Abstract
Breast cancer remains the second leading cancer-related death in women in the United States. Despite improvements in early detection, prevention, and treatment, the mortality rate in breast cancer remains high secondary to the potential for cancer recurrence and the development of metastasis. To minimize breast cancer-related morbidity and mortality, understanding the factors leading to an increased risk of metastasis and developing clinical interventions that reduce this risk is essential. While the association between chronic inflammation and cancer progression is well documented in the literature, the role of acute inflammation and its impact on tumor proliferation and metastasis is less well understood. Here, we will review recently published preclinical studies in mouse models indicating that acute inflammation caused by clinical interventions plays an important role in the risk of peripheral metastases. In addition, we will address the potential impact that these findings may have on the clinical management of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic inflammation and cancer
In 1983 two Australian scientists postulated that gastric cancer may be the result of an inflammatory process caused by chronic Helicobacter pylori (H. pylori) infection in the stomach [1]. Subsequent studies confirmed this correlation and demonstrated that H. pylori infection was associated with a significantly increased risk of gastric cancer [2]. It was at this point of history that viral causes of cancer were also being discovered; for example, the correlation between human papilloma virus (HPV) and cervical cancer [3, 4]. More recently, the mechanism of HPV potentially contributing to the development of oropharyngeal squamous cell cancer has been observed in studies [5, 6]. These findings marked a paradigm shift in the concept of carcinogenesis and opened new pathways for the treatment and prevention of certain cancers. Likewise, chronic ulcerative colitis and Crohn’s disease are well-recognized risk factors for the development of colorectal cancer [7, 8]. Interestingly, even colorectal cancers not associated with inflammatory bowel disease demonstrate an inflammatory response with an increased expression of pro-inflammatory cytokines [9].
Prior studies utilizing mouse models have informed our understanding of the correlation between chronic inflammation and cancer development. These studies expand our understanding of which components of the immune system promote tumor progression relative to those that facilitate efficient elimination of the tumor cells. Immune cells that have been shown to promote tumorigenesis are macrophages, several subtypes of CD4+ cells, and neutrophils.
Macrophages, which are well known for playing a critical role in regulating tissue homeostasis in normal tissues, can cause a maladaptive response in the tumor microenvironment that leads to promotion of tumor growth and invasion into adjacent tissues [10, 11]. Also, several subtypes of CD4+ T cells have been recognized to contribute to tumorigenesis via multiple pathways [12]. Neutrophils, a key component of the acute inflammatory response, have also been shown to contribute to tumor progression and metastasis [13, 14]. At the other end of the spectrum, immunosurveillance and response to tumor-specific antigens via cytotoxic CD8+ T cells is an important mechanism of cancer suppression and regression [15].
The role of the immune system in cancer progression is complex, depending on both the tissue and tumor type involved, and remains somewhat controversial [16]. However, the association between chronic inflammation and tumorigenesis has become more evident.
The concept of acute inflammation and risk of cancer progression
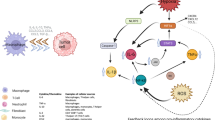
Given the demonstration of chronic inflammation promoting the development and progression of cancer through factors produced within immune cells (e.g. cytokines, growth factors, reactive oxygen species), one can hypothesize that an acute inflammatory response within the tumor may drive cancer progression through a similar mechanism. The presence of acute inflammatory cells in the tumor could play a detrimental role by supporting tumor cell proliferation and, more importantly, metastasis.
In contrast to chronic inflammation, very little is known about the physiological consequences of an acute inflammatory response within tumors. Acute inflammation is clearly triggered by standard diagnostic and therapeutic interventional procedures in cancer treatment [17]. Mechanical trauma induces tissue damage, which results in wound healing, creating an acute local inflammatory state and the recruitment of inflammatory cells (primarily cells from the innate immune system), as well as the production of cytokines, chemokines, and growth factors which may stimulate tumor progression and/or metastasis [18].
Core needle biopsies are standard procedures in breast cancer diagnosis [19]. Although only a small section of the tumor is removed, it is enough to trigger an acute inflammatory reaction as part of the normal wound healing response. This biopsy-mediated acute inflammatory response is theorized to have a proliferative effect on residual tumor cells. This may be relevant given that the period of time between the biopsy and subsequent surgery or the initiation of other cancer treatment can vary from 10 days to 5 weeks, providing time for tumor cell stimulation promoted by the immune response. Likewise, in the case of positive margins following a partial mastectomy, residual tumor cells may be exposed to an inflammatory microenvironment favoring tumor cell progression. Surgical excision of tumors promotes a strong acute inflammatory response at the site of excision. Some have speculated that in cases where re-excision is required for suspected residual tumor, the presence of inflammatory cells during the period between surgeries could provide an opportunity for proliferation or even migration (i.e. metastasis) of residual cancer cells [20, 21].
While studies investigating the potential risk of biopsies and local excision have focused on tumor displacement, no studies have previously addressed the impact of the acute inflammatory response on local and distal tumor recurrence. This may be due to the fact that the concept of acute inflammation being a factor that can promote tumor progression is relatively new. However, with our current knowledge of the link between inflammation and cancer, identifying the potential impact of the acute inflammatory response on tumor cells may offer new insights into our understanding and clinical management of cancer.
Evidences from mouse models supporting the role of acute inflammation in breast cancer metastasis
Very few studies have approached how acute inflammation can promote tumor growth or metastases using animal models. In a rat Lewis-lung model, a sharp spike in angiogenesis stimulators and growth factors to aid in wound healing were shown to lead to an environment fertile for tumor proliferation and metastasis [22]. However, no association with specific inflammatory or immune cells was made. Bouchard et al. studied the affect of radiation-induced inflammation as a possible predictor on cancer cell migration and development of lung metastasis using BALB/c mouse model. Their results showed that pre-irradiation of the mammary gland increased the quantity of circulating cancer cells 2.0-fold, but more importantly, also showed a 2.4-fold increase in the number of lung metastasis associated with the induction of interleukin-6 (IL-6) and cyclooxygenase-2 (COX-2) [23].
To address whether acute inflammation may have an effect on tumor progression or metastasis, we established a mouse model for mammary biopsies where the immune response is physiologically developed (e.g. not in a xenograft model). We used MMTV-PyMT transgenic mice [24] as a mammary tumor mouse model for breast cancer and performed a live surgical punch biopsy as the trigger of acute inflammation. Our study demonstrated that the biopsy triggers an accumulation of inflammatory cells in the tumors within the area of the biopsy. While the biopsy had no strong effect on primary tumor growth, there was an increased ratio of proliferating cells in the tumor area adjacent to the biopsy relative to distal areas of the tumor. The most revealing finding from these studies was the prominent increase (3–5-fold) in the number of newly generated lung metastasis in the mice that underwent biopsy compared with control mice. This effect was associated with the recruitment of inflammatory cells and elevated levels of certain cytokines such as IL-6 in the lung. Anti-inflammatory treatment prior to and after the biopsy reduced the development of metastases triggered by the biopsy. In addition, inhibiting IL-6 production mitigated the number of metastases upon biopsy [25].
Based on early mouse models, we have evidence to support the concept that acute inflammation resulting from ‘wounding’ of primary tumors can increase the rate of metastases. Importantly, the increased development of metastasis promoted by the biopsy could be minimized by administration of anti-inflammatory medication (ibuprofen) at the time of the procedure [25]. These studies in mouse models present a foundation upon which future clinical studies can elucidate the clinical significance that acute inflammation may have on the management of patients with breast cancer. Indeed, recent findings from a retrospective study of 327 consecutive breast cancer patients demonstrated that Non-Steroidal Anti-Inflammatory Drug (NSAID) analgesic used in surgery resulted in improved 5-year disease-free survival, and a reduction of early relapse events [26]. These findings may ultimately have implications on the timing of breast cancer therapy, impact of positive margins, and role of anti-inflammatory therapy in patients undergoing invasive procedures for breast cancer treatment.
Future considerations
In summary, there is emerging evidence that invasive procedures may have an impact on breast cancer progression, including recurrence and metastasis. The significance and impact of these preliminary findings warrant further studies in human breast cancer. Investigating the potential correlation between the acute immune response and tumor progression may further our understanding of carcinogenesis and open channels to novel, immune-based therapeutic strategies to reduce the development of metastasis.
References
Warren JR, Marshall B (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1(8336):1273–1275
Shiotani A, Cen P, Graham DY (2013) Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 23(6 Pt B):492–501. doi:10.1016/j.semcancer.2013.07.004
Brinkman JA, Caffrey AS, Muderspach LI, Roman LD, Kast WM (2005) The impact of anti HPV vaccination on cervical cancer incidence and HPV induced cervical lesions: consequences for clinical management. Eur J Gynaecol Oncol 26(2):129–142
Durst M, Gissmann L, Ikenberg H, zur Hausen H (1983) A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA 80(12):3812–3815
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92(9):709–720
Kruger M, Hansen T, Kasaj A, Moergel M (2013) The correlation between chronic periodontitis and oral cancer. Case Rep Dent 2013:262410. doi:10.1155/2013/262410
Ullman TA, Itzkowitz SH (2011) Intestinal inflammation and cancer. Gastroenterology 140(6):1807–1816. doi:10.1053/j.gastro.2011.01.057
Terzic J, Grivennikov S, Karin E, Karin M (2010) Inflammation and colon cancer. Gastroenterology 138(6):2101–2114.e5. doi:10.1053/j.gastro.2010.01.058
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118(3):285–296. doi:10.1016/j.cell.2004.07.013
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Ruffell B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol 33(3):119–126. doi:10.1016/j.it.2011.12.001
Palucka K, Coussens LM, O’Shaughnessy J (2013) Dendritic cells, inflammation, and breast cancer. Cancer J 19(6):511–516. doi:10.1097/ppo.0000000000000007
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD (2012) Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 19(1):217–224. doi:10.1245/s10434-011-1814-0
Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J (1998) Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol 152(1):83–92
Medler TR, Coussens LM (2014) Duality of the immune response in cancer: lessons learned from skin. J Invest Dermatol 134(e1):E23–E28. doi:10.1038/skinbio.2014.5
Chan CJ, Coussens LM (2013) Poker face no more: cancer recurrence reveals its hand. Nat Med 19(12):1569–1570. doi:10.1038/nm.3410
Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP (2003) Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol 4(12):760–768
Walter ND, Rice PL, Redente EF, Kauvar EF, Lemond L, Aly T, Wanebo K, Chan ED (2011) Wound healing after trauma may predispose to lung cancer metastasis: review of potential mechanisms. Am J Respir Cell Mol Biol 44(5):591–596. doi:10.1165/rcmb.2010-0187RT
Sohn YM, Yoon JH, Kim EK, Moon HJ, Kim MJ (2014) Percutaneous ultrasound-guided vacuum-assisted removal versus surgery for breast lesions showing imaging-histology discordance after ultrasound-guided core-needle biopsy. Korean J Radiol 15(6):697–703. doi:10.3348/kjr.2014.15.6.697
Biglia N, Ponzone R, Bounous VE, Mariani LL, Maggiorotto F, Benevelli C, Liberale V, Ottino MC, Sismondi P (2014) Role of re-excision for positive and close resection margins in patients treated with breast-conserving surgery. Breast 23(6):870–875. doi:10.1016/j.breast.2014.09.009
Russo AL, Arvold ND, Niemierko A, Wong N, Wong JS, Bellon JR, Punglia RS, Golshan M, Troyan SL, Brock JE, Harris JR (2013) Margin status and the risk of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat 140(2):353–361. doi:10.1007/s10549-013-2627-6
Baum M, Demicheli R, Hrushesky W, Retsky M (2005) Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 41(4):508–515. doi:10.1016/j.ejca.2004.09.031
Bouchard G, Bouvette G, Therriault H, Bujold R, Saucier C, Paquette B (2013) Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br J Cancer 109(7):1829–1838. doi:10.1038/bjc.2013.502
Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12(3):954–961
Hobson J, Gummadidala P, Silverstrim B, Grier D, Bunn J, James T, Rincon M (2013) Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis. Breast Cancer Res Treat 139(2):391–401. doi:10.1007/s10549-013-2575-1
Retsky MW, Demicheli R, Hrushesky WJM, Forget P, DeKock M, Gukas I, Rogers RA, Baum M, Sukhatme V, Vaidya JS (2013) Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem 20(33):4163–4176
Acknowledgments
The authors would like to acknowledge the Cabot Wellington Foundation and the VCC-LCCRO for supporting this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szalayova, G., James, T.A. & Rincon, M. A framework for the role of acute inflammation in tumor progression. Breast Cancer Res Treat 151, 235–238 (2015). https://doi.org/10.1007/s10549-015-3392-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3392-5