Abstract
Inflammatory breast cancer (IBC) is the most aggressive form of breast cancer. Despite extensive study, whether inflammation contributes to the tumorigenicity or aggressiveness of IBC remains largely unknown. In this chapter, we will review the potential role played by inflammation in IBC based on the results of in vitro, in vivo, and patient studies. Current evidence suggests that several major inflammatory signaling pathways are constitutively active in IBC and breast cancer. Among them, the NF-κB, COX-2, and JAK/STAT signaling systems seem to play a major role in the tumorigenesis of IBC. Inflammatory molecules such as interleukin-6, tumor necrosis factor alpha (TNF-α), and gamma interferon have been shown to contribute to malignant transformation in preclinical studies of IBC, while transforming growth factor-β, interleukins 8 and 1β, as well as TNF-α appear to play a role in proliferation, survival, epithelial–mesenchymal transition, invasion, and metastasis. In this chapter, we also describe work thus far involving inhibitors of inflammation in the development of prevention and treatment strategies for IBC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast Cancer
- Inflammatory Breast Cancer
- SUM149 Cell
- Inflammatory Breast Cancer Patient
- Inflammatory Breast Cancer Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Breast cancer is the second most common cancer, following skin cancer, among women in America. The American Cancer Society estimates that there will be 232,670 new cases of breast cancer among women in the United States in 2014 (ACS 2014). It is also the second leading cause of death from cancer in women, approximately with 40,000 predicted deaths for 2014. Inflammatory breast cancer (IBC) is the most aggressive form of breast cancer. Although it accounts for an estimated incidence rate of up to 5 % of breast cancers (Anderson et al. 2005; Hance et al. 2005; Jaiyesimi et al. 1992), IBC is responsible for a disproportionate 8–10 % of all breast cancer-related deaths (Hance et al. 2005).
The word “inflammatory” was first applied to the IBC subtype of breast cancer by Haagensen (1971). His description was based on certain presenting features that are unique to this subgroup of patients. IBC presents with rapid onset of breast erythema occupying at least one-third of the breast, accompanied by breast edema leading to the characteristic peau d’orange appearance of the skin. Other features include breast enlargement, pain, and tenderness. Approximately 50 % of patients do not present with a palpable mass or radiographic evidence of cancer (Ueno et al. 1997; Yang et al. 2008). Almost all IBC patients present with lymph node metastasis at the time of diagnosis, and approximately 30 % present with distant metastasis (Jaiyesimi et al. 1992; Li et al. 2011).
For diagnosing IBC, consensus guidelines recommend at a minimum a core biopsy to enable detection of invasive carcinoma and to allow marker study (hormone receptors and HER2). A skin punch biopsy to confirm the presence of dermal lymphatic invasion, one of the hallmarks of IBC, is also strongly recommended in suspected cases (Dawood et al. 2011).
Treatment of IBC, as for other types of breast cancer, involves a multidisciplinary approach that includes surgery, radiation therapy, and medical oncology. Patients are stratified according to extent of disease and the molecular subtype. This approach has been associated with a significant reduction in cancer-related mortality (Ueno et al. 1997; Kesson et al. 2012). Currently, the most active anti-cancer agents include anthracycline and taxanes, in addition to anti-HER2 therapy and endocrine therapy. However, compared with other types of breast cancer, treating IBC has proved to be more challenging mainly because of its rapidly aggressive nature combined with the lack of effective targeting therapy.
Despite extensive study, whether inflammation contributes to the tumorigenicity or aggressiveness of IBC remains unknown. In this chapter, we will review the potential role played by inflammation in IBC based on the results of in vitro, in vivo, and patient studies. We will also describe work thus far involving inhibitors of inflammation in the prevention and treatment of IBC.
3.2 Inflammatory Signaling Pathways Associated with IBC
Several intrinsic pathways driven by oncogenes or tumor suppressor genes have been shown to activate the expression of inflammation-related programs in both IBC and breast cancer in general. These pathways are described below.
NF-κB The nuclear factor of kappaB (NF-κB) family of sequence-specific transcription, known to play a critical role in inflammation and the innate immune response, has recently been implicated in tumorigenesis. It is ubiquitously expressed in all cell types, where, in most cases, it is maintained in an inactive state in the cytoplasm bound to a class of inhibitory proteins known as IκBs (inhibitors of κB). Activation of NF-κB occurs by a variety of stimuli and is regulated in normal cells via two main pathways, the classical (canonical) pathway and the alternative (non-canonical) pathway (Prasad et al. 2010). Both pathways involve kinase-dependent degradation of inhibitory molecules to release NF-κB, but they differ in the inhibitory molecule involved, the activated kinases, and the types of NF-κB proteins as well as the stimuli that trigger them. Upon activation, NF-κB is transported to the nucleus, where it upregulates the expression of target genes that are responsible for a wide variety of effects, including the inflammatory and immune response, proliferation, cell–matrix adhesion, chemotaxis, and angiogenesis (Shostak and Chariot 2011).
In a variety of cancers, including breast cancer, NF-κB undergoes persistent (constitutive) activation (Nakshatri et al. 1997). Laere et al. (2005) performed a genome-wide expression profile using a cDNA microarray to compare IBC and non-IBC tissue samples. The authors reported that an unusually high number of NF-κB target genes were differentially overexpressed in IBC versus non-IBC. In a similar study, the mRNA expression levels of 60 NF-κB-related genes were compared in IBC versus non-IBC samples using real-time quantitative RT-PCR. The authors reported that approximately 60 % of NF-κB-related genes were upregulated in the IBC samples compared to non-IBC samples. The resulting five-gene molecular signature was matched with patient outcomes; it included two genes that are regulated by NF-κB (Lerebours et al. 2008). Collectively, these studies confirm the importance of NF-κB in IBC and its contribution to the aggressive phenotype of IBC.
In a recent study, El-Shinawi et al. (2013) examined the association between evidence of human cytomegalovirus (HCMV) infection in the serum and tissue of IBC and non-IBC patients and whether HCMV was associated with NF-κB activation in IBC. The authors reported significantly higher levels of serum HCMV IgG as well as higher levels of HCMV DNA in the tumor tissue of IBC patients. Infected IBC samples also had enhanced NF-κB/p65 signaling compared to non-IBC controls (El-Shinawi et al. 2013). While these findings suggest an association between IBC and HCMV could exist, the authors noted that the evidence is not conclusive. If the results are confirmed, they may help explain the higher incidence of IBC in some geographic areas (Soliman et al. 2011).
Several studies have suggested complex cross talk between NF-κB and estrogen receptor (ER) in IBC as well as in breast cancer in general. In a separate study, Van Laere et al. reported that NF-κB activation in IBC tumors was associated with ER downregulation, which was linked to both EGFR and/or HER2 overexpression and MAPK hyperactivation (Laere et al. 2007). Additionally, ER seems to be capable of inhibiting both the constitutive and the inducible activation of NF-κB in a dose-dependent manner (Biswas et al. 2004). On the other hand, studies also seem to suggest that in ER-positive patients, cross talk between ER and NF-κB occurs that may either be transrepressive or positive (Kalaitzidis and Gilmore 2005). Theoretically, this could explain how in luminal B subtypes and some ER-positive IBC patients, resistance to hormonal therapy and poorer outcome could result from positive cross talk between NF-κB and ER leading to enhanced ER-mediated expression of genes involved in cell proliferation, survival, and resistance.
COX family The cyclooxygenase (COX) family of enzymes consists of two members, COX-1 and COX-2. Both enzymes catalyze the conversion of arachidonic acid to prostanoids and are also responsible for the generation of eicosanoid products, which are important mediators of pain and inflammation. Tissue upregulation of COX-2 can be triggered by several stimuli, including growth factors and oncogenes (Williams et al. 1999). Aberrant activation of COX/prostaglandin signaling is common in many cancers, especially in colon cancer, where COX-2 is overexpressed in 85 % of tumors (Brown and DuBois 2005). This has also been the case with breast cancer; enzyme levels have been found to be increased in 40 % of breast tumor tissues examined (Yoshimura et al. 2003).
Several studies have documented the role that the PTGS2 gene, which encodes COX-2, plays in cell proliferation, invasion, angiogenesis, and metastasis (Wang and Dubois 2004; Menter et al. 2010). Overexpression of COX-2 in breast cancer correlates with a more aggressive breast cancer profile that is characterized by higher proliferation rates, larger tumors, higher pathologic grade, hormone receptor negativity, and HER2 overexpression (Ristimaki et al. 2002; Subbaramaiah et al. 2002). Compared with non-IBC tumors, the PTGS2 gene is differentially overexpressed in IBC, and it is identified as a key component in the molecular signature for IBC (Laere et al. 2005, 2007). Moreover, prostaglandin E2 (PGE2), which is a product of COX-2 enzymatic activity, is known to be upregulated in primary IBC tumors and metastatic lesions (Robertson et al. 2008). These findings emphasize the role of the COX-2 pathway in IBC and its potential use as a target for disease prevention and treatment.
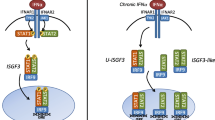
JAK/STAT signaling The JAK/STAT signaling system is the main pathway for a variety of cytokines, including interferon and interleukins (e.g. IL-6), as well as growth factors or other chemical messengers. Depending on both the context and the integrity of the pathway, JAK/STAT signaling can stimulate proliferation and cell migration versus differentiation and apoptosis (Rawlings et al. 2004).
STAT3 is known to be constitutively activated in >50 % of breast cancers and tumor-derived cell lines (Garcia et al. 1997; Diaz et al. 2006). Using small interfering RNA (siRNA) to block STAT3 in both cell culture and xenograft models of breast cancer, investigators were able to show increased apoptosis through the Fas-mediated intrinsic apoptotic pathway, as well as reduced expression of the transmembrane molecule B-cell lymphoma-extra large (Bcl-xL), which promotes survival (Kunigal et al. 2009). Additionally, constitutive activation of STAT led to accelerated mammary tumorigenesis and increased metastatic potential in cancer cells expressing ErbB2 (Barbieri et al. 2010). Moreover, ablation of STAT3 resulted in inhibition of anchorage-independent growth of breast cancer cells, thus limiting their metastatic potential. More recently, however, it was found that JAK2/STAT3 appears to be necessary for growth and survival of tumor cells expressing the cancer stem cell (CSC) phenotype (Ma et al. 2011). More recently, investigators were able to induce cell death in SUM149 IBC tumor spheres by inhibiting STAT3 activation in a dose-dependent manner using a novel JAK2 inhibitor (Ma et al. 2011).
In summary, current evidence suggests that several major inflammatory signaling pathways are constitutively active in IBC and breast cancer. Among them, the NF-κB, COX-2, and JAK/STAT signaling systems seem to play a major role in the tumorigenesis of IBC. Blocking these pathways may prove to be a promising therapeutic strategy owing to their multiple roles in promoting cancer cell survival and metastasis.
3.3 Role of Inflammatory Molecules in the Development of IBC: Evidence From In Vitro Studies
3.3.1 Role of Inflammatory Molecules in Malignant Transformation
Accumulating DNA mutations play a causal role in the process of malignant transformation. Oncogenic insults may result in activation of oncogenes, loss of tumor suppressor genes, or the constitutive activation of membrane receptors or lead to the alteration of critical cellular processes such as the cell cycle or apoptosis (Hanahan and Weinberg 2000). In this setting, inflammation may contribute to carcinogenesis through the activation of the DNA damage response system in response to major oncogenic insults (Martin et al. 2011; Hartman et al. 2011). Moreover, viral infections, such as human papillomavirus (HPV), whose genome has been detected in breast cancer tissue, may also cause DNA damage, resulting in activation of the DNA damage response pathway, and stimulate the formation of a pro-inflammatory tumor microenvironment (Moody and Laimins 2009; Kan et al. 2005).
Interleukin-6 (IL-6), which is overexpressed in the SUM149 preclinical model of IBC (Golen et al. 2000), plays a potent role in malignant transformation. IL-6 was able to convert a non-transformed mammary epithelial cell line (MCF-10A) to the transformed state in 24–36 h (Iliopoulos et al. 2009, 2010).
Additionally, HER2 overexpression, which is known to occur in up to 60 % of IBC tumors and 25 % of non-IBC tumors, is associated with poor outcome (Guerin et al. 1989; Kallioniemi et al. 1991). HER2 amplification was associated with marked increase in IL-6 in breast cancer cells and induced STAT3 activation, suggesting a HER2-IL-6-STAT3 signaling pathway could play a critical role in tumorigenesis (Hartman et al. 2011).
On the other hand, cancer stem cells (CSCs), also known as tumor-initiating cells, are highly tumorigenic cells and are enriched in IBC tumors as well as in preclinical models of IBC (Laere et al. 2010; Charafe-Jauffret et al. 2010; Xiao et al. 2008). IL-6 acts as a growth factor for CSCs and is sufficient to convert non-stem cancer cells to CSCs (Iliopoulos et al. 2011). IL-6 gene expression was found to promote self-renewal, as well as invasive potential, in both normal and MCF-7-derived spheroids (Sansone et al. 2007). IL-6 is also at the center of epigenetic regulation of stem cells (D’Anello et al. 2010; Hodge et al. 2005). IL-6 thus plays a critical role in mediating the epigenetic switch that involves NF-κB and STAT3 and links inflammation to cell transformation (Iliopoulos et al. 2009, 2010).
Additional inflammatory signaling involved in the regulation of CSCs includes the tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) pathways, both of which are upregulated in breast CSCs (Murohashi et al. 2010). Both cytokines are also able to activate the NF-κB pathway (Cheshire and Baldwin 1997; Hayden and Ghosh 2008; Matsumoto et al. 2005). Treatment with the chemokine IL-8 resulted in increased mammosphere formation, whereas IL-8 receptor/CXCR1 blockade depleted the breast CSC population both in vitro and in xenografts (Murohashi et al. 2010; Charafe-Jauffret et al. 2009; Ginestier et al. 2010). Expression of CCL5/RANTES was also found to be upregulated in breast CSC populations (Murohashi et al. 2010).
3.3.2 Role of Inflammatory Molecules in the Survival of IBC Cells
One of the hallmarks of cancer cells is their capacity to acquire resistance to apoptotic signals (Hanahan and Weinberg 2000). Transforming growth factor (TGF)-β is a pro-apoptotic cytokine that normally induces cell cycle arrest in the early phases of tumorigenesis. The mechanisms by which cancer cells escape the inhibitory effects of TGF-β are not fully understood but may include inactivating mutations or homozygous deletions(Kaklamani et al. 2003; Pasche et al. 2004; Dunning et al. 2003) or upregulation in oncogenic expression (Zhang et al. 2003). Once the pro-apoptotic functions of TGF-β are subverted, its tumorigenic potential becomes unhindered, thus stimulating growth, invasion, and angiogenesis (Biswas et al. 2007; Lei et al. 2002).
Likewise, although TNF-α promotes apoptosis in MCF-7 cells (Simstein et al. 2003), a process similar to TGF-β subverts the pro-apoptotic effect of TNF-α (Rivas et al. 2008). HER2 amplification, which is present in up to 60 % of IBC patients, was shown to confer resistance to TNF-α-induced apoptosis in breast cancer cell lines mainly through an Akt/NF-κB anti-apoptotic cascade (Zhou et al. 2000). Likewise, increased expression of claudin-1 was able to reverse TNF-α-induced apoptosis in the MCF-7 breast cancer cell line (Liu et al. 2012). These studies suggest that there are multiple pathways by which breast cancer cells can overcome TNF-α-induced apoptosis, thus promoting cancer cell survival and unleashing the tumorigenic potential of TNF-α.
Using mastectomy samples from patients with either invasive or non-invasive breast cancer as well as tissue from benign controls, both IL-6 protein levels and IL-6 receptor levels, were correlated with tissue levels of B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X (Bax) proteins (Garcia-Tunon et al. 2005). A higher proportion of malignant samples, compared with benign controls, were positive for IL-6, Bcl-2, and Bax by immunohistochemistry. The more invasive samples had a more intense immunoreaction for Bcl-2 than did benign lesions. In addition, infiltrating tumors that were positive for IL-6 were also positive for Bcl-2 with a high degree of correlation between immunoreaction intensities of both antibodies (Garcia-Tunon et al. 2005). These results, along with others, suggest that IL-6 plays a central role in protecting cancer cells against apoptosis as well as the regulation of survival in CSCs via several pathways, including the canonical JAK/STAT3 pathway, and by direct action on Bcl-2 family gene products (Iliopoulos et al. 2011; Hinohara and Gotoh 2010; Heinrich et al. 2003).
3.3.3 Role of Inflammatory Molecules in the Proliferation of IBC Cells
Cancer is fundamentally a disease of inappropriate cell division and proliferation. Cytokines can enhance growth through their interaction with growth factors, e.g., ER, and transcriptional pathways such as IL-6/JAK/STAT3. An in vitro study comparing ER-positive to ER-negative breast cancer cell lines reported higher levels of IL-6-mediated STAT3 phosphorylation in ER-negative versus ER-positive cells. Upon exposure of MCF-7 ER-positive cells to IL-6, tumor cell growth rates were enhanced by >two-fold (Sasser et al. 2007).
IL-1β is a major proinflammatory cytokine that is known to contribute to tumor proliferation, angiogenesis, and local invasion (Apte et al. 2006). Higher IL-1β levels in breast cancer tissue or serum were correlated with more aggressive disease and poorer outcome (Goldberg and Schwertfeger 2010). However, the interaction between IL-1β and ER in tumor growth in breast cancer has been less understood with evidence supporting transcriptional activation of ER by IL-1β (Speirs et al. 1999). A recent study suggested that IL-1 secretion in breast cancer may be regulated by estradiol in vivo and that its release may be inhibited by anti-estrogen therapy (Abrahamsson et al. 2012).
TNF-α, a potent suppressor of proliferation in normal cells, was found to enhance proliferation in the T47D breast cancer cell line through an NF-κB-dependent pathway (Rubio et al. 2006). Proliferating cells were found to express high levels of cyclin D1 (Rivas et al. 2008). Furthermore, the addition of a specific NF-κB inhibitor, Bay 11-7082, was able to block TNF-α-induced tumor promotion and cyclin D1 expression. Additional in vitro studies on MCF-7 cells demonstrated the capacity of TNF-α to upregulate several genes associated with cancer proliferation (Yin et al. 2009). Alternatively, TNF-α can interact with ER as well as other transcription factors in an NF-κB-independent manner to regulate genes that are important for proliferation in breast cancer (Gloire et al. 2006).
3.3.4 Role of Inflammatory Molecules in the Invasion, Metastasis, and Angiogenesis of IBC Cells
Cancer cell progression is a multistep process that involves the acquisition of several characteristics that include epithelial–mesenchymal transition (EMT), cell invasion, migration, intra- and extravasation, and angiogenesis. EMT is the process by which cancer cells lose epithelial properties such as cell polarity and cell-to-cell contact and acquire mesenchymal (fibroblastic) characteristics. This process confers malignant properties such as invasiveness and motility and is essential for cancer cells to metastasize (Thiery 2002; Thiery et al. 2009).
IBC gene expression profiles have revealed the activation of specific stem-cell-related pathways that contribute to the activation of NF-κB, which in turn induces EMT (Laere et al. 2010). Recently, investigators were able to reproduce EMT in IBC cells using a three-dimensional culture system. IBC cells exhibited a reduction in epithelial markers (E-cadherin) and overexpression of mesenchymal marker vimentin. Investigators were able to inhibit EMT by blocking the EGFR pathway using an EGFR tyrosine kinase inhibitor, erlotinib (Zhang et al. 2009).
Overexpression of TGF-β has been associated with several tumors and correlates with aggressive features (Derynck et al. 2001). TGF-β plays a central role in the induction of EMT (Moustakas and Heldin 2007). It inhibits expression of E-cadherin (Xu et al. 2009) and is associated with reduced levels of claudins and occludins, as well as tight-junction degradation (Moustakas and Heldin 2007). Moreover, TGF-β1 induces expression of Mdm2, which results in the destabilization of p53, a critical step in the EMT of breast cancer that is associated with advanced disease (Araki et al. 2010).
In tissue samples of human breast cancers, high levels of TGF-β1 mRNA were associated with increased angiogenesis as measured by microvessel density, features that are common in IBC (de Jong et al. 1998). TGF-β is known to trigger the expression of vascular endothelial growth factor (VEGF) as well as act as a chemo-attractant for monocytes, which in turn release angiogenic factors (Yang and Moses 1990; Ashcroft 1999). Furthermore, TGF-β is also able to induce cell migration through the expression of matrix metalloproteases MMP-2 and MMP-9 (Hagedorn et al. 2001). The results of these studies suggest that TGF-β exerts a broad range of effects that confer invasiveness and metastasis through its regulation of EMT and cell motility (Docherty et al. 2006; Yang et al. 2006).
Higher levels of IL-6 in the SUM149 IBC model have been attributed to regulation by RhoC GTPase, which plays a role in the development of the invasive/angiogenic phenotype of IBC (Golen et al. 2000). In turn, IL-6 activates multiple effectors involved in the process of invasion and metastasis (Heinrich et al. 2003; Tawara et al. 2011). Sullivan et al. (2009) observed that IL-6 induced EMT as well as enhanced invasiveness of MCF-7 cancer cells. Furthermore, IL-6 produced by fibroblasts or stromal adipocytes derived from breast tissue or from metastatic sites promoted invasion in MCF-7 cells (Studebaker et al. 2008; Walter et al. 2009).
The chemokine IL-8 is another inflammatory molecule that is differentially expressed in IBC tumors (Laere et al. 2005, 2007). IL-8 production is amplified in metastatic breast cancer lesions and plays a key role in tumor progression, invasion, and angiogenesis (Freund et al. 2004; Yao et al. 2007; Lin et al. 2004). A similar effect can be seen with increased tumor and serum levels of IL-1β, which were associated with invasiveness in ER-negative breast tumors (Goldberg and Schwertfeger 2010). In ER-positive tumors, IL-1β was found to promote EMT changes as well as cell migration, invasion, angiogenesis, and metastasis (Franco-Barraza et al. 2010; Wang et al. 2005).
Studies in MCF-7 breast cancer cells have also shown TNF-α to promote the expression of a panel of genes that are known to be associated with invasion and metastasis (Yin et al. 2009). Moreover, the chemokine receptor CXCR4 and its ligand CXCL12 (stromal cell-derived factor-1 alpha) are differentially expressed in IBC tumors and are known to regulate interactions between tumor cells and the microenvironment that are critical for the development of organ-specific metastasis (Cabioglu et al. 2007; Clezardin 2011).
3.4 Role of Inflammatory Molecules in the Development of IBC: Evidence From In Vivo Studies
One of the challenges facing IBC research is the development of preclinical models that accurately recapitulate the aggressiveness of the disease. Currently, there is a need for better immunocompetent mouse models of IBC that allow assessment of the molecular and inflammatory mechanisms underlying the disease and the development of effective therapeutic targets.
In a recent study that looked at the role of NF-κB signaling in conferring self-renewal to breast cancer cells, three types of IBC SUM149 cells were prepared and injected into the mammary fat pads of nude mice. These included cells expressing IκBα-SR at low or high density or an empty vector (Kendellen et al. 2013). Investigators assessed self-renewal by measuring the ability of cells when injected at limiting dilutions to establish primary tumors. Cells with deficient NF-κB signaling produced smaller tumors at a much later onset compared to those with empty vector, whereas the low density of SUM149 cells expressing IκBα-SR did not form tumors. The ability to self-renew appears to require both intact canonical and non-canonical NF-κB pathways (Kendellen et al. 2013). This demonstrates the importance of NF-κB for tumorigenesis in xenograft models.
Cyclooxygenase-2 (COX-2) is over-expressed in mammary tumors derived from rodent models of breast cancer. Enhanced COX-2 expression was found to be sufficient to induce mammary gland tumorigenesis in the mouse mammary tumor virus (MMTV)/COX-2 transgenic mouse strain, thus providing evidence for its in vivo oncogenicity (Liu et al. 2001). Additionally, mammary gland involution after weaning was delayed in the transgenic animals compared to controls, suggesting that apoptosis suppression was also involved (Liu et al. 2001).
Studies have also demonstrated that tumor formation in these models can be suppressed either pharmacologically by using anti-inflammatory drugs, including COX inhibitors, or through genetic ablation (Howe 2007; Howe et al. 2001; Howe et al. 2005). COX inhibitors were also evaluated in HER2/neu transgenic mice, which are also ER negative. Celecoxib administration was able to significantly delay tumor formation in the animal model (Howe et al. 2002; Lanza-Jacoby et al. 2003).
To examine the consequences of knocking out COX-2, investigators adopted an approach used in intestinal cancer models (Oshima et al. 1996), by crossing COX-2 knockout mice with mammary tumor virus/neu deletion mutant (MMTV/NDL) mice and comparing tumor multiplicity to HER2/neu transgenic mice that were COX-2 wild type, heterozygous, and null (Howe et al. 2005). Tumor multiplicity and size were significantly reduced in COX-2 knockout mice (heterozygous and null) compared to controls (Howe et al. 2005). Additionally, the authors observed that COX-2 null animals were associated with reduced expression in several angiogenesis factors, which led to a reduction in mammary blood vessel formation. Together, these studies suggest that an intact COX-2 pathway is both necessary and sufficient for the induction of tumorigenesis.
Similarly, the tumorigenicity of TGF-β was assessed by developing a doxycycline-inducible triple transgenic mice model in which doxycycline can be used to induce TGF-β1 expression in polyomavirus middle T antigen (PyVmT) transformed mammary tumors (Muraoka-Cook et al. 2004). TGF-β1 stimulation resulted in rapidly accelerated metastatic progression with >ten-fold increase in lung metastases in as little as 2 weeks. Antisense-mediated inhibition of TGF-β1 resulted in decreased cell motility, survival, anchorage-independent growth, tumorigenicity, and metastasis. Similarly, Criswell et al. looked at the role of TGF-β type III receptors in inducing EMT, cancer cell motility, and invasion of metastatic cancer cells through a similar transgenic model (Criswell et al. 2008).
To address the role of IL-6 in cancer proliferation, investigators looked at whether expression of IL-6 in MCF-7 cells would alter tumor growth rates in immunocompromised mice. Xenografts expressing IL-6 underwent rapid engraftment and expansion relative to MCF-7 xenografts that did not express IL-6 (Sasser et al. 2007). On the other hand, using siRNA to knock down STAT3 expression in nude mice, investigators were able to suppress breast cancer cell growth compared with controls. pRNAi-STAT3 also led to downregulation of STAT3 and Bcl-xL, as well as upregulation of Fas and induction of apoptosis via expression of cleaved caspase-3 (Kunigal et al. 2009; Matthews et al. 2007).
3.5 Evidence From Patients for the Role of Inflammation in IBC
The rarity of IBC as a disease has not allowed the role of inflammation to be systematically examined in clinical studies involving IBC patients; however, numerous clinical reports and observational studies have addressed the role of inflammation in breast cancer in general.
C-reactive protein (CRP) is an acute-phase protein that is considered the classic marker of systemic inflammation. CRP levels in plasma are known to rise rapidly in response to acute inflammation (Black et al. 2004; Casas et al. 2008; Gabay and Kushner 1999), but have also been found to be moderately increased in chronic inflammatory disease (Hirschfield and Pepys 2003). Large epidemiologic studies have suggested a correlation between high circulating levels of CRP and the risk of developing cancer. This observation has not been demonstrated for breast cancer, however. The Women’s Health Study measured baseline plasma CRP levels for 27,919 healthy women aged 45 years or older. After a mean follow-up of 10 years, 892 patients had developed breast cancer; results showed no association between increased CRP levels and the risk of developing breast cancer (Zhang et al. 2007).
Likewise, in a Danish general population study, 10,408 individuals had their CRP levels measured at baseline and were observed for up to 16 years. During follow-up, 1,624 went on to develop cancer, and 998 patients died. Increased CRP levels were associated with an increased risk of cancer of any type and possibly an increased risk of colorectal cancer and lung cancer, but not breast cancer (Allin et al. 2009).
On the other hand, high CRP levels were found to be associated with poor prognosis in several types of cancer, including breast cancer. Allin et al. (2011) looked at CRP levels at baseline in 2,910 breast cancer patients. Higher CRP levels were found to be associated with larger tumor size, development of distant metastases, and poor prognosis. More importantly, the authors reported that breast cancer was the leading cause of death in this cohort, thus excluding the possibility that the outcome was confounded by risk of cardiac disease, for which CRP is an established risk factor (Allin et al. 2011).
Ristimaki and colleagues analyzed the expression of COX-2 protein using immunohistochemistry in tissue specimens of 1,576 patients with breast cancer (Ristimaki et al. 2002). Increased levels were found in approximately 40 % of breast tumors and were associated with shorter distant metastasis-free survival. Tumors with COX-2 expression were associated with negative hormone receptor status as well as the presence of HER2 amplification and axillary nodal metastasis. Additional unfavorable features associated with COX-2 increase include larger tumor size, higher histological grade, high Ki-67 proliferation rates, higher p53 expression, and ductal type histology. However, the differences in outcome between patients with increased COX-2 protein and those without was even more pronounced in patients with more favorable prognostic characteristics, such as ER positivity, low p53 expression, and no HER-2 amplification.
The preclinical model of IBC, a disease known for its aggressive course, expressed increased levels of IL-6 and IL-8 (Golen et al. 2000). High levels of IL-6 were reported to be associated with poorer response to therapy in patients with metastatic breast cancer (Zhang and Adachi 1999). This was confirmed in the clinical setting; breast cancer patients were found to have higher serum levels of IL-6 than do healthy women (Kozlowski et al. 2003; Jiang et al. 2000). Two studies looked at IL-6 in different tumor stages and found higher levels of IL-6 were correlated to advanced cancer stage (Jablonska et al. 2001; Lyons et al. 2011). Others looked at how serum levels correlated with recurrence and outcome in the metastatic setting (Nishimura et al. 2000; Bozcuk et al. 2004; Salgado et al. 2003). One study analyzed the association between IL-6 serum levels and response to therapy as designated by the Response Evaluation Criteria in Solid Tumors (RECIST); higher levels were associated with poor objective response to therapy (Zhang and Adachi 1999).
Similarly, TGF-β levels in plasma in breast cancer patients were found to be increased and predictive of lymph node and distant metastasis (Ivanovic et al. 2009; Yu et al. 2010). Additionally, increased levels of IL-1β in the tumor and in the serum of ER-negative breast tumors were correlated with tumor invasiveness and poor outcome (Studebaker et al. 2008). The production of IL-8 in ER-positive breast cancer patients was associated with shorter relapse-free survival (Freund et al. 2003). Furthermore, increased circulating levels of TNF-α were correlated with increased lymph node metastasis and breast cancer stage (Sheen-Chen et al. 1997).
3.6 Inhibitors of Inflammation for the Prevention and Treatment of IBC
Management of IBC consists of tri-modality therapy: neoadjuvant chemotherapy, then modified radical mastectomy, followed by locoregional radiotherapy. Prior to the era of multimodality therapy, the 5-year overall survival rate was less than 5 % (Robbins et al. 1974). In a more recent study, patients who received all components of tri-modality therapy achieved an overall survival rate at 5 years of 51 %, versus 24 % for patients who did not receive all three components (Bristol et al. 2008).
One of the biggest challenges in the treatment of IBC thus far has been the lack of clinically relevant treatment targets. In a retrospective analysis, 316 IBC patients were assigned according to ER and HER2 status into four groups: ER positive (33 %), ER positive/HER2 positive (12 %), HER2 positive (26 %), and triple negative (29 %) (Li et al. 2011). The triple-negative subtype was found to predict the worst overall survival and high recurrence rates. Hence, the search for potential treatment targets has become a priority in particular for patients with triple-negative IBC. One promising tactic has been to target the inflammatory pathways in the adjuvant setting or in combination with systemic therapy (Pierga et al. 2010; Agrawal and Fentiman 2008).
Pan et al. looked at the activity of tetrathiomolybdate, a copper chelator, in tumors derived from SUM149 IBC cells. Tetrathiomolybdate was shown to effectively suppress angiogenesis and motility in IBC cell line tumors through its inhibitory effects on NF-κB signaling (Pan et al. 2002, 2003). This was accompanied by reduced levels of VEGF, basic fibroblast growth factor (bFGF), IL-6, IL-1α, and IL-8, as well as decreased tumor volume (Pan et al. 2002). Another compound that is known to inhibit the NF-κB pathway is pyrrolidinedithiocarbamate (Zhou et al. 2008). Inhibition of the NF-κB pathway, which is upregulated in IBC, is one of the most promising areas of research.
The chemokine CXCR4/CXCL12 receptor/ligand pair has been observed to promote angiogenesis as well as confer survival on CSCs (Duda et al. 2011; Greenfield et al. 2010). The CXCR4 antagonist, CTCE 9908, in combination with paclitaxel, was evaluated in a SUM149 preclinical model of triple-negative IBC (Singh et al. 2010). CTCE-9908 as a single agent inhibited skeletal metastases but failed to prevent primary tumor growth or pulmonary metastasis.
Owing to their unacceptable cardiotoxicity, the use of selective COX-2 inhibitors has been limited despite initial enthusiasm regarding promising epidemiologic results and their anti-cancer activities (Psaty and Furberg 2005; Graham et al.2005). Interest has instead shifted to searching for alternative COX-2-targeted agents. One such target is the family of prostanoid receptors, particularly EP4, that bind with PGE2, which is a product of COX-2 (Jones et al. 2009). EP4 was found to mediate invasion and metastasis in both inflammatory and non-IBCs, and EP3 suppressed angiogenesis in IBC tumors (Robertson et al. 2008, 2010). None of the available EP4 antagonists have yet been tested in cancer patients.
Apricoxib is a novel selective COX inhibitor analog that is currently under evaluation in breast cancer in combination with lapatinib and capecitabine in the treatment of HER2/neu-positive advanced breast cancer (Health NIo 2001). Tranilast is another compound under investigation and is known as a potent inhibitor of PGE2. It was shown to suppress tumorigenesis in both xenograft mammary tumors and human triple-negative breast cancer cells (Chakrabarti et al. 2009; Subramaniam et al. 2010, 2011).
Another agent under evaluation in breast cancer is fish oil. Fish oils contain the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which ultimately lead to the inhibition of inflammation in the body (Weaver et al. 2009). This is suspected to occur through inhibition of COX/PGE2 production (Wendel and Heller 2009).
Recently, a new role for statins as a preventive agent against cancer has emerged. Recent evidence has suggested that in addition to their lipid-lowering effects, statins exert powerful anti-inflammatory effects by acting on multiple inflammatory gene pathways (Jain and Ridker 2005). The anti-cancer effects of statins have also been linked to the mevalonate pathway, which in turn leads to the inhibition of many downstream growth factors (Nielsen et al. 2012). Large observational studies have pointed toward the potential role this pathway has against cancer in general as well as breast cancer specifically (Nielsen et al. 2012; Ahern et al. 2011). Brewer et al. have also addressed the potential role of statins in improving the survival of patients with IBC (Brewer et al. 2012).
Curcumin is the principal derivative of turmeric, the popular Indian spice and a member of the ginger family. Various preclinical studies have looked into its role in breast cancer as a chemosensitizer and radiosensitizer to agents such as doxorubicin, 5-fluorouracil, and paclitaxel (Goel and Aggarwal 2010). Curcumin is a known potent inhibitor of NF-κB, STAT3, and COX-2 as well as other growth factors and anti-apoptotic proteins (Goel and Aggarwal 2010).
3.7 Conclusions and Future Directions
Current evidence supports that inflammation plays a central role in the process of tumor formation in IBC at various levels. Several important inflammatory gene pathways are differentially upregulated in IBC and contribute to the formation of a pro-inflammatory feedback loop that is critical for malignant transformation (Hartman et al. 2011). On the other hand, downstream cytokines and chemokines are involved at every step of tumorigenesis/carcinogenesis, including initiation, transformation, proliferation, cancer cell survival, invasion, angiogenesis, and metastasis. Several pharmacological compounds that target the inflammatory signaling pathways are currently being tested in the laboratory and in the clinical setting. There is a demand for better immunocompetent IBC mouse models for more accurate in vivo testing and drug development. Proteomic analysis of IBC offers the opportunity to conduct a quantitative and functional evaluation of protein activity in the various signaling networks involved (Chen et al. 2002). It allows assessment of posttranslational modifications, complementing gene expression studies in IBC (Bichsel et al. 2001). New approaches such as high-throughput screening may help identify novel agents that inhibit key signaling pathways. Ultimately, the clinical role of targeting inflammation in IBC needs to be tested prospectively.
References
Abrahamsson A, Morad V, Saarinen NM, Dabrosin C (2012) Estradiol, tamoxifen, and flaxseed alter IL-1beta and IL-1Ra levels in normal human breast tissue in vivo. J Clin Endocrinol Metab 97(11):E2044–E2054
ACS (2014) Cancer facts and figures 2014. American Cancer Society, Atlanta, GA. Accessed 25 Mar 2014
Agrawal A, Fentiman IS (2008) NSAIDs and breast cancer: a possible prevention and treatment strategy. Int J Clin Pract 62(3):444–449
Ahern TP, Pedersen L, Tarp M et al (2011) Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst 103(19):1461–1468
Allin KH, Bojesen SE, Nordestgaard BG (2009) Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol: Off J Am Soc Clin Oncol 27(13):2217–2224
Allin KH, Nordestgaard BG, Flyger H, Bojesen SE (2011) Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res 13(3):R55
Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH (2005) Epidemiology of inflammatory breast cancer (IBC). Breast Dis 22:9–23
Apte RN, Krelin Y, Song X et al (2006) Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer 42(6):751–759
Araki S, Eitel JA, Batuello CN et al (2010) TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Investig 120(1):290–302
Ashcroft GS (1999) Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect/Ins Pasteur 1(15):1275–1282
Barbieri I, Pensa S, Pannellini T et al (2010) Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of cten. Cancer Res 70(6):2558–2567
Bichsel VE, Liotta LA, Petricoin EF 3rd (2001) Cancer proteomics: from biomarker discovery to signal pathway profiling. Cancer J 7(1):69–78
Biswas DK, Shi Q, Baily S et al (2004) NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 101(27):10137–10142
Biswas S, Guix M, Rinehart C et al (2007) Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Investig 117(5):1305–1313
Black S, Kushner I, Samols D (2004) C-reactive Protein. J Biol Chem 279(47):48487–48490
Bozcuk H, Uslu G, Samur M et al (2004) Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine 27(2–3):58–65
Brewer TM, Masuda H, Iwamoto T et al (2012) Statin use and improved survival outcome in primary inflammatory breast cancer: retrospective cohort study. Paper presented at: CTRC-AACR: san antonio breast cancer symposium, 4–8 Dec 2012, San Antonio, TX
Bristol IJ, Woodward WA, Strom EA et al (2008) Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys 72(2):474–484
Brown JR, DuBois RN (2005) COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol: Official J Am Soc Clin Oncol 23(12):2840–2855
Cabioglu N, Gong Y, Islam R et al (2007) Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 18(6):1021–1029
Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB (2008) C-reactive protein and coronary heart disease: a critical review. J Intern Med 264(4):295–314
Chakrabarti R, Subramaniam V, Abdalla S, Jothy S, Prud’homme GJ (2009) Tranilast inhibits the growth and metastasis of mammary carcinoma. Anti-Cancer Drugs 20(5):334–345
Charafe-Jauffret E, Ginestier C, Iovino F et al (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69(4):1302–1313
Charafe-Jauffret E, Ginestier C, Iovino F et al (2010) Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 16(1):45–55
Chen G, Gharib TG, Huang CC et al (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 1(4):304–313
Cheshire JL, Baldwin AS Jr (1997) Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaB beta degradation. Mol Cell Biol 17(11):6746–6754
Clezardin P (2011) Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res: BCR 13(2):207
Criswell TL, Dumont N, Barnett JV, Arteaga CL (2008) Knockdown of the transforming growth factor-beta type III receptor impairs motility and invasion of metastatic cancer cells. Cancer Res 68(18):7304–7312
D’Anello L, Sansone P, Storci G et al (2010) Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer 9:300
Dawood S, Merajver SD, Viens P et al (2011) International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 22(3):515–523
de Jong JS, van Diest PJ, van der Valk P, Baak JP (1998) Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol 184(1):53–57
Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29(2):117–129
Diaz N, Minton S, Cox C et al (2006) Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res: Off J Am Assoc Cancer Res 12(1):20–28
Docherty NG, O’Sullivan OE, Healy DA et al (2006) TGF-beta1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol: Ren Physiol 290(5):F1202–F1212
Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK (2011) CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res: Off J Am Assoc Cancer Res 17(8):2074–2080
Dunning AM, Ellis PD, McBride S et al (2003) A transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res 63(10):2610–2615
El-Shinawi M, Mohamed HT, El-Ghonaimy EA et al (2013) Human cytomegalovirus infection enhances NF-kappaB/p65 signaling in inflammatory breast cancer patients. PLoS ONE 8(2):e55755
Franco-Barraza J, Valdivia-Silva JE, Zamudio-Meza H et al (2010) Actin cytoskeleton participation in the onset of IL-1beta induction of an invasive mesenchymal-like phenotype in epithelial MCF-7 cells. Arch Med Res 41(3):170–181
Freund A, Chauveau C, Brouillet JP et al (2003) IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene 22(2):256–265
Freund A, Jolivel V, Durand S et al (2004) Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene 23(36):6105–6114
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 340(6):448–454
Garcia R, Yu CL, Hudnall A et al (1997) Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ: Mol Biol J Am Assoc Cancer Res 8(12):1267–1276
Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M (2005) IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 47(1):82–89
Ginestier C, Liu S, Diebel ME et al (2010) CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Investig 120(2):485–497
Gloire G, Dejardin E, Piette J (2006) Extending the nuclear roles of IkappaB kinase subunits. Biochem Pharmacol 72(9):1081–1089
Goel A, Aggarwal BB (2010) Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 62(7):919–930
Goldberg JE, Schwertfeger KL (2010) Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets 11(9):1133–1146
Graham DJ, Campen D, Hui R et al (2005) Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 365(9458):475–481
Greenfield JP, Cobb WS, Lyden D (2010) Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas. J Clin Investig 120(3):663–667
Guerin M, Gabillot M, Mathieu MC et al (1989) Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer: J Int du Cancer 43(2):201–208
Haagensen C (1971) Diseases of the breast, 2nd edn. Saunders, Philadelphia
Hagedorn HG, Bachmeier BE, Nerlich AG (2001) Synthesis and degradation of basement membranes and extracellular matrix and their regulation by TGF-beta in invasive carcinomas (review). Int J Oncol 18(4):669–681
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH (2005) Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 97(13):966–975
Hartman ZC, Yang XY, Glass O et al (2011) HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res 71(13):4380–4391
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132(3):344–362
Health NIo (2001) APRiCOT-B: Study to Evaluate Apricoxib in Combination With Lapatinib and Capecitabine in the Treatment of HER2/Neu+ Breast Cancer (TP2001-202). http://clinicaltrials.gov/show/NCT00657137. Accessed on 4 Apr 2013
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374(Pt 1):1–20
Hinohara K, Gotoh N (2010) Inflammatory signaling pathways in self-renewing breast cancer stem cells. Curr Opin Pharmacol 10(6):650–654
Hirschfield GM, Pepys MB (2003) C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM: Monthly J Assoc Phys 96(11):793–807
Hodge DR, Peng B, Cherry JC et al (2005) Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res 65(11):4673–4682
Howe LR (2007) Inflammation and breast cancer, cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res: BCR 9(4):210
Howe LR, Subbaramaiah K, Brown AM, Dannenberg AJ (2001) Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer 8(2):97–114
Howe LR, Subbaramaiah K, Patel J et al (2002) Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res 62(19):5405–5407
Howe LR, Chang SH, Tolle KC et al (2005) HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res 65(21):10113–10119
Iliopoulos D, Hirsch HA, Struhl K (2009) An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139(4):693–706
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K (2010) STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 39(4):493–506
Iliopoulos D, Hirsch HA, Wang G, Struhl K (2011) Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA 108(4):1397–1402
Ivanovic V, Dedovic-Tanic N, Milovanovic Z et al (2009) Quantification of transforming growth factor beta 1 levels in metastatic axillary lymph node tissue extracts from breast cancer patients: a new specimen source. Anal Quant Cytol Histol/Int Acad Cytol Am Soc Cytol 31(5):288–295
Jablonska E, Kiluk M, Markiewicz W, Piotrowski L, Grabowska Z, Jablonski J (2001) TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunologiae et Ther Exp 49(1):63–69
Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms Nature reviews. Drug Discov 4(12):977–987
Jaiyesimi IA, Buzdar AU, Hortobagyi G (1992) Inflammatory breast cancer: a review. J Clin Oncol: Off J Am Soc Clin Oncol 10(6):1014–1024
Jiang XP, Yang DC, Elliott RL, Head JF (2000) Reduction in serum IL-6 after vacination of breast cancer patients with tumour-associated antigens is related to estrogen receptor status. Cytokine 12(5):458–465
Jones RL, Giembycz MA, Woodward DF (2009) Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol 158(1):104–145
Ma LZ, Walgren B, Clayton RA, Burkholder JR (2011) T.P. LY2784544, a small molecule JAK2 inhibitor, induces apoptosis in inflammatory breast cancer spheres through targeting IL-6-JAK-STAT3 pathway. Paper presented at: Proceedings of the annual meeting of the American Association for Cancer Research; 2–6 Apr 2011, Orlando
Kaklamani VG, Hou N, Bian Y et al (2003) TGFBR1*6A and cancer risk: a meta-analysis of seven case-control studies. J Clin Oncol: Off J Am Soc Clin Oncol 21(17):3236–3243
Kalaitzidis D, Gilmore TD (2005) Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinology and Metabolism: TEM 16(2):46–52
Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ (1991) Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. I J Cancer: J Int du Cancer 49(5):650–655
Kan CY, Iacopetta BJ, Lawson JS, Whitaker NJ (2005) Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer 93(8):946–948
Kendellen MF, Bradford JW, Lawrence CL, Clark KS, Baldwin AS (2013) Canonical and non-canonical NF-kappaB signaling promotes breast cancer tumor-initiating cells. Oncogene, 11 Mar 2013
Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS (2012) Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ 344:e2718
Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ (2003) Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 48:82–84
Kunigal S, Lakka SS, Sodadasu PK, Estes N, Rao JS (2009) Stat3-siRNA induces Fas-mediated apoptosis in vitro and in vivo in breast cancer. Int J Oncol 34(5):1209–1220
Lanza-Jacoby S, Miller S, Flynn J et al (2003) The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev 12(12):1486–1491
Lei X, Bandyopadhyay A, Le T, Sun L (2002) Autocrine TGFbeta supports growth and survival of human breast cancer MDA-MB-231 cells. Oncogene 21(49):7514–7523
Lerebours F, Vacher S, Andrieu C et al (2008) NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer 8:41
Li J, Gonzalez-Angulo AM, Allen PK et al (2011) Triple-negative subtype predicts poor overall survival and high locoregional relapse in inflammatory breast cancer. Oncologist 16(12):1675–1683
Lin Y, Huang R, Chen L et al (2004) Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. J Int du Cancer 109(4):507–515
Liu CH, Chang SH, Narko K et al (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276(21):18563–18569
Liu Y, Wang L, Lin XY et al (2012) Anti-apoptotic effect of claudin-1 on TNF-alpha-induced apoptosis in human breast cancer MCF-7 cells. Tumour Biol 33(6):2307–2315
Lyons TR, O’Brien J, Borges VF et al (2011) Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 17(9):1109–1115
Martin OA, Redon CE, Dickey JS, Nakamura AJ, Bonner WM (2011) Para-inflammation mediates systemic DNA damage in response to tumor growth. Communicative Integr Biol 4(1):78–81
Matsumoto G, Namekawa J, Muta M et al (2005) Targeting of nuclear factor kappaB Pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin Cancer Res 11(3):1287–1293
Matthews JR, Watson SM, Tevendale MC, Watson CJ, Clarke AR (2007) Caspase-dependent proteolytic cleavage of STAT3alpha in ES cells, in mammary glands undergoing forced involution and in breast cancer cell lines. BMC Cancer 7:29
Menter DG, Schilsky RL, DuBois RN (2010) Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical Cancer Res 16(5):1384–1390
Moody CA, Laimins LA (2009) Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog 5(10):e1000605
Moustakas A, Heldin CH (2007) Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 98(10):1512–1520
Muraoka-Cook RS, Kurokawa H, Koh Y et al (2004) Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res 64(24):9002–9011
Murohashi M, Hinohara K, Kuroda M et al (2010) Gene set enrichment analysis provides insight into novel signalling pathways in breast cancer stem cells. Br J Cancer 102(1):206–212
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr (1997) Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 17(7):3629–3639
Nielsen SF, Nordestgaard BG, Bojesen SE (2012) Statin use and reduced cancer-related mortality. New Engl J Med 367(19):1792–1802
Nishimura R, Nagao K, Miyayama H et al (2000) An analysis of serum interleukin-6 levels to predict benefits of medroxyprogesterone acetate in advanced or recurrent breast cancer. Oncology 59(2):166–173
Oshima M, Dinchuk JE, Kargman SL et al (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87(5):803–809
Pan Q, Kleer CG, van Golen KL et al (2002) Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 62(17):4854–4859
Pan Q, Bao LW, Merajver SD (2003) Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFkappaB signaling cascade. Mol Cancer Res 1(10):701–706
Pasche B, Kaklamani V, Hou N et al (2004) TGFBR1*6A and cancer: a meta-analysis of 12 case-control studies. J Clin Oncol 22(4):756–758
Pierga JY, Delaloge S, Espie M et al (2010) A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat 122(2):429–437
Prasad S, Ravindran J, Aggarwal BB (2010) NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 336(1–2):25–37
Psaty BM, Furberg CD (2005) COX-2 inhibitors–lessons in drug safety. New Engl J Med 352(11):1133–1135
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(Pt 8):1281–1283
Ristimaki A, Sivula A, Lundin J et al (2002) Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62(3):632–635
Rivas MA, Carnevale RP, Proietti CJ et al (2008) TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res 314(3):509–529
Robbins GF, Shah J, Rosen P, Chu F, Taylor J (1974) Inflammatory carcinoma of the breast. Surg Clin North Am 54(4):801–810
Robertson FM, Simeone AM, Mazumdar A et al (2008) Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Ther Oncol 7(4):299–312
Robertson FM, Simeone AM, Lucci A, McMurray JS, Ghosh S, Cristofanilli M (2010) Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4. Cancer 116(11):2806–2814
Rubio MF, Werbajh S, Cafferata EG et al (2006) TNF-alpha enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene 25(9):1367–1377
Salgado R, Junius S, Benoy I et al (2003) Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer: J Int du Cancer 103(5):642–646
Sansone P, Storci G, Tavolari S et al (2007) IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Investig 117(12):3988–4002
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM (2007) Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J: Off Publ Fed Am Soc Exp Biol 21(13):3763–3770
Sheen-Chen SM, Chen WJ, Eng HL, Chou FF (1997) Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res Treat 43(3):211–215
Shostak K, Chariot A (2011) NF-kappaB, stem cells and breast cancer: the links get stronger. Breast Cancer Res: BCR 13(4):214
Simstein R, Burow M, Parker A, Weldon C, Beckman B (2003) Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med 228(9):995–1003
Singh B, Cook KR, Martin C et al (2010) Evaluation of a CXCR4 antagonist in a xenograft mouse model of inflammatory breast cancer. Clin Exp Metastasis 27(4):233–240
Soliman AS, Kleer CG, Mrad K et al (2011) Inflammatory breast cancer in North Africa: comparison of clinical and molecular epidemiologic characteristics of patients from Egypt, Tunisia, and Morocco. Breast Dis 33(4):159–169
Speirs V, Kerin MJ, Newton CJ et al (1999) Evidence for transcriptional activation of ERalpha by IL-1beta in breast cancer cells. Int J Oncol 15(6):1251–1254
Studebaker AW, Storci G, Werbeck JL et al (2008) Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res 68(21):9087–9095
Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ et al (2002) Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem 277(21):18649–18657
Subramaniam V, Chakrabarti R, Prud’homme GJ, Jothy S (2010) Tranilast inhibits cell proliferation and migration and promotes apoptosis in murine breast cancer. Anti-Cancer Drugs 21(4):351–361
Subramaniam V, Ace O, Prud’homme GJ, Jothy S (2011) Tranilast treatment decreases cell growth, migration and inhibits colony formation of human breast cancer cells. Exp Mol Pathol 90(1):116–122
Sullivan NJ, Sasser AK, Axel AE et al (2009) Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 28(33):2940–2947
Tawara K, Oxford JT, Jorcyk CL (2011) Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res 3:177–189
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Ueno NT, Buzdar AU, Singletary SE et al (1997) Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol 40(4):321–329
van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD (2000) RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia 2(5):418–425
Van Laere S, Van der Auwera I, Van den Eynden GG et al (2005) Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res Treat 93(3):237–246
Van Laere SJ, Van der Auwera I, Van den Eynden GG et al (2007) NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br J Cancer 97(5):659–669
Van Laere S, Limame R, Van Marck EA, Vermeulen PB, Dirix LY (2010) Is there a role for mammary stem cells in inflammatory breast carcinoma?: a review of evidence from cell line, animal model, and human tissue sample experiments. Cancer 116(11 Suppl):2794–2805
Walter M, Liang S, Ghosh S, Hornsby PJ, Li R (2009) Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 28(30):2745–2755
Wang D, Dubois RN (2004) Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol 31(1 Suppl 3):64–73
Wang FM, Liu HQ, Liu SR, Tang SP, Yang L, Feng GS (2005) SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1beta in vivo and in vitro. Breast Cancer Res Treat 89(1):5–14
Weaver KL, Ivester P, Seeds M, Case LD, Arm JP, Chilton FH (2009) Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem 284(23):15400–15407
Wendel M, Heller AR (2009) Anticancer actions of omega-3 fatty acids–current state and future perspectives. Anti-Cancer Agents Med Chem 9(4):457–470
Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18(55):7908–7916
Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH (2008) The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol 173(2):561–574
Xu J, Lamouille S, Derynck R (2009) TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19(2):156–172
Yang EY, Moses HL (1990) Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol 111(2):731–741
Yang Y, Pan X, Lei W, Wang J, Song J (2006) Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition and apoptosis via a cell cycle-dependent mechanism. Oncogene 25(55):7235–7244
Yang WT, Le-Petross HT, Macapinlac H et al (2008) Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat 109(3):417–426
Yao C, Lin Y, Chua MS et al (2007) Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer: J Int du Cancer 121(9):1949–1957
Yin Y, Chen X, Shu Y (2009) Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacotherapie 63(6):421–428
Yoshimura N, Sano H, Okamoto M et al (2003) Expression of cyclooxygenase-1 and -2 in human breast cancer. Surg Today 33(11):805–811
Yu Y, Wang Y, Ren X et al (2010) Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor beta contributes to chemoresistance in breast cancer cells. Mol Cancer Res 8(12):1633–1642
Zhang GJ, Adachi I (1999) Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anti-Cancer Res 19(2B):1427–1432
Zhang F, Lundin M, Ristimaki A et al (2003) Ski-related novel protein N (SnoN), a negative controller of transforming growth factor-beta signaling, is a prognostic marker in estrogen receptor-positive breast carcinomas. Cancer Res 63(16):5005–5010
Zhang SM, Lin J, Cook NR et al (2007) C-reactive protein and risk of breast cancer. J Natl Cancer Inst 99(11):890–894
Zhang D, LaFortune TA, Krishnamurthy S et al (2009) Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 15(21):6639–6648
Zhou BP, Hu MC, Miller SA et al (2000) HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 275(11):8027–8031
Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y (2008) NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 111(3):419–427
Acknowledgments
Grant Support: State of Texas Rare and Aggressive Breast Cancer Research Program Grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Basel
About this chapter
Cite this chapter
Fouad, T.M., Kogawa, T., Reuben, J.M., Ueno, N.T. (2014). The Role of Inflammation in Inflammatory Breast Cancer. In: Aggarwal, B., Sung, B., Gupta, S. (eds) Inflammation and Cancer. Advances in Experimental Medicine and Biology, vol 816. Springer, Basel. https://doi.org/10.1007/978-3-0348-0837-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-0348-0837-8_3
Published:
Publisher Name: Springer, Basel
Print ISBN: 978-3-0348-0836-1
Online ISBN: 978-3-0348-0837-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)