxidation of the D-ring and removal of the C-21 glucose moiety, and (3) oxidation of ring E [43]. Plausible camptothecin precursors, such as pumiloside and 3(S)-deoxypumiloside, were isolated from Ophiorrhiza species [18, 19]. Pumiloside has been found also in C. acuminata [44].
Predicted camptothecin biosynthetic pathway in O. pumila. The enzymes are as follows: TDC, tryptophan decarboxylase; G10H, geraniol 10-hydroxylase; CPR, NADPH:cytochrome P450 reductase; SLS, secologanin synthase; STR, strictosidine synthase. Plausible intermediates of camptothecin biosynthesis are provided in parentheses
The cloning of complementary DNAs (cDNAs) from O. pumila hairy roots has been successfully performed to isolate the genes encoding the biosynthetic enzymes involved in the upper part of camptothecin biosynthesis, including STR, tryptophan decarboxylase (TDC) [45], and nicotinamide adenine dinucleotide phosphate, reduced form (NADPH):cytochrome P450 reductase (CPR), in this species [46] (Fig. 3.5). The full-length STR cDNA sequence isolated from O. pumila (OpSTR) contained a 1,056-bp open reading frame (ORF) encoding a protein of 351 amino acids with a molecular mass of 38.9 kDa. The deduced amino acid sequence of OpSTR exhibited 55 % and 51 %identities with STRs from Rauwolfia serpentina [41] and Catharanthus roseus [47], respectively. OpSTR most likely localizes to the vacuole, as predicted by the PSORT program. Southern blot analysis suggested that a single STR-encoding gene is present in the genome of O. pumila. The highest OpSTR expression occurred in hairy roots , followed by the root, and the stem, whereas OpSTR was apparently not expressed in leaves. STR enzymatic activity was detected in the protein extracts of stems, roots, and hairy roots; however, no activity was detected in leaf and callus extracts. The distribution of STR activity correlated with the messenger RNA (mRNA) accumulation pattern and the camptothecin concentrations in O. pumila tissues, with the exception of the young leaves, suggesting that roots and stems are the main tissues for camptothecin biosynthesis [34] .
Tryptamine, a precursor of strictosidine, is formed by the decarboxylation of tryptophan by the enzyme TDC. The cDNA clone encoding TDC was first isolated from C. roseus [48]. The full-length TDC cDNA sequence isolated from O. pumila (OpTDC) contained a 1,521-bp ORF encoding a protein of 506 amino acids with a molecular mass of 56.6 kDa. The deduced amino acid sequence of OpTDC showed high identity to TDCs from C. acuminata [49] and C. roseus [48] (71 and 67 %, respectively). Southern blot analysis suggested that at least TDC-encoding genes are present in the genome. The expression patterns of OpSTR and OpTDC were nearly the same.
The enzyme CPR is essential for the activity of cytochrome P450 monooxygenases, such as geraniol 10-hydroxylase (G10H) and secologanin synthase (SLS), which are involved in camptothecin biosynthesis [50] (Fig. 3.5). The full-length CPR cDNA sequence isolated from O. pumila (OpCPR) contained a 2,073-bp ORF encoding a protein of 690 amino acids with a molecular mass of 76.6 kDa. The deduced amino acid sequence of OpCPR showed high identity with Arabidopsis thaliana, Petroselinum crispum, Pisum sativum, and Triticum aestivum CPRs (72, 66, 65, and 67 %, respectively). Southern blot analysis suggested that only a single CPR-encoding gene was present in the genome of O. pumila. Mirroring the general importance of the enzyme, OpCPR was expressed in all tissues.
Studies have been performed to investigate the effects of stress compounds, such as methyl jasmonate (MeJA), salicylic acid (SA), and yeast extract (YE), on the expression of OpSTR, OpTDC, and OpCPR in O. pumila hairy roots [46]. The changes in the expression patterns of OpSTR and OpTDC in response to these various compounds were highly similar. In particular, OpSTR and OpTDC expression was repressed by SA and YE treatments but unaffected by MeJA. Meanwhile, no treatment resulted in the induction or repression of OpCPR transcripts. In addition, no change in STR activity was observed after treatment with either stress compounds or phytohormones .
3.3.2 In Silico and In Vitro Tracer Studies with [1-13C]glucose
Both the mevalonate (MVA) pathway [51] and the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway [52–54] have been recognized for their role in the formation of isopentenyl diphosphate, the precursor of terpenoid biosynthesis. Yamazaki et al. [55] investigated the incorporation of [1-13C]glucose into camptothecin in the hairy roots of O. pumila by in silico computation using the Atomic Reconstruction of Metabolism (ARM) [56] program and by in vivo tracer experiments. The 13C-nuclear magnetic resonance (13C-NMR) analysis clearly showed that the secologanin moiety of camptothecin was synthesized via the MEP pathway . Furthermore, in O. pumila hairy root cultures, treatment with fosmidomycin, a specific inhibitor of the MEP pathway, resulted in a significant decrease in camptothecin production. These results support the conclusion that the secologanin moiety of camptothecin is derived from the MEP pathway .
3.4 Metabolic Modification in Hairy Roots of O. pumila by RNA Interference
A detailed understanding of camptothecin production, including the enzymatic pathway for its biosynthesis, will be essential to the ultimate goal of the metabolic engineering of this compound. In Papaver somniferum (opium poppy), genetic approaches using antisense RNA [57, 58] or RNA interference (RNAi)-mediated silencing [59] of biosynthetic enzymes have been performed, leading to rapid progress in the metabolic engineering of benzylisoquinoline alkaloids. Therefore, it is considered that RNAi technology is an effective strategy for investigating camptothecin biosynthesis. In our study, the production of camptothecin, strictosidine, and camptothecin-related alkaloids was suppressed in a TDC expression-dependent manner in RNAi hairy roots . Among the hairy root-specific peaks correlated with TDC expression in the liquid chromatography/Fourier transform ion cyclotron resonance mass spectrometry (LC-FTICR-MS) analysis, two unknown peaks with a positive correlation were annotated as alkaloids and six unknown peaks with a negative correlation, as flavonoids. The exact mass of several non-annotated peaks was similar to those of predicted intermediates in camptothecin biosynthesis, suggesting that most peaks that positively correlated with TDC expression could be intermediates in camptothecin biosynthesis [60].
References
Kutchan TM (1995) Alkaloid biosynthesis: the basis for metabolic engineering of medicinal plants. Plant Cell 7:1059–1070
Raskin I, Ribnicky DM, Momarnytsky S et al (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20:522–531
Wall ME, Wani MC, Cook CE et al (1966) Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88:3888–3890
Hsiang YH, Hertzberg R, Hecht S et al (1985) Camptothecin induces proteinlinked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878
Giovanella BC, Stehlin JS, Wall ME et al (1989) DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science 246:1046–1048
Pizzolato JF, Saltz LB (2003) The camptothecins. Lancet 361:2235–2242
Priel E, Showalter SD, Blair DG (1991) Inhibition of human immunodeficiency virus (HIV-1) replication in vitro by noncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res Hum Retroviruses 7:65–72
Bodley AL, Cumming JN, Shapiro TA (1998) Effects of camptothecin, a topoisomerase I inhibitor, on Plasmodium falciparum. Biochem Pharmacol 55:709–711
Govindachari TR, Viswanathan N (1972) Alkaloids of Mappia foetida. Phytochemistry 11:3529–3531
Springob K, Saito K (2002) Metabolic engineering of plant secondary metabolism: a promising approach to the production of pharmaceuticals. Sci Cult 68:76–85
Lorence A, Nessler CL (2004) Camptothecin, over four decades of surprising findings. Phytochemistry 65:2735–2749
Arisawa M, Gunasekera SP, Cordell GA et al (1981) Plant anticancer agents XXI. Constituents of Merrilliodendron megacarpum. Planta Med 43:404–407
Zhou B-N, Hoch JM, Johnson RK et al (2000) Use of COMPARE analysis to discover new natural product drugs: isolation of camptothecin and 9-methoxycamptothecin from a new source. J Nat Prod 63:1273–1276
Gunasekera SP, Badawi MM, Cordell GA et al (1979) Plant anticancer agents X. Isolation of camptothecin and 9-methoxycamptothecin from Ervatamia heyneana. J Nat Prod 42:475–477
Dai J-R, Halloch YF, Cardellina II JH et al (1999) 20-O-β-Glucopyranosyl camptothecin from Mostuea brunonis: a potential camptothecin pro-drug with improved solubility. J Nat Prod 62:1427–1429
Tafur S, Nelson JD, DeLong DC et al (1976) Antiviral components of Ophiorrhiza mungos. Isolation of camptothecin and 10-methoxycamptothecin. Lloydia 39:261–262
Arbain D, Putra DP, Sargent MV (1993) The alkaloids of Ophiorrhiza filistipula. Aust J Chem 46:977–985
Aimi N, Nishimura M, Miwa A et al (1989) Pumiloside and deoxypumiloside; plausible intermediates of camptothecin biosynthesis. Tetrahedron Lett 30:4991–4994
Kitajima M, Fujii N, Yoshino F et al (2005) Camptothecin and two new monoterpene glucosides from Ophiorrhiza liukiuensis. Chem Pharm Bull 53:1355–1358
Darwin SP (1976) The pacific species of Ophiorrhiza L. (Rubiaceae). Lyonia 1:47–102
Arbain D, Dachriyanus, Firmansyah et al (1998) Unusual indole alkaloids from Ophiorrhiza blumeana Korth. J Chem Soc Perkin Trans 1:2537–2540
Aimi N, Hoshino H, Nishimura M et al (1990) Chaboside, first natural glycocamptothecin found from Ophiorrhiza pumila. Tetrahedron Lett 31:5169–5172
Aimi N, Tsuyuki T, Murakami H et al (1985) Structures of ophiorines A and B; novel type gluco indole alkaloids isolated from Ophiorrhiza spp. Tetrahedron Lett 26:5299–5302
Aimi N, Murakami H, Tsuyuki T et al (1986) Hydrolytic degradation of β-carboline-type monoterpenoid glucoindole alkaloids: a possible mechanism for harman formation in Ophiorrhiza and related Rubiaceous plants. Chem Pharm Bull 34:3064–3066
Hayata B (1912) Ophiorrhiza Linn. In: Icones of the plants of Formosa, and materials for a flora of the island, based on a study of the collections of the botanical survey of the government of Formosa, fasc. 2. Bureau of Productive Industries, Taihoku, pp 85–92
Makino T (1906) Observations on the flora of Japan. Bot Mag 20:1–156
van Hengel AJ, Buitelaar RM, Wichers HJ (1994) VII Camptotheca acuminata Decne: in vitro cultures and the production of camptothecin. In: Bajaj YPS (ed) Medicinal and aromatic plants VII. Biotechnology in agriculture and forestry, Vol. 28. Springer, Berlin, pp 98–112
Sakato K, Tanaka H, Mukai N et al (1974) Isolation and identification of camptothecin from cells of Camptotheca acuminata suspension cultures. Agric Biol Chem 38:217–218
Wiedenfeld H, Furmanowa M, Roeder E et al (1997) Camptothecin and 10-hydroxycamptothecin in callus and plantlets of Camptotheca acuminata. Plant Cell Tissue Organ Cult 49:213–218
Roja G, Heble MR (1994) The quinoline alkaloids camptothecin and 9-methoxycamptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochemistry 36:65–66
Ciddi V, Shuler ML (2000) Camptothecine from callus cultures of Nothapodytes foetida. Biotechnol Lett 22:129–132
Fulzele DP, Satdive RK, Pol BB (2001) Growth and production of camptothecin by cell suspension cultures of Nothapodytes foetida. Planta Med 67:150–152
Kitajima M, Fisher U, Nakamura M et al (1998) Anthraquinones from Ophiorrhiza pumila tissue and cell cultures. Phytochemistry 48:107–111
Yamazaki Y, Urano A, Sudo H et al (2003) Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants. Phytochemistry 62:461–470
De Luca V, Saint-Pierre B (2000) The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci 5:168–173
Kitajima M, Nakamura M, Takayama H et al (1997) Constituents of regenerated plants of Ophiorrhiza pumila; formation of a new glycocamptothecin and predominant formation of (3R)-deoxypumiloside over (3S)-congener. Tetrahedron Lett 38:8997–9000
Saito K, Sudo H, Yamazaki M et al (2001) Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep 20:267–271
Asano T, Watase I, Sudo H et al (2004) Camptothecin production by in vitro cultures of Ophiorrhiza liukiuensis and O. kuroiwai. Plant Biotech 21:275–281
Watase I, Sudo H, Yamazaki M et al (2004) Regeneration of transformed Ophiorrhiza pumila plants producing camptothecin. Plant Biotech 21:337–342
Stöckigt J, Zenk MH (1977) Strictosidine (isovincoside): the key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J Chem Soc Chem Commun 18:646–648
Kutchan TM, Hampp N, Lottspeich F et al (1988) The cDNA clone for strictosidine synthase from Rauvolfia serpentina. DNA sequence determination and expression in Escherichia coli. FEBS Lett 237:40–44
Stöckigt J, Ruppert M (1999) Strictosidine—the biosynthetic key to monoterpenoid indole alkaloids. In: Barton SD, Nakanishi K, Meth-Cohn O (eds) Comprehensive natural products chemistry, Vol. 4. Pergamon, Oxford, pp 109–138
Hutchinson CR, Heckendorf AH, Straughn JL et al (1979) Biosynthesis of camptothecin. 3. Definition of strictosamide as the penultimate biosynthetic precursor assisted by 13C and 2H NMR spectroscopy. J Amer Chem Soc 101:3358–3369
Carte BK, DeBrosse C, Eggleston D et al (1990) Isolation and characterization of a presumed biosynthetic precursor of camptothecin from extracts of Camptotheca acuminata. Tetrahedron 46:2747–2760
Noé W, Mollenschott C, Berlin J (1984) Tryptophan decarboxylase from Catharanthus roseus cell suspension cultures: purification, molecular and kinetic data of the homogenous protein. Plant Mol Biol 3:281–288
Yamazaki Y, Sudo H, Yamazaki M et al (2003) Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44:395–403
McKnight TD, Roessner CA, Devagupta R et al (1990) Nucleotide sequence of a cDNA encoding the vacuolar protein strictosidine synthase from Catharanthus roseus. Nucl Acids Res 18:4939
De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of a cDNA encoding a plant tryptophan decarboxylase: comparison with animal DOPA decarboxylases. Proc Natl Acad Sci U S A 86:2582–2586
López-Meyer M, Nessler CL (1997) Tryptophan decarboxylase is encoded by two autonomously regulated genes in Camptotheca acuminata which are differentially expressed during development and stress. Plant J 11:1167–1175
Meijer AH, Lopes Cardoso MIL, Voskuilen JT et al (1993) Isolation and characterization of a cDNA clone from Catharanthus roseus encoding NADPH:cytochrome P450 reductase, an enzyme essential for reactions catalysed by cytochrome P450 mono-oxygenases in plants. Plant J 4:47–60
Cane DE (1999) Isoprenoid biosynthesis overview. In: Cane DE (ed) Comprehensive natural product chemistry, vol. 2. Elsevier, Amsterdam, pp 1–13
Rohmer M (1999) A mevalonate-independent route to isopentenyl diphosphate. In: Cane DE (ed) Comprehensive natural product chemistry, vol. 2. Elsevier, Amsterdam, pp 45–67
Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130: 1079–1089
Kuzuyama T, Seto H (2003) Diversity of the biosynthesis of isoprene units. Nat Prod Rep 20: 171–183
Yamazaki Y, Kitajima M, Arita M et al (2004) Biosynthesis of camptothecin. In silico and in vivo tracer study from [1-13C]glucose. Plant Physiol 134:161–170
Arita M (2003) In silico atomic tracing by substrate-product relationships in Escherichia coli intermediary metabolism. Genome Res 13:2455–2466
Park SU, Yu M, Facchini PJ (2002) Antisense RNA-mediated suppression of benzophenanthridine alkaloid biosynthesis in transgenic cell cultures of California poppy. Plant Physiol 128:696–706
Frick S, Chitty JA, Kramell R et al (2004) Transformation of opium poppy (Papaver somniferum L.) with antisense berberine bridge enzyme gene (anti-bbe) via somatic embryogenesis results in an altered ratio of alkaloids in latex but not in roots. Transgenic Res 13:607–613
Allen RS, Millgate AG, Chitty JA et al (2004) RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat Biotechnol 22:1559–1566
Asano T, Kobayashi K, Kashihara E et al (2013) Suppression of camptothecin biosynthetic genes results in metabolic modification of secondary products in hairy roots of Ophiorrhiza pumila. Phytochemistry 91:128–139
Acknowledgments
This work was supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), and by CREST of the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yamazaki, M., Asano, T., Saito, K. (2013). Camptothecin Production and Biosynthesis in Plant Cell Cultures. In: Gang, D. (eds) 50 Years of Phytochemistry Research. Recent Advances in Phytochemistry, vol 43. Springer, Cham. https://doi.org/10.1007/978-3-319-00581-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-00581-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00580-5
Online ISBN: 978-3-319-00581-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)