Abstract
Gasotransmitters and gaseous-signaling molecules are hugely important for controlling cell function and especially so during stress challenges. Past research has concentrated on molecules such as nitric oxide (NO) and hydrogen sulfide (H2S), although others such as ethylene and carbon monoxide (CO) are also important. Here, molecular hydrogen (H2) is added to the mix. H2 has been shown to ameliorate responses to a range of stressors in plants, including exposure to heavy metals, salinity, extreme temperatures, and UV radiation. Clearly, H2 is an important gas, which may be useful for enhancing plant growth and food security in the future. Exogenous treatments with H2 are easy in the form of hydrogen-rich water (HRW), but there are still issues with its wide-spread use. Furthermore, the molecular basis of the action of H2 in cells is still not clear. Here, aspects of the use and the action of H2 in plants are discussed, along with what might be learnt from other species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
In 1987, work in animals showed that endothelial-derived relaxing factor (EDRF) was in fact the gas nitric oxide (NO) (Palmer et al. 1987). This opened the door to studies not only on reactive nitrogen species (RNS) in biological systems, but also observations on other physiological gasotransmitters. Such analyses also sparked work on other small reactive compounds which could be involved in cell signaling, including nongaseous reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) (Veal et al. 2007), and reactive sulfur compounds such as hydrogen sulfide (H2S) (Aroca et al. 2018). The year 2019 marked the fortieth anniversary of NO studies in plants (Klepper 1979; Kolbert et al. 2019), but more recently a new player has been added to the list, molecular hydrogen (H2) (Wilson et al. 2017), which can alter plant cell activity (for example, Chen et al. 2017a), and may play a role in stress responses. As plants are sessile, they require convoluted strategies to overcome a range of stress challenges, which include exposure to UV light (Hideg et al. 2013), heavy metals (Morkunas et al. 2018), extreme temperature, both high (Niu and Xiang 2018) and low (Lyons 2012), drought (Farooq et al. 2009), flooding (Loreti et al. 2016), and salinity (Fahad et al. 2015).
The strategy for plants when under stress is to induce signal transduction pathways, which often lead to altered gene expression, and hence the complement of cellular proteins, enabling enhanced or new activities. Such actions are allowing the cells to manage the current stress, or even future stress challenges. The signaling invoked in plants involves a range of phytohormones (Khan et al. 2012), but it also involves numerous gasotransmitters, which are important mediators in other organisms as well. These include NO (Nabi et al. 2019), H2S (Pandey and Gautam 2020), carbon monoxide (CO; Cui et al. 2012) and ethylene (Debbarma et al. 2019). Plant stress has a major impact on plant growth and productivity, and gasotransmitters are instrumental in the responses mounted by plants. Often there is an interaction and/or co-ordination of the signaling mediated by such molecules (Hancock and Whiteman 2016; Singh et al. 2020; Bhuyan et al. 2020). Here, the interactions of H2 with other gaseous signaling molecules are discussed, with the focus on how H2 alleviates plant stress.
5.2 H2 Treatment of Plants
Hydrogen gas is hard to administer to plants. In mammals, hydrogen gas mixtures can be inhaled, and there are many examples of its use (Ge et al. 2017; Wu et al. 2019a, b), including in the treatment for COVID-19 (Chen et al. 2021a; Russell et al. 2021). However, the gas is highly flammable, raising safety issues, and is lighter than air, so H2 will rapidly disperse into the upper atmosphere, making treatment of ground-level plants unpragmatic. Therefore, treatment of plants often involves the creation and diluting of a saturated solution of hydrogen in what is referred to as hydrogen-rich water (HRW). However, H2 is not very soluble (Molecular Hydrogen Institute n.d.; Wilhelm et al. 1977) and will rapidly revert to the gaseous phase and be lost. This then may necessitate a frequent re-application of HRW to the plant tissues, either directly onto the leaves, or into the root feed water, to illicit an effect. However, as can be seen in Table 5.1, there are many examples of the use of HRW in plants. With such a range of responses, including to heavy metals, temperature stress and light stress it is clear that plants can perceive and react to the presence of H2 or HRW. Interestingly, one of the potentially significant uses of H2 application is in post-harvest, where it may be useful to prolong storage of crops, particularly fruits (Hu et al. 2014) and flowers (Ren et al. 2017b). A new twist on the use of HRW is the formation of hydrogen nanobubble water (HNW) (Li et al. 2021b). This is suggested to increase the solubility of H2 and prolong H2 delivery.
For H2 usage to be useful in practice, new and easier-to-use applications for the delivery of H2 may need to be developed. These may come from disparate industries (Mayorga et al. 2020), for example, one potential donor is magnesium hydride (MgH2) (Li et al. 2020b), a compound proposed for use in the solar-energy sector (Mathew et al. 2021). The kinetics of release of H2 are slower and more sustained than just using HRW, but it was found to be more efficient when used in a citrate buffer. Another recently used compound for releasing H2 in plants is AB@hMSN, an ammonia borane-loaded hollow mesoporous silica nanoparticle (Wang et al. 2021). However, there is a caveat here. If donor molecules are used, they are likely to leave behind by-products, and this could severely compromise the biologically safe use of H2.
5.3 Molecular Targets of H2
The hydrogen molecule is extremely small (relative to other signaling molecules) and relatively inert. Therefore, it is difficult to envisage how it is perceived by cells and acted on. Classical hormone-type signaling, for example with chemokines (D’Ambrosio et al. 2003), would use a protein receptor, but this is unlikely with a molecule such as H2. Some signaling molecules, such as NO, will react with proteins, either through the prosthetic groups or via reacting with thiol groups (Feng et al. 2019). However, again, it is hard to see how this type of reaction would apply to H2 because unlike NO, which is polar and a reactive free radical, H2 is non-polar and not reactive. Therefore, other mechanisms must exist to account for the biological effects seen with H2 administration.
One of the main thrusts of the argument regarding H2 action is that it affects the antioxidant levels in cells. Many of these effects are indirect, with expression or accumulation of enzymes involved in the antioxidant capacity of the cell being altered (for example Zhao et al. 2017; Chen et al. 2017b). However, this can only happen if there is a direct perception of the H2 molecule, and usually that is the aspect that is skirted in the literature.
It was reported that H2 does have direct effects as an antioxidant by reacting with hydroxyl radicals (⋅OH) but not with other ROS, such as the superoxide anion (O2⋅−) or H2O2 (Ohsawa et al. 2007). ⋅OH are known to be involved in plant stress responses, such as during heavy metal challenge (Cuypers et al. 2016), paraquat treatment (Babbs et al. 1989), and chilling and drought stresses (Shen et al. 1997). Therefore, the removal of ⋅OH by a radical scavenger, suggested here to be H2, could account for some of the effects seen. This being said, a later paper has suggested that a close investigation of the kinetics of this reaction does not support this notion (Penders et al. 2014), and in fact, it was suggested that the ⋅OH would react with other biomolecules before H2, so that the effects of ⋅OH would not be mitigated against by H2 addition. In a similar way, a second direct target was suggested to be the peroxynitrite molecule (ONOO−). This would be produced by the reaction of superoxide (O2⋅−) with NO, and as both are temporally and spatially produced together during stress responses, the presence of ONOO− is very likely. If H2 removes ONOO−, this could account for the effects seen. However, a close examination of the kinetics again, seems to rule out ONOO− as a direct H2 target (Penders et al. 2014; LeBaron et al. 2019a).
With both ⋅OH and ONOO− being ruled out, it was suggested that a possible target could be the ferric (Fe3+) ion (Penders et al. 2014). This would not be out of kilter with what has been reported for other gasotransmitters. One of the main actions of NO is the activation of soluble guanylyl cyclase (sGC) by a direct interaction of the NO with the heme prosthetic group of the enzyme (Xiao et al. 2019). With a foray into this area (Penders et al. 2014), the reduction of the iron by H2 in myoglobin, cytochrome P450 and putidaredoxin was investigated, but it was concluded that there was no reduction of heme or iron-sulfur (Fe/S) clusters in these proteins. However, with a redox midpoint potential of −414 mV [relative to the Standard Hydrogen Electrode (SHE)], H2 could thermodynamically reduce a range of heme groups in a variety of enzymes, and this is suggested as a focus of future investigation (Hancock et al. 2021). As discussed, enzymes such as the NAPDH oxidase homologues would be particularly interesting as they are known to be involved in a range of stress responses (for example, He et al. 2017). It is not inconceivable that sGC may be an H2 target too. Clearly much more work is needed here, using a wide range of plant proteins which contain heme or Fe/S prosthetic groups, before such a mechanism can be ruled out. Nevertheless, there may need to be some caution here, as it cannot always be assumed that signaling pathways determined in animal systems are the same in plants. For example, the action of NO on a sCG has been thrown into doubt in plants (Astier et al. 2019). Therefore, the action of H2 may be different too, although the use of other biological systems to advance plant science is a powerful tool, as discussed below.
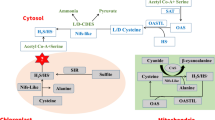
Several effects of H2 have been reported to be mediated by the enzyme heme-oxygenase (HO-1) (Jin et al. 2013; Lin et al. 2014). This enzyme catalyzes the breakdown of heme in a reaction which (1) involves oxygen, (2) uses NADPH as a cofactor and (3) produces biliverdin, CO and iron ions (Wilks 2002). However, the exact reaction with H2 has yet to be reported, so it may be a consequence of downstream signaling which is yet to be determined. Another enzyme thought to mediate H2 effects is glutathione peroxidase, an enzyme instrumental in the maintenance of intracellular redox. By the use of genetically deficient strains and inhibitors, it was shown that glutathione peroxidase was needed to mediate H2 action in the Ganoderma lucidum fungus (Ren et al. 2017a). The enzyme is a selenium containing protein, making this an interesting potential H2 target, unless the direct action of H2 is upstream of the enzyme itself.
Lastly, it has been suggested that because H2 has two spin states that this could be a way for H2 to influence other biomolecules (Hancock and Hancock 2018). However, to date, there is no experimental evidence of this.
As yet, no definitive mechanism of how H2 interacts directly with biological systems has been identified, although several mechanisms have been suggested (Table 5.2). Therefore, much more work needs to be undertaken in this area. Despite this there clearly are effects in plants (Table 5.1), and this phenomenon can be exploited in the absence of a molecular mechanism, particularly as there appear to be no reports that H2 application is harmful to neither plants nor animals. No H2 mechanisms seem to leave by-products and so there seems to be no ramifications for food safety.
5.4 Signaling and Effects of H2
Cell signaling events in plants, as with all species, is crucial for the organism to thrive and to survive stress challenges. The perception of an external signal, perhaps a biotic or abiotic stress, and the signal transduction pathway, leading to a response, involves a range of proteins and small molecules, and instrumental in many of these pathways are the small relatively reactive gasotransmitters, such as NO (Nabi et al. 2019) and H2S (Pandey and Gautam 2020). Although, as discussed, it is hard to envisage how H2 may have a direct interaction and effect on polypeptides, there is a body of evidence that shows that H2 interacts, or has effects on, signaling events that involve other gasotransmitters and small redox compounds. Some of the evidence is discussed below.
5.4.1 Nitric Oxide, Stress and Hydrogen Gas Treatment
It has been known for several decades that NO is produced by plants and has a profound effect on controlling plant function (Kolbert et al. 2019). There is no doubt that NO has a central role in controlling cell function (Kumar and Pathak 2018), whilst more recently, it has been found that H2 interacts in the NO pathways.
Decreased NO generation was reported when HRW was used to alleviate aluminum stress in alfafa (Chen et al. 2014). Fifty percent saturated HRW reduced the effects of a NO donor, suggesting that NO may mediate H2 effects. In contrast, H2 increased the NO production in tomato seedlings when root growth was being investigated. This was reduced by the NO scavenger 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), which suggests that H2 was not directly scavenging NO. The conclusion was that auxin-induced H2 generation was then mediated by NO production from the enzyme nitrate reductase (NR) (Cao et al. 2017). Similar results were reported with cucumber, where HRW increased root growth and NO accumulated. Both HRW and NO increased the expression of cell cycle genes: CycA (A-type cyclin); CycB (B-type cyclin); CDKA (cyclin-dependent kinase A); and CDKB (cyclin-dependent kinase B). The effects were reduced by inhibitors of NR and nitric oxide synthase (NOS)-like enzymes, and NO scavengers (Zhu et al. 2016). NO also mediated root growth induced by H2 in cucumber, where downstream proteins were identified as a plasma membrane H+-ATPase and 14-3-3 proteins (Fu et al. 2000; Mhawech 2005; Li et al. 2020a, b). The latter being key regulatory proteins of such intracellular signaling cascades as mitogen activated protein kinase (MAPK) and p53. The enzyme NR was also found to be involved in NO generation when root formation was induced by a H2 releasing donor AB@hMSN (Wang et al. 2021).
H2 has the potential to be useful for postharvest storage of plant materials. One percent HRW [2.2 μM H2] (calculated from the authors’ information) and sodium nitroprusside (SNP: 150 μM) improved vase-life of cut lilies and these effects were reduced when NO was removed. It was also found, in a study of the genes expressed, that the chloroplast ATP synthase CF1 alpha subunit (AtpA) may be important in mediating these effects (Huo et al. 2018). Furthermore, nitrate accumulation was reduced in tomatoes by H2, and this may have implications for the way fruits are stored (Zhang et al. 2019).
It is clear therefore, that H2 has effects on, and is mediated, by NO metabolism, and it appears that this is not due to a direct scavenging of NO by H2, which would be in line with what was previously reported (Ohsawa et al. 2007). However, H2 may have effects through ROS too, which may also impinge on NO metabolism. To exemplify, abscisic acid (ABA) induced the accumulation of H2 in Arabidopsis thaliana, which led to better drought tolerance. However, the effects also involved ROS and NO accumulation, with the enzymes NR and NADPH oxidase being used. In fact, it was found that the promotion of NO accumulation by H2 was dependent on ROS production, showing what a complex and interdependent system H2 is involved in (Xie et al. 2014).
5.4.2 Reactive Oxygen Species, Antioxidants and Hydrogen Gas
It is clear that ROS metabolism needs to be considered when the effects of H2 are in question, especially as H2 may have antioxidant and pro-oxidant effects (LeBaron et al. 2019b).
Even though the direct scavenging of O2⋅− and H2O2 were ruled out (Ohsawa et al. 2007), and the scavenging of ⋅OH was also cast into doubt (Penders et al. 2014), many reports suggest that H2 has affects in plants through the modulation of the antioxidant capacity of cells. The postharvest treatment of Chinese chive with H2 reduced oxidative damage and increased the activity of several antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD) and ascorbate peroxidase (APX) (Jiang et al. 2021), resulting in an increased shelf life of the chives. Oxidative stress in Medicago sativa was also alleviated by HRW following UV-B exposure, and this too was mediated by changes in antioxidants, particularly flavonoids (Xie et al. 2015). HRW also allowed better tolerance to light stress in Zea mays, again mediated by antioxidant enzymes (Zhang et al. 2015). These included SOD, CAT and APX, which reduced the accumulation of O2⋅− and H2O2.
The tolerance bestowed on plants by H2 administration to other stress challenges is also mediated by antioxidants. This includes aluminum tolerance in maize, where HRW altered the cellular levels of CAT, APX, SOD, and POD (Zhao et al. 2017). In rice seedlings exposed to cold stress, the SOD levels were altered, which appeared to be mediated by changes in the miRNA levels, in particular miR-398 transcripts. The authors suggested that this was imperative to maintaining the redox homeostasis of the cells (Xu et al. 2017b).
The changes in antioxidant activity observed in cells will not only relieve the tangible aspects of oxidative stress, as seen with less lipid peroxidation and protein oxidation, but it will also be part of the system which maintains the redox poise of the cell, which will be part of the complex interplay used in signaling (Shao et al. 2008). Lowering ROS will mean that reactions with NO will potentially be reduced, and so reducing the production of ONOO−, which acts as a downstream signaling molecule of NO (Speckmann et al. 2016). It is known that ROS will also act on glutathione, a molecule instrumental in maintaining the cellular redox balance. It has been suggested that the redox of a cell is carefully kept in a “Goldilocks zone” (Alleman et al. 2014), and therefore any changes in intracellular redox molecules will feed into this. A good example of how such redox active molecules interact to give the effects in plants is seen with the legume–Rhizobium symbiosis system (Pauly et al. 2006), where GSH, NO and ROS were studied.
5.4.3 Hydrogen Gas and Ethylene Signaling
One of the most well-known gasotransmittters is ethylene (C2H4; H2C=CH2). It is involved in a range of physiological systems in plants, such as plant growth (Dubois et al. 2018), but is probably best known for its role in fruit ripening (Barry and Giovannoni 2007).
The interaction of ethylene with other gasotransmitters is not novel. For example, NO and ethylene has been reported to work together in the root development of cucumber (Xu et al. 2017a). Therefore, an interaction of H2 and ethylene is no surprise. Postharvest senescence of rose flowers was reduced by H2 application and this was mediated by changes in ethylene signaling. There was a reduction of substrates and biosynthetic enzymes: 1-aminocyclopropene-1-carboxylate (ACC); ACC synthase (ACS); and ACC oxidase (ACO). Gene expression of Rh-ACS3 and Rh-ACO1 transcripts, encoding biosynthesis enzymes, was also reduced. Interestingly, expression of the ethylene receptor, Rh-ETR1, was increased (Wang et al. 2020). These data clearly show that there is an influence of H2 on ethylene metabolism and concomitant signaling.
A proteomic study also showed that H2 and ethylene cooperated in signaling (Huang et al. 2020). Using cucumber roots as the model system, it was shown that inhibitors of ethylene signaling, AgNO3 and aminoethoxyvinylglycine (AVG), reduced the adventitious root development induced by H2 treatment. The proteomic analysis, using 2D-gel electrophoresis coupled with mass spectroscopic analysis, showed that HRW induced the up-regulation of nine proteins and the down-regulation of fifteen. The authors concluded that ethylene was downstream of H2 and that six proteins were worthy of note and were probably mediating H2 effects. These were RuBisCO, oxygen-evolving enhancer protein (OEE1), sedoheptulose-1,7-bisphosphatase (SBPase), threonine dehydratase (TDH), cytosolic ascorbate peroxidase (cAPX), and protein disulfide-isomerase (PDI).
5.4.4 Hydrogen Gas and Hydrogen Sulfide Signaling
H2S is recognized as being toxic (Truong et al. 2006), but it is also now accepted as being a therapeutic gasotransmitter controlling key events in physiology and cell function (Wang 2003; Gadalla and Snyder 2010). However, as with the other small reactive compounds, H2S does not act alone but is part of the complex interaction in which these molecules partake. It has been suggested that H2S may act as a brake on some of the other signaling pathways (Hancock and Whiteman 2014). Alongside this, H2S has also reported to be part of the H2 signaling taking place in cells.
With the expression in Arabidopsis of a hydrogenase gene from Chlamydomonas reinhardtii (CrHYD1), which leads to H2 biosynthesis, it was shown that endogenous H2 was needed for osmotic stress tolerance in plants. Exposure to H2 stimulated the production of H2S and it was suggested that, to cause the modulation of the stomatal apertures, leading to the tolerance observed, H2S was downstream of H2 (Zhang et al. 2020a). A similar result was found with cut flowers. In a study of cut carnations, it was shown that a MgH2 and citrate solution increased H2S generation. The redox homeostasis was maintained whilst the expression of senescence genes was repressed (Li et al. 2020b). Hypotaurine, a H2S scavenger, reversed the effects and it was suggested that the downstream effects of H2 were mediated by H2S, which is in line with the study on stomata (Zhang et al. 2020a).
It can be seen therefore, that H2 is involved in the signaling pathways of a range of gasotransmitters, including NO, ethylene and H2S.
5.5 What Might Be Learnt from Other Species
Working across the kingdoms of organisms can be rewarding, but it does come with some caveats. To exemplify, the characterization of the NAPDH oxidases from humans (Schröder 2020) has greatly helped advance the research on homologues of these enzymes in plants (Qu et al. 2017). Indeed, oxidase proteins from plants and animals could be combined to reconstitute activity in vitro (Desikan et al. 1996). On the other hand, the discovery of a NOS in animals (Bredt and Snyder 1990) has only led to controversy in plant science (Astier et al. 2018). Furthermore, the lack of a sGC signaling pathway in plants, so well characterized in animals, further emphasizes the caution that may need to be used (Astier et al. 2019). Having said that, deliberated below is how much can be learnt about the role of H2 in biological systems by taking a broad approach.
If H2 is able to enhance stress responses in plants, there needs to be an increase in the H2 concentration in the relevant cells. This can be achieved via two mechanisms: either the endogenous production of H2 can be increased, or the H2 can be supplied exogenously.
Probably one of the most well-known endogenous biological systems for the production of H2 is in the algae Chlamydomonas (Vargas et al. 2018). This organism is so good at generating H2 that it has been suggested to be used as a biofuel (Scranton et al. 2015). Generation of H2 is via a hydrogenase enzyme, and such mechanisms have been recently reviewed (Russell et al. 2020). If enzyme-based H2 production can be increased in plants, either by the manipulation of the control of such enzymes, or by increasing the expression and relevant polypeptide accumulation, then targeted H2 signaling can be used to enhance plant growth and survival. Model organisms such as Chlamydomonas, and then higher plant models such as Arabidopsis, will be instrumental in such work.
Alternatively, H2 can be supplied exogenously. As discussed above, this might be from anthropogenic activity such as the application of HRW. However, plants, like many organisms, are likely to be in synergy or symbiotically with prokaryotes and fungi, which themselves can produce H2. In humans, it has been suggested that increased H2 production by gut microflora may enhance health (Ostojic 2020). Therefore, an increase in the prokaryotic production of H2 around the root system of plants may have beneficial effects. On the other hand, H2 oxidizing soil bacteria have also been shown to be beneficial (Zhang et al. 2020b). Manipulation of the soil bacterial flora therefore may be complicated but changing the H2 metabolisms in the vicinity of the root system might have future benefits.
A study of bacteria may also help unravel how H2 works. As the H2 couple has a very reducing mid-point potential (−414 mV relative to SHE), then reduction of many protein prosthetic groups may be thermodynamically possible. This principle is exemplified by the reports on the reduction of cytochrome c3 in Desulfovibrio desulfuricans (Peck 1959). Interestingly, following the redox reactions which may proceed downstream of this reduction, it was suggested that H2S could be produced, which is known to be an important gasotransmitter in plants (Aroca et al. 2018), including under stress conditions (Singh et al. 2020), relevant to the discussion here. The study by Peck (1959) shows two important things. Firstly, the reduction of a heme group by H2 is possible in biological systems. Secondly, once the heme is reduced there are possible downstream reactions which could potentially yield signaling molecules. As already mooted (Hancock et al. 2021), this needs to be explored further in plants and animals, not just in prokaryotes.
One of the biggest areas where other species can be useful to study is in the biomedical arena. Here, H2 has been shown to have a benefit in a variety of diseases, including those listed in Table 5.3. H2 has been found to relieve symptoms of COVID-19 and has been used for clinical trials (Guan et al. 2020). It has also been found to be of benefit in neurodegenerative disease (Chen et al. 2021b), rheumatoid arthritis (Yang et al. 2020) and diabetes (Yang et al. 2020). Therefore, it is clear that H2 has a range of benefits for human health and for alleviating disease symptoms. Moreover, if mechanisms are known for H2 action in the biomedical arena, can this be translated across and used in plant science?
It is not only the support that data such as that in Table 5.3 gives to the argument that H2 has profound effects in biological systems, but it is the manner in which H2 has its effects that is relevant here. Clearly, some of the effects and proposed mechanisms in animals are not directly relevant to plants. For example, a reduction in IL-6 levels or a dampening of a cytokine storm is not a mechanism which would be seen in plants. However, H2 may have effects on analogous intercellular signaling molecules in plants, such as ethylene (Wang et al. 2020). Other effects may be much more relevant. As previously mentioned, H2 may work through the action it has on antioxidants, an effect which has already been seen in plants. Accordingly, changes in antioxidants and a dampening of oxidative stress are a common feature in neurogenerative disease alleviation, the reductive effects on diabetes, cancer therapies, in mood alterations and in Hepatitis B (Ichihara et al. 2015). This is also a common feature of how H2 alleviates plant stress (e.g., Zhang et al. 2015; Jiang et al. 2021). The biochemistry of animal and plant cells differs in detail but remains the same in principle. Therefore, a close study of the research on H2 from the animal kingdom may be very beneficial to plant science in the future and vice versa. With the list of conditions for which H2 may benefit human health, it is no surprise that H2 has been mooted as a future therapy for humans (Ge et al. 2017; Wu et al. 2019a). With a focus on respiratory diseases at the present time because of the COVID-19 pandemic, the research and application of H2 is likely to be of continued interest in the biomedical field (Russell et al. 2021). On the other hand, H2 application has already been suggested to be hugely beneficial to agriculture (Zeng et al. 2014; Li et al. 2021a). The responses to H2 are likely to be supported by common molecular mechanisms in plants and animals, be that through antioxidants or Fe3+ reduction, or other means. Plant science might have a lot to learn from the work being carried out on prokaryotes and higher animals, and vice versa.
5.6 Conclusions and Future Perspectives
It seems clear now that H2 is a useful treatment for plants, alleviating a range of stress challenges (Table 5.1), as well as a potential regimen for the post-harvest storage of fruits and flowers, where it evidently delays senescence (Hu et al. 2014; Ren et al. 2017b). However, there are many aspects of the biochemistry of H2 which are simply not clear. Firstly, it is not known what the direct targets of H2 are in cells, even though several mechanisms have been suggested (Table 5.2), including scavenging radicals and other reactive signals, or acting through HO-1. Secondly, the full range of effects are not known, even though there are numerous reports of H2 application being beneficial (Table 5.1).
The redox mid-point potential of H2 is relatively low when compared to other biomolecules. Thermodynamically, it would be possible for H2 to reduce Fe3+ to Fe2+ and this would have ramifications for many enzymes, suggesting the reduction of prosthetic groups, particularly many heme groups, is theoretically possible, although not widely reported. Additionally, selenium-containing enzymes may be targets. However, such reactions are likely not to be kinetically feasible without the certain environments that could lower the activation energy for such a reduction to take place. However, clearly, a comprehensive study of the proteins controlled by H2 is required, even if it is simply to rule them out as being involved. Unlike the work with NO and H2S (Baty et al. 2005; Hawkins and Davies 2019), a proteomic approach would seem to not be feasible with H2 as no direct covalent post-translational modification of proteins have yet been reported for this molecule. On the other hand, downstream post-translational protein modification will occur, and a full compendium of such effects would be useful to know.
Although endogenous generation of H2 in some plants is possible and may be able to be manipulated, manipulation of exogenous sources of H2 would be a better approach as it would be easier. The presence of H2 may be dictated by the surrounding microflora, but H2 may be applied to plants as a treatment. Using H2 as a gas is unlikely to be of use, but the generation of HRW or HNW may allow application to either foliage or roots, or both. Clearly, there are safety aspects from a physical point-of-view, as H2 is extremely flammable, but from a biological viewpoint H2 appears to be safe to use, both for plants and animals. As with other similar molecules, for example H2S (Song et al. 2014), donor molecules may open up the better use of H2 in the future, and the use of some are already being reported, such as MgH2 (Li et al. 2020b) and AB@hMSN (Wang et al. 2021).
H2 use in agriculture and horticulture has yet to be widely adopted, but there is a growing interest in this biologically safe treatment. As more is known about how it works, and the significance of any effects are more widely reported, H2 may become an accepted way to enhance plant growth and crop storage in the future.
References
Alleman RJ, Katunga LA, Nelson MA, Brown DA, Anderson EJ (2014) The “goldilocks zone” from a redox perspective—adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol 5:358
Aroca A, Gotor C, Romero LC (2018) Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci 9:1369
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3411
Astier J, Mounier A, Santolini J, Jeandroz S, Wendehenne D (2019) The evolution of nitric oxide signalling diverges between animal and green lineages. J Exp Bot 70:4355–4364
Babbs CF, Pham JA, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90:1267–1270
Barry CS, Giovannoni JJ (2007) Ethylene and fruit ripening. J Plant Growth Regul 26:143–159
Baty JW, Hampton MB, Winterbourn CC (2005) Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem J 389:785–795
Bhuyan MB, Hasanuzzaman M, Parvin K, Mohsin SM, Al Mahmud J, Nahar K, Fujita M (2020) Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul 90:409–424
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87:682–685
Cao Z, Duan X, Yao P, Cui W et al (2017) Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int J Mol Sci 18:2084
Chen M, Cui W, Zhu K, Xie Y, Zhang C, Shen W (2014) Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J Hazard Mater 267:40–47
Chen Q, Zhao X, Lei D, Hu S, Shen Z, Shen W, Xu X (2017a) Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul 83:69–82
Chen H, Zhang J, Hao H, Feng Z, Chen M, Wang H, Ye M (2017b) Hydrogen-rich water increases postharvest quality by enhancing antioxidant capacity in Hypsizygus marmoreus. AMB Express 7:1–10
Chen K, Lin W, Kuo H (2021a) Chemical and biochemical aspects of molecular hydrogen in treating Kawasaki disease and COVID-19. Chem Res Toxicol 34:952–958
Chen W, Zhang H, Qin S (2021b) Neuroprotective effects of molecular hydrogen: a critical review. Neurosci Bull 37:389–404
Cui WT, Liu RT, Lin, YT, Xie YJ, Shen WB (2012) Carbon monoxide: a new member of the gaseous signalling molecule in plants. J Nanjing Agri Univ: 05
Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260:715–724
Cui W, Fang P, Zhu K, Mao Y, Gao C, Xie Y, Wang J, Shen W (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111
Cuypers A, Hendrix S, Amaral Dos Reis R, De Smet S, Deckers J, Gielen H, Jozefczak M, Loix C, Vercampt H, Vangronsveld J, Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7:470
D’Ambrosio D, Panina-Bordignon P, Sinigaglia F (2003) Chemokine receptors in inflammation: an overview. J Immunol Methods 273:3–13
Debbarma J, Sarki YN, Saikia B, Boruah HPD, Singha DL, Chikkaputtaiah C (2019) Ethylene response factor (ERF) family proteins in abiotic stresses and CRISPR–Cas9 genome editing of ERFs for multiple abiotic stress tolerance in crop plants: a review. Mol Biotechnol 61:153–172
Desikan R, Hancock JT, Coffey MJ, Neill SJ (1996) Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett 382:213–217
Dubois M, Van den Broeck L, Inzé D (2018) The pivotal role of ethylene in plant growth. Trends Plant Sci 23:311–323
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404
Farooq M, Wahid A, Kobayashi NSMA, Fujita DBSMA, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Sustain Agri:153–188
Feng J, Chen L, Zuo J (2019) Protein S-nitrosylation in plants: current progresses and challenges. J Integr Plant Biol 61:1206–1223
Fu H, Subramanian RR, Masters SC (2000) 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 40:617–647
Gadalla MM, Snyder SH (2010) Hydrogen sulfide as a gasotransmitter. J Neurochem 113:14–26
Ge L, Yang M, Yang NN, Yin XX, Song WG (2017) Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget 8:102653–102673
Guan W, Wei C, Chen A, Sun X, Guo G, Zou X, Shi J, Lai P, Zheng Z, Zhong N (2020) Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with coronavirus disease 2019 in a recent multicentre, open-label clinical trial. J Thorac Dis 12:3448–3452
Hancock JT, Hancock TH (2018) Hydrogen gas, ROS metabolism, and cell signaling: are hydrogen spin states important? Reactive Oxygen Species 6:389–395
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Hancock JT, Whiteman M (2016) Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann NY Acad Sci 1365:5–14
Hancock JT, LeBaron TW, Russell G (2021) Molecular hydrogen: redox reactions and possible biological interactions. React Oxyg Species (Apex) 11:17–25
Hawkins CL, Davies MJ (2019) Detection, identification, and quantification of oxidative protein modifications. J Biol Chem 294:19683–19708
He H, Yan J, Yu X, Liang Y, Fang L, Scheller HV, Zhang A (2017) The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem Biophys Res Commun 491:834–839
Hideg É, Jansen MA, Strid Å (2013) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci 18:107–115
Hirano S, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F (2021) Potential therapeutic applications of hydrogen in chronic inflammatory diseases: possible inhibiting role on mitochondrial stress. Int J Mol Sci 22:2549
Hu H, Li P, Wang Y, Gu R (2014) Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem 156:100–109
Huang D, Bian B, Zhang M, Wang C, Li C, Liao W (2020) The role and proteomic analysis of ethylene in hydrogen gas-induced adventitious rooting development in cucumber (Cucumis sativus L.) explants. PeerJ 8:e8896
Huo J, Huang D, Zhang J, Fang H, Wang B, Wang C, Ma Z, Liao W (2018) Comparative proteomic analysis during the involvement of nitric oxide in hydrogen gas-improved postharvest freshness in cut lilies. Int J Mol Sci 19:3955
Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K (2015) Beneficial biological effects and the underlying mechanisms of molecular hydrogen-comprehensive review of 321 original articles. Med Gas Res 5:1–21
Ishibashi T, Sato B, Shibata S, Sakai T, Naritomi Y, Koyanagi S, Hara H, Nagao T (2014) Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: a randomized, double-blind, placebo-controlled pilot study. Int Immunopharmacol 21:468–473
Jiang K, Kuang Y, Feng L, Liu Y, Wang S, Du H, Shen W (2021) Molecular hydrogen maintains the storage quality of Chinese chive through improving antioxidant capacity. Plan Theory 10:1095
Jin Q, Zhu K, Cui W, Xie Y, Han BIN, Shen W (2013) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ 36:956–969
Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M, Adachi T, Obayashi H, Yoshikawa T (2008) Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 28:137–143
Kang K, Kang Y, Choi I, Gu Y, Kawamura T, Toyoda Y, Nakao A (2011) Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res 1:11
Khan NA, Nazar R, Iqbal N, Anjum NA (eds) (2012) Phytohormones and abiotic stress tolerance in plants. Springer, Berlin/Heidelberg
Klepper L (1979) Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmos Environ (1967) 13:537–542
Kolbert Z, Barroso JB, Brouquisse R, Corpas FJ, Gupta KJ, Lindermayr C, Loake GJ, Palma JM, Petřivalský M, Wendehenne D, Hancock JT (2019) A forty year journey: the generation and roles of NO in plants. Nitric Oxide 93:53–70
Kumar P, Pathak S (2018) Nitric oxide: a key driver of signaling in plants. MOJ Eco Environ Sci 3:145–148
LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J (2019a) A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 24:2076
LeBaron TW, Laher I, Kura B, Slezak J (2019b) Hydrogen gas: from clinical medicine to an emerging ergogenic molecule for sports athletes. Can J Physiol Pharmacol 97:797–807
LeBaron TW, Singh RB, Fatima G, Kartikey K, Sharma JP, Ostojic SM, Gvozdjakova A, Kura B, Noda M, Mojto V, Niaz MA, Slezak J (2020) The effects of 24-week, high-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with metabolic syndrome: a randomized controlled trial. Diabetes Metab Syndr Obes 13:889–896
Li C, Huang D, Wang C, Wang N, Yao Y, Li W, Liao W (2020a) NO is involved in H2-induced adventitious rooting in cucumber by regulating the expression and interaction of plasma membrane H+-ATPase and 14-3-3. Planta 252:1–16
Li L, Liu Y, Wang S, Zou J, Ding W, Shen W (2020b) Magnesium hydride-mediated sustainable hydrogen supply prolongs the vase life of cut carnation flowers via hydrogen sulfide. Front Plant Sci 11:595376
Li L, Lou W, Kong L, Shen W (2021a) Hydrogen commonly applicable from medicine to agriculture: from molecular mechanisms to the field. Curr Pharm Des 27:747–759
Li L, Yin Q, Zhang T, Cheng P, Xu S, Shen W (2021b) Hydrogen nanobubble water delays petal senescence and prolongs the vase life of cut carnation (Dianthus caryophyllus L.) flowers. Plants 10:1662
Lin Y, Zhang W, Qi F, Cui W, Xie Y, Shen W (2014) Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol 171:1–8
Loreti E, van Veen H, Perata P (2016) Plant responses to flooding stress. Curr Opin Plant Biol 33:64–71
Lyons J (ed) (2012) Low temperature stress in crop plants: the role of the membrane. Elsevier, Saint Louis
Mathew A, Nadim N, Chandratilleke TT, Humphries TD, Paskevicius M, Buckley CE (2021) Performance analysis of a high-temperature magnesium hydride reactor tank with a helical coil heat exchanger for thermal storage. Int J Hydrog Energy 46:1038–1055
Mayorga MA, Cadavid J, Lopez C, Suarez O, Bonilla J, Vargas J, Narvaez P, Rodriguez LI (2020) Selection of hydrogen donors for the production of renewable diesel by in situ catalytic deoxygenation of palm oil. Chem Eng Trans 80:61–66
Mhawech P (2005) 14-3-3 proteins—an update. Cell Res 15:228–236
Mizuno K, Sasaki AT, Ebisu K, Tajima K, Kajimoto O, Nojima J, Kuratsune H, Hori H, Watanabe Y (2017) Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res 7:247–255
Molecular Hydrogen Institute (n.d.) http://www.molecularhydrogeninstitute.com/concentration-and-solubility-of-h2 (accessed on xx/xx/2021)
Morkunas I, Woźniak A, Mai VC, Rucińska-Sobkowiak R, Jeandet P (2018) The role of heavy metals in plant response to biotic stress. Molecules 23:2320
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N (2010) Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome – an open label pilot study. J Clin Biochem Nutr 46:140–149
Nishimaki K, Asada T, Ohsawa I, Nakajima E, Ikejima C, Yokota T, Kamimura N, Ohta S (2018) Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr Alzheimer Res 15:482–492
Niu Y, Xiang Y (2018) An overview of biomembrane functions in plant responses to high-temperature stress. Front Plant Sci 9:915
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688
Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, Tamaki M, Takeshita H, Futatuki T, Ohishi W, Ishiguro T, Okamoto S, Ishii S, Takanami H (2017) Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis 26:2587–2594
Ostojic SM (2020) Exercise-driven increase in gut microbial hydrogen production as a possible factor of metabolic health. Front Physiol 11:1065
Palmer RM, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Pandey AK, Gautam A (2020) Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol Plant 168:511–525
Pauly N, Pucciariello C, Mandon K, Innocenti G, Jamet A, Baudouin E, Hérouart D, Frendo P, Puppo A (2006) Reactive oxygen and nitrogen species and glutathione: key players in the legume–rhizobium symbiosis. J Exp Bot 57:1769–1776
Peck HD (1959) The ATP-dependent reduction of sulfate with hydrogen in extracts of Desulfovibrio desulfuricans. Proc Natl Acad Sci USA 45:701–708
Penders J, Kissner R, Koppenol WH (2014) ONOOH does not react with H2: potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free Radic Biol Med 75:191–194
Qu Y, Yan M, Zhang Q (2017) Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signal Behav 12:e1356970
Ren A, Liu R, Miao ZG, Zhang X, Cao PF, Chen TX, Li CY, Shi L, Jiang AL, Zhao MW (2017a) Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in Ganoderma lucidum. Environ Microbiol 19:566–583
Ren PJ, Jin X, Liao WB, Wang M, Niu LJ, Li XP, Xu XT, Zhu YC (2017b) Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic Environ Biotechnol 58:576–584
Russell G, Zulfiqar F, Hancock JT (2020) Hydrogenases and the role of molecular hydrogen in plants. Plan Theory 9:1136
Russell G, Nenov A, Hancock J (2021) Oxy-hydrogen gas: the rationale behind its use as a novel and sustainable treatment for COVID-19 and other respiratory diseases. Eur Med J. https://doi.org/10.33590/emj/21-00027
Schröder K (2020) NADPH oxidases: current aspects and tools. Redox Biol:101512
Scranton MA, Ostrand JT, Fields FJ, Mayfield SP (2015) Chlamydomonas as a model for biofuels and bio-products production. Plant J 82:523–531
Shao HB, Chu LY, Shao MA, Jaleel CA, Hong-mei M (2008) Higher plant antioxidants and redox signaling under environmental stresses. C R Biol 331:433–441
Shen B, Jensen RG, Bohnert HJ (1997) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiology 115:527–532
Singh S, Kumar V, Kapoor D, Kumar S, Singh S, Dhanjal DS, Datta S, Samuel J, Dey P, Wang S, Prasad R (2020) Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol Plant 168:301–317
Song G, Li M, Sang H, Zhang L, Li X, Yao S, Yu Y, Zong C, Xue Y, Qin S (2013) Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res 54:1884–1893
Song ZJ, Ng MY, Lee ZW, Dai W, Hagen T, Moore PK, Huang D, Deng LW, Tan CH (2014) Hydrogen sulfide donors in research and drug development. Med Chem Comm 5:557–570
Speckmann B, Steinbrenner H, Grune T, Klotz LO (2016) Peroxynitrite: from interception to signaling. Arch Biochem Biophys 595:153–160
Su N, Wu Q, Liu Y, Cai J, Shen W, Xia K, Cui J (2014) Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. J Agric Food Chem 62:6454–6462
Suzuki Y, Sano M, Hayashida K, Ohsawa I, Ohta S, Fukuda K (2009) Are the effects of α-glucosidase inhibitors on cardiovascular events related to elevated levels of hydrogen gas in the gastrointestinal tract? FEBS Lett 583:2157–2159
Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ (2006) Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38:733–744
Vargas SR, Santos PVD, Giraldi LA, Zaiat M, Calijuri MDC (2018) Anaerobic phototrophic processes of hydrogen production by different strains of microalgae Chlamydomonas sp. FEMS Microbiol Lett 365:fny073
Veal EA, Day AM, Morgan BA (2007) Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14
Wang R (2003) The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5:493–501
Wang C, Fang H, Gong T, Zhang J, Niu L, Huang D, Huo J, Liao W (2020) Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star’ by antagonizing ethylene. Plant Mol Biol 102:271–285
Wang Y, Lv P, Kong L, Shen W, He Q (2021) Nanomaterial-mediated sustainable hydrogen supply induces lateral root formation via nitrate reductase-dependent nitric oxide. Chem Eng J 405:126905
Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev 77:219–262
Wilks A (2002) Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal 4:603–614
Wilson HR, Veal D, Whiteman M, Hancock JT (2017) Hydrogen gas and its role in cell signaling. CAB Reviews 12:2–3
Wu Q, Su N, Cai J, Shen Z, Cui J (2015) Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J Plant Physiol 175:174–182
Wu Y, Yuan M, Song J, Chen X, Yang H (2019a) Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano 13:8505–8511
Wu X, Zhu ZB, Chen JH, Huang YF, Liu ZL, Zou JW, Chen YH, Su NN, Cui J (2019b) Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis L.) by exogenous hydrogen-rich water. Chemosphere 216:684–697
Wu Q, Huang L, Su N, Shabala L, Wang H, Huang X, Wen R, Yu M, Cui J, Shabala S (2020a) Calcium-dependent hydrogen peroxide mediates hydrogen-rich water-reduced cadmium uptake in plant roots. Plant Physiol 183:1331–1344
Wu Q, Su N, Shabala L, Huang L, Yu M, Shabala S (2020b) Understanding the mechanistic basis of ameliorating effects of hydrogen rich water on salinity tolerance in barley. Environ Exp Bot 177:104136
Wu X, Su N, Yue X, Fang B, Zou J, Chen Y, Shen Z, Cui J (2021) IRT1 and ZIP2 were involved in exogenous hydrogen-rich water-reduced cadmium accumulation in Brassica chinensis and Arabidopsis thaliana. J Hazard Mater 407:124599
Xia C, Liu W, Zeng D, Zhu L, Sun X, Sun X (2013) Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic Hepatitis B. Clin Transl Sci 6:372–375
Xiao S, Li Q, Hu L, Yu Z, Yang J, Chang Q, Chen Z, Hu G (2019) Soluble guanylate cyclase stimulators and activators: where are we and where to go? Mini Rev Med Chem 19:1544–1557
Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165:759–773
Xie Y, Zhang W, Duan X, Dai C, Zhang Y, Cui W, Wang R, Shen W (2015) Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso) flavonoids and antioxidant defence in Medicago sativa. Funct Plant Biol 42:1141–1157
Xu S, Zhu S, Jiang Y, Wang N, Wang R, Shen W, Yang J (2013) Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 370:47–57
Xu XT, Jin X, Liao WB, Dawuda MM, Li XP, Wang M, Niu LJ, Ren PJ, Zhu YC (2017a) Nitric oxide is involved in ethylene-induced adventitious root development in cucumber (Cucumis sativus L.) explants. Sci Hortic 215:65–71
Xu S, Jiang Y, Cui W, Jin Q, Zhang Y, Bu D, Fu J, Wang R, Zhou F, Shen W (2017b) Hydrogen enhances adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant Soil 414:53–67
Yang M, Dong Y, He Q, Zhu P, Zhuang Q, Shen J, Zhang X, Zhao M (2020) Hydrogen: a novel option in human disease treatment. Oxidative Med Cell Longev 2020:1–17
Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N (2013) Pilot study of H2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord 28:836–839
Zeng J, Ye Z, Sun X (2014) Progress in the study of biological effects of hydrogen on higher plants and its promising application in agriculture. Medical Gas Research 4:1–4
Zhang X, Zhao X, Wang Z et al (2015) Protective effects of hydrogen-rich water on the photosynthetic apparatus of maize seedlings (Zea mays L.) as a result of an increase in antioxidant enzyme activities under high light stress. Plant Growth Regul 77:43–56
Zhang X, Su N, Jia L, Tian J, Li H, Huang L, Shen Z, Cui J (2018) Transcriptome analysis of radish sprouts hypocotyls reveals the regulatory role of hydrogen-rich water in anthocyanin biosynthesis under UV-A. BMC Plant Biol 18:1–14
Zhang Y, Zhao G, Cheng P, Yan X, Li Y, Cheng D, Wang R, Chen J, Shen W (2019) Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int J Food Prop 22:1425–1438
Zhang Y, Cheng P, Wang Y, Li Y, Su J, Chen Z, Yu X, Shen W (2020a) Genetic elucidation of hydrogen signaling in plant osmotic tolerance and stomatal closure via hydrogen sulfide. Free Radic Biol Med 161:1–14
Zhang W, Niu Y, Li YX, Zhang F, Zeng RJ (2020b) Enrichment of hydrogen-oxidizing bacteria with nitrate recovery as biofertilizers in the mixed culture. Bioresour Technol 313:123645
Zhao X, Chen Q, Wang Y, Shen Z, Shen W, Xu X (2017) Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol Environ Saf 144:369–379
Zhu Y, Liao W, Niu L, Wang M, Ma Z (2016) Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol 16:146
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hancock, J.T., LeBaron, T.W., May, J., Thomas, A., Russell, G. (2023). Alleviation of Plant Stress by Molecular Hydrogen. In: Aftab, T., Corpas, F.J. (eds) Gasotransmitters Signaling in Plants under Challenging Environment. Plant in Challenging Environments, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-031-43029-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-43029-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43028-2
Online ISBN: 978-3-031-43029-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)