Abstract
In mammalian cells, hydrogen sulfide (H2S) has been identified as the third gasotransmitter after nitric oxide and carbon monoxide. Overwhelming evidence has proven that H2S also participates in diverse physiological and biochemical processes within the organism and exert specific functions in plants. A number of reports illustrated that H2S could improve plants ability of adapting to the multiple environmental stimuli by alleviating injuries and toxicities caused by the stressful conditions. It also participated in specific physiological, developmental and metabolic processes, such as the regulation of stomatal movement and drought tolerance, senescence and maturation, and lateral root formation. In this article, latest research progresses in biosynthetic and metabolic pathways of H2S in plants as well as corresponding physiological functions were summarized. We also discussed the potential molecular mechanism of interaction between H2S and other signaling molecules as well as the H2S-modifying protein activities. Finally, we prospected possible future work for H2S in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S), a small colorless gas with a characteristic odor of rotten eggs, has long been notorious as an environmental toxin with its unpleasant smell. For instance, H2S in high concentration is toxic to mitochondrial respiration inhibiting the activity of mitochondrial cytochrome C oxidase (Dorman et al. 2001; Mancardi et al. 2009). However, H2S has been proposed as the third gas messenger after nitric oxide (NO) and carbon monoxide (CO; Wang 2002). Similar to NO and CO, endogenously released H2S can freely cross cell membranes without relying on receptors and be involved in signal transduction processes as signaling molecule (Baskar and Bian 2011). H2S has been shown to play various roles in diverse physiological processes in animals, such as neuro-modulation, inflammation, apoptosis, cardio-protection, etc. (Kabil et al. 2014). Recently, mounting results on detecting the release of endogenous H2S in vivo imply that H2S may have a critical role in physiological and metabolic processes in plants. H2S has been revealed as a significant participant in the regulation of a range of physiological responses, including plant growth, development, stomata movement, flower senescence, etc. (García-Mata and Lamattina 2010; Lisjak et al. 2010; Zhang et al. 2010, 2011; Scuffi et al. 2014). Here, in this review, we discussed endogenous H2S synthesis and metabolism, different physiological functions of H2S and cross-talk of H2S with other signals in plants.
Endogenous H2S synthesis and metabolism
Endogenous H2S synthesis and metabolism in animals
H2S is endogenously produced in mammalian tissues through both enzymatic and non-enzymatic pathways, and the former is the main pathway (Wang 2002). In mammalian cells, H2S can be produced endogenously through the function of enzymes such as cystathionine-γ-lyase (CSE, EC 4.4.1.1), cystathionine-β-synthase (CBS, EC 4.2.1.22), cysteine aminotransferase (CAT, EC 2.6.1.3) and 3-mercapto pyruvate sulfurtransferase (3-MST, EC 2.8.1.2) (Olas 2015). CSE and CBS have been consistently demonstrated to produce H2S from the degradation of sulfur-containing amino acids, such as l-cysteine, l-homocysteine and l-cystathionine (Wang 2002; Xu et al. 2014). Pyridoxal 5′-phosphate (vitamin B6) is used as the cofactor (Yang et al. 2013). H2S is also produced by CAT and 3-MST from l-cysteine in the presence of α-ketoglutarate (Shibuya et al. 2009). It has been known that the production of H2S by CSE and CAT/3-MST pathway is regulated by Ca2+ (Kimura 2014). By contrast, non-enzymatic pathway only accounts for a small portion of physiologically generated H2S in mammalian cells. For example, glucose could react with methionine, homocysteine or cysteine to produce gaseous sulfur compounds—methanethiol and H2S (Kolluru et al. 2013).
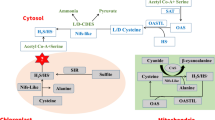
Endogenous H2S synthesis and metabolism in plants
Identification of cysteine desulfhydrase (CDes) in plants
We have searched NCBI database (http://www.ncbi.nlm.nih.gov/) to look for potential homolog proteins of mammalian CBS, CSE, MST, or CAT in plants. Two CBS domain-containing proteins were found in Arabidopsis thaliana, namely cystathionine-β-synthase domain-containing protein 1 and 2 (CBSX1/2). Interestingly, rather than catalyzing the biosynthesis of H2S generation, the main biological function of CBSX1/2 was to maintain intracellular homeostasis (Yoo et al. 2011). It could activate ferredoxin/NADP-thioredoxin systems, thereby regulating Calvin cycle and endogenous hydrogen peroxide (H2O2) level. This implies that mechanism of endogenous H2S generation in plants may be completely different from that in animals. Cysteine desulfhydrases (CDes) is considered a key enzyme of endogenous H2S generation in plants, which degrades cysteine into H2S, pyruvate, and ammonium, requiring pyridoxal 5′-phosphate as a cofactor.
At least two major categories of candidate cysteine-degrading enzymes have been characterized in different plant species (Table 1). Harrington and Smith (1980) reported the production of H2S from l-cysteine by an l-cysteine desulfhydrase (l-CDes; EC 4.4.1.1) in tobacco cells. They utilized S35-labeled l-cysteine, and found sulfide and pyruvate were produced in equimolar amounts from l-cysteine catalyzed by l-CDes. Later, l-CDes activity was detected and existed in leaf discs of some other plants, such as Cucurbita pepo, Cucumis sativus and pumpkin (Rennenberg 1983; Rennenberg and Filner 1983; Rennenberg et al. 1987). By contrast, Nagasawa et al. (1985, 1988) reported that the activity of d-cysteine desulfhydrase (d-CDes), which decomposed d-cysteine into pyruvate, H2S and ammonium in a stoichiometric ratio of 1:1:1, was detectable in Escherichia coli and Pseudomonas putida (Table 1). The activity of d-CDes was also detected in C. pepo, C. sativus, Chlorella fusca, spinach, tobacco and Arabidopsis (Schmidt 1982; Rennenberg 1983; Schmidt and Erdle 1983; Rennenberg et al. 1987; Riemenschneider et al. 2005). Therefore, l/d-CDes could catalyze the formation of H2S from l- or d-cysteine, respectively (Rennenberg et al. 1987; Álvarez et al. 2012a). Besides different substrates, their enzymatic inhibitors are also diverse. The activity of l-CDes could be inhibited by aminooxyacetic acid (AOA; 100 μM), which had no effects on d-CDes. In addition, their sub-cellular localization is also different. d-CDes was predominantly localized in cytoplasm, whereas l-CDes activity was found in chloroplast and mitochondria (Rennenberg et al. 1987). Interestingly, l/d-CDes exhibited differential enzymatic kinetics parameters as well. For example, the l-CDes had a sharp pH optimum of 8 and preliminary kinetic data indicated that the K m is 0.2 mM in tobacco cells (Harrington and Smith 1980). By contrast, an apparent K m of 0.14 mM or 0.25 mM was found for d-CDes in C. fusca or Arabidopsis (Schmidt 1982; Riemenschneider et al. 2005).
Catalytic mechanism and physiological functions of CDes in plants
The reaction catalyzed by l-CDes has been recognized as a side reaction of the l-cysteine biosynthetic pathway for a long time (Aida et al. 1969). Cysteine is a metabolic precursor for numerous important bio-molecules. The last step of cysteine biosynthetic metabolism is catalyzed by O-acetylserine(thiol)lyase (OASTL), which incorporates sulfur under reduction state with O-acetylserine thus produces cysteine (Álvarez et al. 2012a). However, early views implied that OASTL also possessed the activity of l-CDes, which catalyzed the release of H2S from degradation of l-cysteine as a side reaction (Aida et al. 1969). Three O-acetylserine(thiol)lyase isoenzymatic sub-families were characterized in Arabidopsis (Hell et al. 1994; Barroso et al. 1995; Hesse et al. 1999; Jost et al. 2000; Burandt et al. 2002; Wirtz et al. 2004), namely OASTL A1 (At4g14880), OASTL A2 (At3g22460), OASTL B (At2g43750), and OASTL C (At3g59760). Preliminary kinetic data revealed that the cysteine synthesizing activity of OASTL B was 25 µmol min−1mg protein−1 at pH 8.0, whereas corresponding H2S formation activity in the presence of dithiothreitol (DTT) was about 250 nmol min−1mg protein−1 at pH 9.0, further illustrating that the molar ratio of cysteine synthesis and H2S formation was nearly 10–1. Consequently, the importance of identification of true plant l-CDes was neglected, regardless of the fact that l-CDes activity has been detected in plants.
A novel gene (AT5G28030) has been cloned and identified as l-CDes by (Álvarez et al. 2010), which was expected to encode a cysteine synthase-like protein (CS-LIKE) in A. thaliana (Table 1). By comparing the amino acid sequences of CS-LIKE with that of Arabidopsis OASTL protein, it was found that partial characteristic sequences had varied. For example, 74–78 amino acid residues of OAS-A1 was TSGNT, a highly conserved sequence which might be involved in sulfur incorporation into cysteine through a formulation a loop (Bonner et al. 2005). However, in that of CS-LIKE protein, the 75 amino acid residue, serine, was replaced by glycine. In addition, some amino acid residues are non-conservative in the β8A–β9A loop of CS-LIKE (Álvarez et al. 2010), while the loop of OASTL is highly conserved and has been demonstrated to be important for the interaction with serine acetyltransferase (SAT; EC 2.3.1.30). The highly conserved β8A–β9A surface loop was considered as the action site with SAT in true OASTL enzymes, but CS-LIKE has non-conservative amino acid changes in this loop and this change resulted in its inability to interact with SAT. Further research has found that CS-LIKE catalyzes the degradation of l-cysteine rather than its biosynthesis. The K m value for l-cysteine in the CS-LIKE reaction is 13-fold lower than that for OAS in the OASTL reaction, demonstrating a much higher affinity of CS-LIKE for l-cysteine as a substrate (Álvarez et al. 2010). Based on theses experimental data, Álvarez et al. (2010) proposed CS-LIKE was a novel l-CDes (EC 4.4.1.1) and designated it DES1, further study discovered that its catalytic activity required pyridoxal-5′-phosphate as a cofactor.
The biological significance of DES1 in cysteine metabolism was investigated by the phenotype of the T-DNA insertion mutants, des1–1 and des1–2 (Álvarez et al. 2010). It was found that compared with wild types, the total DES activity in leaves (5-week-old) or seedlings (2-week-old) of des1–1 and des1–2 plants was reduced by 20 % or 25 %, respectively. Furthermore, compared with wild types, mutation of DES1 gene led to premature leaf senescence. Through real-time quantitative PCR analysis of senescence-associated genes SEN1 and SAG21 as well as NAP (a member of NAC transcription factor gene family), the conclusion was drawn that expression of senescence-associated genes and transcription factors was increased in des1–1 and des1–2 mutants compared with their respective wild types (Col-0 and No-0). In addition, a cadmium (Cd) tolerance test comparing des1–1 and des1–2 mutants with their respective wild types was performed. Under 175 mM Cd, wild type Col-0 and No-0 seed germination rate was 18 and 14 %, respectively, with a growth of chlorotic leaves; while des1–1 and des1–2 mutant seed germination rate was 88 and 75 %, with a growth of green leaves. Furthermore, under 250 mM Cd, seed germination rate of wild type Col-0 and No-0 was only about 4 and 2 % while that of des1–1 and des1–2 mutants was 47 and 34 %. These data indicated that des1 mutants had enhanced tolerance to Cd, further research revealed that des1 mutants enhanced antioxidant defenses under Cd stress (Álvarez et al. 2010). Another explanation was that the total intracellular cysteine concentration increased by approximately 25 %, which is a precursor of glutathione synthesis (Álvarez et al. 2012b; Romero et al. 2013a, b).
Álvarez et al. (2012b) reported that mutation of DES1 disrupted H2S generation in the Arabidopsis cytoplasm as well as damage plant metabolism. ATG8 protein, which was involved in autophagy, has been analyzed through immunoblot analysis to verify whether the existence of senescence-associated vacuoles (SAVs) in des1 mutants was correlated with autophagic mechanism. Results indicated that deficient in DES1 protein function promoted accumulation and lipidation of SAVs as well as activation of autophagy. Subsequent study found that mutation of DES1 impacted H2S generation in the Arabidopsis cytosol, but capacity of H2S generation could be restored through exogenous addition sources (Na2S or sodium hydrosulfide, NaHS) or by genetic complementation, which could further eliminate the phenotypic differences between the des1 mutant and wild-type plants.
Interestingly, DES influenced plant immune response through cooperating with OASTL to regulate cysteine metabolism sequentially (Álvarez et al. 2012b). Study demonstrated that cysteine was closely related to the plant immune response. des1 mutants showed an increase of 20–30 % in cysteine content compared with their respective wild types, whereas oas-a1 mutant expressed 24–31 % less cysteine content compared with wild types. Further research suggested that des1 mutant had an increasing resistance to biotrophic and necrotrophic pathogens, while oas-a1 knockout mutants were more sensitive. These results implied that DES and OASTL play a fundamental role in regulating response to oxidative stress and resisting to pathogens through mediating cysteine metabolism.
Recently, besides DES1, several other genes encoding l/d-CDes have been discovered in Arabidopsis and Brassica napus (Table 1). For instance, AtNFS1/AtNifS (A. thaliana nitrogen fixation S, At5g65720), which is localized in the mitochondria, plays a role in Fe/S cluster assembly in cell (Kushnir et al. 2001), while AtNFS2/AtSUF (Arabidopsis chloroplastic nitrogen fixation S, At1g08490) catalyzes the formation of elemental sulfur and alanine from cysteine or of elemental selenium (Se) and alanine from seleno-Cys in the plastids (Leon et al. 2002; Pilon-Smits et al. 2002). l-CDesI (l-cysteine desulfhydrase, At3g62130) catalyzes the degradation of cysteine into H2S, pyruvate, and ammonium in the nucleus (Papenbrock et al. 2007). In addition to Arabidopsis, a novel gene encoding l-CDes from B. napus was identified and designated it as BnDES1 by Xie et al. (2013a, b).
Physiological functions of H2S in plants
In the past few years, growing evidence showed that H2S could alleviate damage in plants challenged with numerous abiotic stresses via the improvement of antioxidant systems (Table 2; Chen et al. 2013; Dawood et al. 2012; Singh et al. 2015; Wang et al. 2010; Sun et al. 2013; Fang et al. 2014; Zhang et al. 2008; Jin et al. 2011; Li et al. 2011, 2013a, b, 2014; Shan et al. 2014; Lai et al. 2014; Wang et al. 2012). Besides, H2S is also involved in the regulation of stomata movement (Lisjak et al. 2010; Zhang et al. 2010; García-Mata and Lamattina 2010; Scuffi et al. 2014), as well as plant growth, development and anti-aging (Zhang et al. 2011). Interestingly, it has been demonstrated that in the above-mentioned physiological processes, some other signaling molecules are also involved, such as Ca2+ and NO. NaHS and GYY4137 (morpholin-4-ium 4 methoxyphenyl phosphinodithioate) are usually used as the donors of exogenous H2S in related studies. NaHS solution, of which the H2S concentration can be regulated close to that under physiological conditions, is the most widely used (Wang et al. 2012). GYY4137 is a kind of phosphorodithioate derivatives. It can generate H2S slowly and steadily under physiological conditions (Li et al. 2008). H2S gas and H2S saturated solution can also be donors of exogenous H2S, however, due to the toxicity and low controllability of H2S gas, as well as the inability to simulate the concentration of H2S under physiological conditions (H2S saturated solution), they are not used commonly.
Heavy metal and other ion stresses
Heavy metals exhibit a strong inhibitory effects on plant growth and development (Schützendübel and Polle 2002). Several studies illustrated that H2S could alleviate toxic effects of these non-essential metal elements through different strategies. For example, cadmium (Cd) is one of the most toxic heavy metals and exerts deteriorated responses to almost every aspects of plant physiology. An over-accumulation of ROS was induced in Populus euphratica upon Cd challenging, resulting in oxidative damage and programmed cell death (Sun et al. 2013). Pretreatment with NaHS could alleviate Cd toxicity via the following mechanisms. First, exogenous H2S could reduce ROS over-production thus alleviate oxidative damage through up-regulating the activity of antioxidant enzymes such as glutathione reductase (GR) and catalase. What is more, it was also found that exogenous H2S could prevent Cd flowing into cells through H2O2-activated Ca2+ channels on plasma membrane, while promoted cadmium inside cells enter vacuoles. Therefore, the accumulation of cadmium in cytoplasm was reduced.
Boron is an essential micronutrient for plants. However, excess boron poses toxic effect to plants. Wang et al. (2010) discovered that excess boron caused a significant root growth inhibition of cucumber seedlings. This inhibited tendency was closely related to the repression of the up-regulated gene expression of pectin micronutrient (CsPME, an enzyme which controls the formation of cell wall through catalysis of cell wall pectin methyl esterification) as well as expansin (CsExp, an enzyme catalyzing expansion of cell wall) in cucumber seedlings induced by excess boron. Pretreatment with NaHS, a donor of exogenous H2S, significantly alleviated the root growth inhibition. Importantly, the up-regulation of expression of CsPMEs, CsExp and PME activity was also inhibited, further implying that H2S alleviated root growth inhibition induced by boron toxicity in cucumber seedlings via the regulation of cell wall biosynthesis.
Cu is also an essential microelement for plant growth and development, involved in electron transfer chains, catalytic enzymes related to protein trafficking, etc. (Yruela 2005). According to the research results of Zhang et al. (2008), excess copper triggered an accumulation of ROS, such as H2O2 and superoxide anion (O2 −) in wheat seeds, resulting in oxidative damage and subsequent germination inhibition. Pretreatment with NaHS up-regulated activities of antioxidant enzymes, such as catalase and superoxide dismutase (SOD), thereby reducing the Cu-induced overproduction of ROS and oxidative damage, and seed germination rate was subsequently improved. Importantly, this research also found that pretreatment with NaHS could slightly reduce copper over-accumulation, suggesting that H2S might be involved in the regulation of copper uptake, and specific mechanism remained to be further elucidated.
Some other metal elements such as chromium (Cr) and aluminum (Al) also have a detrimental effect on plants. Fang et al. (2014) reported that Cr triggered cell death in foxtail millet root tip, which was alleviated by exogenous H2S pretreatment. Interacting with Ca2+, H2S alleviated the accumulation of Cr in foxtail millet cell through down-regulating genes encoding proteins promoting heavy metals uptake expression (ZIP1, ZIP3, ZIP4, and ZIP6), as well as up-regulating genes encoding proteins promoting heavy metals efflux expression such as HMA3–1, HMA3–2, MTPC1, MTPC2. However, the relationship and interaction mechanism between H2S and Ca2+ remains to be further studied at genetic level. Dawood et al. (2012) found that Al stress led to growth inhibition as well as ROS accumulation in barley seedling, which in turn caused oxidative damage. NaHS pretreatment could up-regulate antioxidant enzyme activity such as SOD and CAT in barley seedling, through which oxidative damage caused by Al was eased. Moreover, the activity of plasma membrane ATPase was decreased by aluminum, whereas NaHS pretreatment alleviated this tendency, implying that the physiological function of H2S-improved aluminum tolerance in barley seedling may be related to the regulation of ATPase activity. In addition, Chen et al. (2013) discovered that expression of the gene encoding citrate transporter (HvAACT1) was activated under Al stress in barley seedling, thus activating the secretion of citrate. NaHS pretreatment up-regulated the expression of HvAACT1, in turn leading to increased citrate secretion. These results showed that there might be a relationship between H2S alleviating Al toxicity and citrate secretion in barley seedlings.
Arsenate can also induce an ROS over-accumulation in plants, resulting in oxidative damage, growth and photosynthesis inhibition as well as reduction of nitrogen content. Ascorbic acid (ASA)–glutathione (GSH) cycle, in which multiple enzymes are involved such as monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), GR, etc., is an important defence mechanism in plants to scavenge over-accumulated ROS. Arsenate stress brought oxidative damage, disorganization of lipids, proteins and plasma membrane as well as growth inhibition to pea seedlings (Singh et al. 2015). Pretreatment with NaHS enhanced the activities of MDHAR, DHAR and GR, which participated in ASA–GSH cycle in pea seedlings, thereby rescued arsenate-induced declined levels of ASA and GSHs reduced/oxidized ratios. Besides, they also discovered that the activities of cysteine desulfhydrase and nitrate reductase (NR) were decreased under arsenate stress, resulting in a decreased production of endogenous nitric oxide. However, NaHS pretreatment led to up-regulated activities of DES and NR, as well as an increased content of NO, implying that NO was involved in the process of H2S enhancing arsenate tolerance of pea seedlings.
Heat stress
Many studies have suggested that high temperatures could result in protein denaturation, aggregation and increased fluidity of membrane lipids, thus cause inactivation of enzymes in chloroplast and mitochondria, impeding protein synthesis, degradation and eventually lead to cellular injury and even trigger cell death (Knight 2000; Larkindale and Knight 2002; Wahid et al. 2007; Hanumappa and Nguyen 2010). The bio-protective behavior of H2S on plant heat tolerance was universal, and a potent crosstalk between H2S and other signaling molecules was discovered. Li et al. (2011) found that pretreatment with NaHS alleviated heat-induced decrease of survival rate in tobacco suspension cultured cells. Moreover, this physiological function of NaHS was further promoted by exogenous Ca2+, whereas significantly inhibited by ethylene diamine tetraacetic acid (EDTA, a plasma membrane channel blocker), as well as calmodulin (CaM) antagonists. These results indicated that H2S-enhanced heat tolerance of tobacco suspension cultured cells may be related to the transmembrane transport and signal transduction of Ca2+.
It was also demonstrated that NaHS pretreatment significantly increased germination percentage of seeds as well as survival percentage of seedlings of maize upon heat stress challenging, as well as the alleviation of increased electrolyte leakage in roots (Li et al. 2013a, b). Meanwhile, the activity of 1-pyrroline-5-carboxylate synthetase (P5CS), the key enzyme of glutamate pathways through which proline was accumulated, was enhanced, whereas that of proline dehydrogenase (ProDH) was down-regulated. Thus an increase of the accumulation of proline occurred. These results revealed that H2S could enhance heat tolerance of maize, in which proline synthesis might be involved. Li et al. (2013a, b) further reported that pretreatment with sodium nitroprusside (SNP), an exogenous NO donor, significantly increased survival percentage of maize seedlings under heat stress. Meanwhile, the activity of l-CDes, the key enzyme of generation of endogenous H2S, was increased, resulting in an increase of H2S content. What is more, the function that NO enhanced heat tolerance of maize seedlings was enhanced by application of GYY4137, a H2S donor, whereas inhibited by inhibitors of H2S synthesis as well as H2S scavengers. These results suggested that H2S interacted with NO as a downstream signaling molecule, alleviating the damage caused by heat stress in maize seedlings. The interrelationship between two signaling molecules remained to be further studied.
Salt stress
Salt stress is one of the major abiotic stress factors that limits seed germination, seedling growth, plant growth and productivity (Shi et al. 2007; Ferreira-Silva et al. 2012). High salinity causes imbalance of ion and redox homeostasis, leading to an increased ROS content and caused oxidative damage (Li et al. 2014; Shan et al. 2014; Wang et al. 2012). ROS overproduction and a change of the redox states of AsA and GSH, which protected matabolic precesses against ROS, was caused by salt stress in maize leaves, thus triggered an oxidative damage. Exogenous H2S increased antioxidant capacity of maize seedlings and alleviated the oxidative damage under salt stress by up-regulating the activities of DHAR and GR which were involved in AsA and GSH metabolism (Shan et al. 2014). In addition, pretreatment with exogenous H2S up-regulated the expression of antioxidant enzymes such as SOD, CAT and guaiacol peroxidase (Wang et al. 2012). This contributed to the reduction of the ROS accumulation upon salt treatment, thus alleviating oxidative damage as well as seed germination and seedling growth inhibition of Medicago sativa caused by salt stress. Meanwhile, exogenous H2S treatment produced an increase of endogenous NO production, however, the functions mentioned above of H2S were inhibited by NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide (cPTIO), indicating that NO was required for H2S enhancing Medicago sativa tolerance against salt stress.
NaCl also interrupted the ion homeostasis in Medicago sativa seedling roots (Lai et al. 2014), by inducing plasma membrane depolarization, resulting in an activation of depolarization-activated shaker-like K+ outward-rectifying K channels (SKOR), thus leading to a K+ loss which caused an imbalance of K/Na ratio. Further study indicated that NaHS pretreatment caused down-regulated SKOR expression, thus inhibited the loss of K+ in plants cells and maintained K+/Na+ homeostasis. Based on these researches, H2S could enhance salt tolerance by differential mechanisms in several plant species, such as up-regulating the expressions of antioxidant enzymes and maintaining ion homeostasis, which contributes to achieve redox and ion balance. The regulation behavior of H2S on ion homeostasis was also proved in Arabidopsis. Exogenous H2S obviously mitigated the increase of Na+/K+ caused by salt tress, thus alleviated the growth inhibition of Arabidopsis root (Li et al. 2014). Further research indicated that pretreatment with exogenous H2S promoted endogenous H2O2 accumulation through regulating the activities of glucose-6-phosphate dehydrogenase (G6PDH) and plasma membrane NADPH oxidase, which were involved in H2O2 production. However, the G6PDH inhibitor could remove the alleviating effect of H2S against salt stress, indicating that H2O2 was required for H2S-improved salinity resistance in Arabidopsis root.
Regulation of drought and osmotic stress as well as stomatal movement
Drought stress is one of the important environmental factors which greatly restricted crop production (Boyer 1982). Jin et al. (2011) has found endogenous H2S production was increased by the up-regulation activity of l-CDes and d-CDes under drought stress, leading to induced stomatal closure and improvement of drought resistance in A. thaliana consequently. Later, further research indicated that pre-treatment with H2S donor NaHS can enhance drought tolerance via interacting with abscisic acid (ABA) in the regulation of stomatal closure in Arabidopsis (Jin et al. 2013; Lisjak et al. 2011) investigated osmotic stress by treating bermudagrass with PEG6000, and found that endogenous H2S production was increased under osmotic stress. Pretreatment with NaHS up-regulated the activities of antioxidant enzymes such as CAT, peroxidase (POD), contributing to a reduced accumulation of H2O2 and superoxide anion, thereby alleviating osmotic stress-induced oxidative damage in bermudagrass.
Stomata are small pores bounded by a pair of guard cells on the surfaces of leaves, controlling about 90 % of gas exchange between the interior of the leaves and the external atmosphere (Hetherington and Woodward 2003). Stomata express major contributions to the ability of plants to control their water relations and to gain carbon, and play a crucial role in photosynthesis, transpiration, and plant drought tolerance (Hetherington and Woodward 2003; Chaerle et al. 2005). Stomata close when osmotic pressure of the guard cells drops, which in turn reduce water loss and contribute to the up-regulating of osmotic pressure. Recently, the regulations of guard cells and stomatal movement have been well-studied, particularly abscisic acid (ABA) signaling pathways. ABA plays an important role in signaling processes, not only induces stomatal closure and inhibits stomatal opening, but also regulates the expressions and activities of many effectors in the signaling cascade (García-Mata and Lamattina 2013). The ABA-dependent signaling pathways in guard cells involves numerous second messengers, including potassium ion, Ca2+, protein phosphatases, guanylate cyclase/cyclic ADP ribose, H2O2, NO, etc. (Hetherington and Woodward 2003). Stomatal movement regulated by ABA has become a model system for the study of signaling processes in plants.
H2S has been proposed to be involved in stomatal movement (García-Mata and Lamattina 2010; Lisjak et al. 2010; Zhang et al. 2010; Scuffi et al. 2014). H2S donor NaHS and GYY4137 both caused stomatal opening in Arabidopsis thaliana whether in dark or light (Lisjak et al. 2010). Further research found that the treatment to leaves with either ABA or darkness could cause the production of NO, while exogenous addition of NaHS or GYY4137 significantly reduced NO accumulation. It has been reported that NO participated in ABA-triggered stomatal closure (Bright et al. 2006). Therefore, H2S may cause stomatal opening through reducing ABA-mediated accumulation of NO. Interestingly, early ideas already proposed that in animal systems H2S inhibited NO generation through inhibition of nitric oxide synthase (Kubo et al. 2007).
In contrast to the above-mentioned observation, H2S-induced stomatal closure was observed in Vicia faba, A. thaliana and Impatiens walleriana (Zhang et al. 2010). Moreover, García-Mata and Lamattina (2010) also reported H2S could induce stomatal closure in A. thaliana and V. faba. Exogenous H2S released by H2S donors NaHS induced stomatal closure in a dose-dependent manner, reaching the maximum effect at 100 μM. Further research indicated that both H2S donors, NaHS and GYY4137, induced stomatal closure in the same pattern. In addition, NaHS-induced stomatal closure was partially blocked in guard cells by H2S scavenger, hypotaurine (HT), reaching the maximum block effect at 200 μM. Interestingly, ABA-dependent stomatal closure was partially blocked by HT pretreatment suggesting that H2S might be involved in ABA signal inducing stomatal closure. The different results between Lisjak et al. (2010) and García-Mata and Lamattina (2010) may be because of differences in isolation of epidermal strips and the timing of the treatments leading to the differential response of the guard cells to H2S donors.
Genetic experiment was further performed to elucidate the role of H2S in ABA-induced stomatal closure. Scuffi et al. (2014) studied the role of DES1 in the cross-talk between H2S and NO in the ABA-dependent signaling pathways in guard cells. This study indicated that ABA fails to induce stomatal closure in isolated epidermal strips of des1 mutants, demonstrating that DES1 was required for ABA-dependent stomatal closure. Subsequent study showed that the ABA-hyposensitivity of des1 mutants was restored through the addition of exogenous H2S from either NaHS or GYY4137. More importantly, complementing with the full-length DES1 cDNA in des1 mutant also resulted in the similar restored results, suggesting that DES1-related H2S production participate in ABA-dependent stomatal closure. NO-specific scavenger cPTIO impaired H2S-dependent induction of stomatal closure was observed through treating the epidermal strips of wild-type plants with NaHS in the presence of cPTIO, indicating NO is involved in H2S-dependent stomatal closure. In addition, NaHS also failed to trigger stomatal closure in the NR1/2 double mutant (nia1/nia2) plants, which could be restored by exogenous addition of NO specific donor S-nitroso-N-acetylpenicillamine (SNAP). These pharmacological and genetic evidences illustrated that the depletion of endogenous NO blocked H2S-mediated induction of stomatal closure, implying the interaction of H2S and NO in stomatal movement processes. Moreover, this study also demonstrated that NO was downstream of DES1-produced H2S in the ABA-dependent stomatal closure. The stomata of wild type and des1 mutant responded to SNAP at the same level. Furthermore, H2S donors NaHS or GYY4137 had significant increased endogenous NO fluoresce level in wild type compared with des1 mutant, indicating H2S has an effect on endogenous NO production. However, this change was not obvious in des1 mutants and Col-0 plants when treated with ABA together with the H2S scavenger HT, demonstrating that DES1 is required for ABA-induced NO production.
Delaying plant senescence
Senescence is a complex process that involves lipid peroxidization, causes oxidant damage induced by ROS, resulting in membrane structural and biophysical changes, eventually leading to interference of cellular homeostasis and even cause cell death (Borochov and Woodson 1989; Paliyath and Droillard 1992; Beja-Tal and Borochov 1994; Borochov et al. 1994; Rubinstein 2000). NaHS-pretreatment delayed senescence, flower abscission and browning of some cut flowers, such as Erigeron annuus, Euonymus maackii Rupr, Hibiscus syriacus L., Liriope spicata, Loropetalum chinense, Punica granatum L., Rosa chinensis Jacq, and Salix matsudana Koidz (Zhang et al. 2011). In addition, this study indicated that in explants, the content of malondialdehyde (MDA), which as an indicator of the oxidative damage degree of cells, showed an inverse correlation to endogenous H2S concentration. Further research showed aging plants displayed higher levels of MDA and lower amounts of H2S. Besides, NaHS pretreatment up-regulated the activities of CAT, SOD, ascorbate peroxidase (APX) and POD thus maintained much lower levels of H2O2, indicating H2S delayed plant senescence through alleviating oxidative damage.
Interaction of H2S with other signaling molecules
It has been known that H2S extensively interacts with other signaling molecules in plants, such as NO (Lisjak et al. 2010; Wang et al. 2012; Scuffi et al. 2014; Singh et al. 2015; Shi et al. 2014; Hancock and Whiteman 2014; Calderwood and Kopriva 2014), ROS (Hancock and Whiteman 2014), H2O2 (Li et al. 2014), CO (Lin et al. 2012), indole acetic acid (IAA; Zhang et al. 2009), gibberellic acid (GA; Xie et al. 2013a, b), jasmonic acid (JA; Hou et al. 2011) and ABA (García-Mata and Lamattina 2010; Scuffi et al. 2014; Jin et al. 2011). These interactions resulted in a complex signaling network in plant biology. However, the definite roles of H2S in signal transduction networks and the mechanism of interaction between H2S and other signal molecules are still poorly understood. For example, the molecular mechanism of the interaction between H2S and NO still needs further research. Wang et al. (2012) reported that in M. sativa, salt tolerance was enhanced by exogenous addition of the H2S donor, meanwhile an increase of endogenous NO production was observed. Whereas, the above function of H2S was inhibited by NO scavenger cPTIO, indicating that NO was required for salt damage alleviation function in M. sativa. Similar case was observed by Li et al. (2012) that NO participated in H2S enhancing tolerance against Cd stress in M. sativa. Interestingly, Hancock and Whiteman (2014) suggested that H2S is not working as a signaling molecule in modulating the levels and effects of ROS and NO, rather than indicating that H2S is acting as a referee to ensure that the over-accumulation of such compounds is not causing damage to cells and tissues.
In animals, H2S exerts its functions by protein S-sulfhydration under physiological conditions in organisms. H2S can modify specific targets through protein sulfhydration which involves the posttranslational modification of protein cysteine residues (Mustafa et al. 2009). Simultaneously, a major physiological effect of NO is executed by protein S-nitrosylation, a reversible posttranslational modification by covalent addition of an NO molecule onto a cysteine thiol to form S-nitrosothiol (Jaffrey et al. 2001; Stamler et al. 2001). Several studies demonstrated that the functions of proteins in NO signaling pathways were regulated through S-nitrosylation by numerous mechanisms (Hess et al. 2005; Wang et al. 2006; Astier et al. 2011; Gupta 2011; Hess and Stamler 2012). In animals, Mustafa et al. (2009) indicated that protein posttranslational modification can be regulated by competition between the sulfhydration and nitrosylation of the same cysteine residues and this competition can be observed on GAPDH protein. In addition, Sen et al. (2012) suggested that sulfhydration and nitrosylation both appear to regulate p65 subunit of nuclear factor κB and do so reciprocally. Compared with animals, current research of the role of S-sulfhydration and S-nitrosylation as protein signaling modality in plants is still in its infancy. For example, 176 S-sulfhydrated proteins have been identified in Arabidopsis leaves, and several proteins are involved in electron transport and energy pathways such as tricarboxylic acid cycle (Romero et al. 2013a, b). 63 S-nitrosylated proteins from S-nitrosoglutathione (GSNO)-treated cell culture extracts and 23 S-nitrosylated proteins from NO-treated Arabidopsis leaves were identified by a biotin switch method (Lindermayr et al. 2005). Recently, Hu et al. (2015) identified 1195 endogenously S-nitrosylated peptides in 926 proteins from the Arabidopsis by a site-specific nitrosoproteomic approach. 106 S-sulfhydrated proteins were identified in Arabidopsis by biotin switch method (Aroca et al. 2015). Considering the fact that NO signaling participates in H2S signal transduction (Lisjak et al. 2010; Wang et al. 2012; Scuffi et al. 2014; Singh et al. 2015), in vivo synthesis of H2S and NO may interact with each other through S-sulfhydration and S-nitrosylation, respectively, consequently exert functions under physiological and biochemical conditions in plants. Therefore, the cross-talk between H2S-meidated S-sulfhydration and NO-mediated S-nitrosylation needs to be further studied, and further genetic evidence should be provided.
Conclusions and perspectives
As a newly emerged gaseous signal molecule, H2S has long been known primarily as an environmental toxin because it can inhibit the activity of mitochondrial cytochrome C oxidase at high concentrations (Dorman et al. 2001). However, recent papers indicated that H2S is a mitochondrial substrate at low concentrations and is a poison to mitochondria at high concentration (Helmy et al. 2014; Mancardi et al. 2009). Sulfide is regarded as an important intermediate in sulfur metabolism. Hydrogen sulfide can serve as an alternative source of sulfur for plants (Calderwood and Kopriva 2014).
Over these years, numerous papers have provided evidence for the various biological roles of H2S in mammals and plants (Kabil et al. 2014; Polhemus and Lefer 2014; Cooper and Brown 2008; Zhao et al. 2001; Kimura 2000; Kida et al. 2011; Kimura and Kimura 2004; Lisjak et al. 2013; García-Mata and Lamattina 2013; Scuffi et al. 2014). Studies on endogenous synthesis and physiological functions of H2S in plants have gained great achievements. However, many research issues remained to be further explored, such as, the target molecules of H2S when it participated in the above physiological and biochemical processes. In addition, the downstream cascade reactions triggered by H2S signaling still need characterization. The studies in animals may provide the valuable references for that in plants, as there might exist similar physiological and biochemical processes between in animals and in plants. For example, in animals, H2S realizes signal transduction by activation of cyclic adenosine monophosphate (cAMP) pathways which is different from NO and CO (Wang 2002). Meanwhile, although it is gradually considered that H2S acts as a novel signaling molecule and participates in signal transduction, further studies are still needed to verify its possibility. Whether H2S really plays a role as a signaling molecule and takes part in signal transduction pathways when it is involved in physiological processes such as stomatal movement and stress responses, or it is merely a response of plants to the existence of H2S. Moreover, if it is determined that H2S is a true signaling molecule in plants, then, whether the signal transduction pathway of H2S in plants is similar to that in animals, that is, whether H2S competes signal transduction via cAMP pathways as well. These questions remain to be answered by further researches.
In addition, most of physiological data in plants came from treatment with exogenous H2S donors, scavenger or synthetic inhibitor. Most importantly, the H2S-metabolism related genetic researches are very poor. Although mutations of the DES1 gene interfered H2S generation in the cytosol and strongly affect plant metabolism. The leaf endogenous H2S concentrations in DES1 mutants are only 30 % less than the quantified amount in the wild types (Álvarez et al. 2012b), remaining 70 % endogenous H2S production is unclear. Thus, the mechanism of endogenous H2S production in plants and signal transduction pathways between plant cells and tissues need to be further explored. Importantly, the endogenous H2S production has been lack of effective measuring approaches for a long time, the detection of H2S mainly via optical densitometry method at present, which still cannot real-time monitoring the sub-localization and dynamic spatial and temporal changes of H2S in cell. Taken together, some aspects that need to be deeply studied in the future as a research priority, such as developing a high efficiency, convenient fluorescent dyes, utilizing ethyl methyl sulfone-induced mutations to conduct large-scale screening of H2S-related locus, and exploring cross-talk between H2S and other signaling molecules, etc.
Author contribution statement
Hongming Guo, Tianyu Xiao, and Yanjie Xie collated all the input, drafted the entire manuscript, and contributed to critical reading of this article. Heng Zhou and Wenbiao Shen prepared the table and contributed to the English language corrections. All the authors read and approved the manuscript in its final form.
Abbreviations
- ABA:
-
Abscisic acid
- Al:
-
Aluminum
- AOA:
-
Aminooxyacetic acid
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- AtNFS1/AtNifS:
-
Arabidopsis thaliana nitrogen fixation S
- AtNFS2/AtSUF:
-
Arabidopsis chloroplastic nitrogen fixation S
- CaM:
-
Calmodulin
- cAMP:
-
Cyclic adenosine monophosphate
- CAT:
-
Cysteine aminotransferase
- CBS:
-
Cystathionine-β-synthase
- CBSX:
-
Cystathionine-β-synthase domain-containing protein
- CDes:
-
Cysteine desulfhydrases
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide
- CSE:
-
Cystathionine-γ-lyase
- DAF-FM-DA:
-
4,5-Diaminoflorescein diacetate
- DHAR:
-
Dehydroascorbate reductase
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylene diamine tetraacetic acid
- FTS:
-
Ferredoxin-Trx system
- G6PDH:
-
Glucose-6-phosphate dehydrogenase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GSH:
-
Glutathione
- GSNO:
-
S-Nitrosoglutathione
- GR:
-
Glutathione reductase
- GYY4137:
-
Morpholin-4-ium 4 phosphinodithioate
- H2S:
-
Hydrogen sulfide
- H2O2 :
-
Hydrogen peroxide
- HT:
-
Hypotaurine
- IAA:
-
Indole acetic acid
- l-DES:
-
l-Cystine desulfydrase
- MDA:
-
Malondialdehyde
- 3-MST:
-
3-Mercapto pyruvate sulfurtransferase
- MDHAR:
-
Monodehydroascorbate reducatase
- NaHS:
-
Sodium hydrosulfide
- NR:
-
Nitrate reductase
- nia1/2:
-
Nitrate reductase 1/2
- NO:
-
Nitric oxide
- NTS:
-
NADP-Trx system
- OASTL:
-
O-Acetylserine(thiol)lyase
- O2 − :
-
Superoxide anion
- POD:
-
Peroxidase
- PME:
-
Pectin micronutrient
- ProDH:
-
Proline dehydrogenase
- P5CS:
-
1-Pyrroline-5-carboxylate synthetase
- ROS:
-
Reactive oxygen species
- SAVs:
-
Senescence-associated vacuoles
- SAT:
-
Serine acetyltransferase
- SKOR:
-
Shaker-like K+ outward-rectifying K channels
- SNAP:
-
S-Nitroso-N-acetylpenicillamine
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
References
Aida K, Tokuyama T, Uemura T (1969) The role of cysteine desulphhydrase and cysteine synthase in the evolution of hydrogen sulphide in pantothenic acid deficient yeast. Antonie Van Leeuwenhoek 35(Suppl 1):15–16
Álvarez C, Bermúdez MÁ, Romero LC, Gotor C, García I (2012a) Cysteine homeostasis plays an essential role in plant immunity. New Phytol 193:165–177
Álvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with l-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669
Álvarez C, García I, Moreno I, Pérez-Pérez ME, Crespo JL, Romero LC, Gotor C (2012b) Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 24:4621–4634
Aroca A, Serna A, Gotor C, Romero LC (2015) S-Sulfhydration: a cystein posttranslational modification in plant systems. Plant Physiol 168:334–342
Astier J, Rasul S, Koen E, Manzoor H, Besson-Bard A, Lamotte O, Jeandroz S, Durner J, Lindermayr C, Wendehenne D (2011) S-Nitrosylation: an emerging post-translational protein modification in plants. Plant Sci 181:527–533
Barroso C, Vega JM, Gotor C (1995) A new member of the cytosolic O-acetylserine(thiol)lyase gene family in Arabidopsis thaliana. FEBS Lett 365:1–5
Baskar R, Bian J (2011) Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol 656:5–9
Beja-Tal S, Borochov A (1994) Age-related changes in biochemical and physical properties of carnation petal plasma membranes. J Plant Physiol 143:195–199
Bonner ER, Cahoon RE, Knapke SM, Jez JM (2005) Molecular basis of cysteine biosynthesis in plants: structural and functional analysis of O-acetylserine sulfhydrylase from Arabidopsis thaliana. J Biol Chem 280:38803–38813
Borochov A, Cho MH, Boss WF (1994) Plasma membrane lipid metabolism of petunia petals during senescence. Physiol Plant 90:279–284
Borochov A, Woodson WR (1989) Physiology and biochemistry of flower petal senescence. Hortic Rev 11:15–43
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bright J, Desikan R, Hancock JT, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Burandt P, Schmidt A, Papenbrock J (2002) Three O-acetyl-l-serine(thiol)lyase isoenzymes from Arabidopsis catalyse cysteine synthesis and cysteine desulfuration at different pH values. J Plant Physiol 159:111–119
Calderwood A, Kopriva S (2014) Hydrogen sulfide in plants: from dissipation of excess sulfur to signaling molecule. Nitric Oxide 41:72–78
Chaerle L, Saibo N, Van Der Straeten D (2005) Tuning the pores: towards engineering plants for improved water use efficiency. Trends Biotechnol 23:308–315
Chen J, Wang WH, Wu FH, You CY, Liu TW, Dong XJ, He JX, Zheng HL (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Cooper CE, Brown GC (2008) The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40:533–539
Dawood M, Cao F, Jiahanqir MM, Zhang G, Wu F (2012) Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater 209–210:121–128
Dorman DC, Moulin FJ-M, McManus BE, Mahle KC, Jmmes RA, Struve MF (2001) Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65:18–25
Fang HH, Pei YX, Tian BH, Zhang LP, Qiao ZJ, Liu ZQ (2014) Ca2+ participates in H2S induced Cr6+ tolerance in Setaria italica. Chin J Cell Biol 36:758–765
Ferreira-Silva SL, Voigt EL, Silva EN, Maia JM, Aragão TC, Silveira JAG (2012) Partial oxidative protection by enzymatic and non-enzymatic components in cashew leaves under high salinity. Biol Plant 56:172–176
García-Mata C, Lamattina L (2010) Hydrogen sulfide, a novel gasotransmitter involved in guard cell signaling. New Phytol 188:977–984
García-Mata C, Lamattina L (2013) Gssotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201(202):66–73
Gupta KJ (2011) Protein S-nitrosylation in plants: photorespiratory metabolism and NO signaling. Sci Signal 4(154):jc1. doi:10.1126/scisignal.2001404
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Hanumappa M, Nguyen HT (2010) Genetic approaches toward improving heat tolerance in plants. In: Jenks MA, Wood AJ (eds) Genes for plant abiotic stress. Blackwell Publishing, Oxford, UK, pp 221–260
Harrington HM, Smith IK (1980) Cysteine metabolism in cultured tobacco cells. Plant Physiol 65:151–155
Hell R, Bork C, Bogdanova N, Frolov I, Hauschild R (1994) Isolation and characterization of two cDNAs encoding for compartment specific isoforms of O-acetylserine (thiol) lyase from Arabidopsis thaliana. FEBS Lett 351:257–262
Helmy N, Prip-Buus C, Vons C, Lenoir V, Abou-Hamdan A, Guedouari-Bounihi H, Lombès A, Bouillaud F (2014) Oxidation of hydrogen sulfide by human liver mitochondria. Nitric Oxide 41:105–112
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166
Hess DT, Stamler JS (2012) Regulation by S-nitrosylation of protein posttranslational modification. J Biol Chem 287:4411–4418
Hesse H, Lipke J, Altmann T, Höfgen R (1999) Molecular cloning and expression analyses of mitochondrial and plastidic isoforms of cysteine synthase (O-acetylserine(thiol)lyase) from Arabidopsis thaliana. Amino Acids 16:113–131
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Hou Z, Liu J, Hou L, Li X, Liu X (2011) H2S may function downstream of H2O2 in jasmonic acid-induced stomatal closure in Vicia faba. Chin Bull Bot 46:396–406
Hu JL, Huang XH, Chen LC, Sun XW, Lu CM, Zhang LX, Wang YC, Zuo JR (2015) Site-specific nitrosoproteomic identification of endogenously S-nitrosylated proteins in Arabidopsis. Plant Physiol 167:1734–1746
Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH (2001) Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3:193–197
Jin ZP, Shen JJ, Qiao ZJ, Yang GD, Wang R, Pei YX (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414:481–486
Jin ZP, Xue SW, Luo YN, Tian BH, Fang HH, Li H, Pei YX (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Jost R, Berkowitz O, Wirtz M, Hopkins L, Hawkesford MJ, Hell R (2000) Genomic and functional characterization of the oas gene family encoding O-acetylserine (thiol) lyases, enzymes catalyzing the final step in cysteine biosynthesis in Arabidopsis thaliana. Gene 253:237–247
Kabil O, Motl N, Banerjee R (2014) H2S and its role in redox signaling. Biochim Biophys Acta 1844:1355–1366
Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, Ichinose F (2011) Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid Redox Signal 15:343–352
Kimura H (2000) Hydrogen sulfide induces cyclic AMP and modulates the NMDA recrptor. Biochem Biophys Res Commun 267:129–133
Kimura H (2014) The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41:4–10
Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167
Knight H (2000) Calcium signaling during abiotic stress in plants. Int Rev Cytol 195:269–324
Kolluru GK, Shen X, Bir SC, Kevil CG (2013) Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35:5–20
Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A (2007) Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology 241:92–97
Kushnir S, Babiychuk E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G, Lill R, Van Montagu M (2001) A Mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13:89–100
Lai DW, Mao Y, Zhou H, Li F, Wu M, Zhang J, He Z, Cui W, Xie Y (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:628–695
Leon S, Tournaine B, Briat JF, Lobreaux S (2002) The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulphurase. Biochem J 366:557–564
Li ZG, Ding XJ, Du PF (2013a) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li ZG, Gong M, Xie H, Yang L, Li J (2011) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185–186:185–189
Li J, Jia H, Wang J, Cao Q, Wen Z (2014) Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251:899–912
Li L, Wang YQ, Shen WB (2012) Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 25:617–631
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK (2008) Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117:2351–2360
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013b) Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Lin YT, Li MY, Cui WT, Lu W, Shen WB (2012) Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul 31:519–528
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulphide: environmental factor or signaling molecule? Plant Cell Environ 36:1607–1616
Lisjak M, Teklic T, Wilson ID, Wood ME, Whiteman M, Hancock JT (2011) Hydrogen sulfide effects on stomatal apertures. Plant Signal Behav 6:1444–1446
Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P (2009) Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta 1787:864–872
Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang GD, Wang R, Snyder SH (2009) H2S Signals through protein S-sulfhydration. Sci Signal 2:72
Nagasawa T, Ishii T, Kumagai H, Yamada H (1985) d-Cysteine desulfhydrase of Escherichia coli. Purification and characterization. Eur J Biochem 153:541–551
Nagasawa T, Ishii T, Yamada H (1988) Physiological comparison of d-cysteine desulfhydrase of Escherichia coli with 3-chloro-d-alanine dehydrochlorinase of Pseudomonas putida CR 1–1. Arch Microbiol 149:413–416
Olas B (2015) Hydrogen sulfide in signaling pathways. Clin Chim Acta 439:212–218
Paliyath G, Droillard MJ (1992) The mechanisms of membrane deterioration and disassembly during senescence. Plant Physiol Biochem 30:789–812
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants: from the field to the test tube and back. Plant Biol 9:582–588
Pilon-Smits EA, Garifullina GF, Abdel-Ghany S, Kato S, Mihara H, Hale KL, Burkhead JL, Esaki N, Kurihara T, Pilon M (2002) Characterization of a NifS-like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiol 130:1309–1318
Polhemus DJ, Lefer DJ (2014) Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114:730–737
Rennenberg H (1983) Cysteine desulfhydrase activity in cucurbit plants: simulation by preincubation with l- and d-cysteine. Phytochemistry 22:1557–1560
Rennenberg H, Arabatzis N, Grundel I (1987) Cysteine desulphydrase activity in higher plants: evidence for the action of l- and d-cysteine specific enzymes. Phytochemistry 26:1583–1589
Rennenberg H, Filner P (1983) Developmental changes in the potential for H2S emission in cucurbit plants. Plant Physiol 71:269–275
Riemenschneider A, Weqele R, Schmidt A, Papenbrock J (2005) Isolation and characterization of a d-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J 272:1291–1304
Romero LC, Aroca MA, Serna A, Gotor C (2013a) Proteomic analysis of endogenous S-sulfhydration in Arabidopsis thaliana. Nitric Oxide 31:S23
Romero LC, Garcia I, Gotor C (2013b) l-Cysteine desulfhydrase1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal Behav 8:e24007
Rubinstein B (2000) Regulation of cell death in flower petals. Plant Mol Biol 44:303–318
Schmidt A (1982) A cysteine desulfhydrase from spinach leaves specific for d-cysteine. Zeitschrift fr Pflanzenphysiologie 107:301–312
Schmidt A, Erdle I (1983) A cysteine desulfhydrase specific for d-cysteine from the green alga Chlorella fusca. Zeitschrift für Naturforschung C 38:428–435
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Scuffi D, Núñez A, Laspina N, Gotor C, Lamattina L, Garcia-Mata C (2014) Hydrogen sulfide generated by l-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166:2065–2076
Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kin S, Snyder SH (2012) Hydrogen sulfide-linked Sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45(1):13–24
Shan C, Liu H, Zhao L, Wang X (2014) Effects of exogenous hydrogen sulfide on the redox states of ascorbate and glutathione in maize leaves under salt stress. Biol Plant 58:169–173
Shi Q, Ding F, Wang X, Wei M (2007) Exogenous nitric oxide protects cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem 45:542–550
Shi HT, Ye TT, Chan ZL (2014) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 74:99–107
Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11:703–714
Singh VP, Singh S, Kumar J, Prasad SM (2015) Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: possible involvement of nitric oxide. J Plant Physiol 181:20–29
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation, the prototypic redox-based signaling mechanism. Cell 106:675–683
Sun J, Wang R, Zhang X, Yu Y, Zhao R, Li Z, Chen S (2013) Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol Biochem 65:67–74
Wahid A, Gelani S, Ashrf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang R (2002) Two’s compay, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Wang Y, Li L, Cui W, Xu S, Shen W, Wang R (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351:107–119
Wang BL, Shi L, Li YX, Zhang WH (2010) Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 231:1301–1309
Wang Y, Yun BW, Kwon E, Hong JK, Yoon J, Loake GJ (2006) S-Nitrosylation: an emerging redox-based post-translational modification in plants. J Exp Bot 57:1777–1784
Wirtz M, Droux M, Hell R (2004) O-Acetylserine(thiol)lyase: an enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J Exp Bot 55:1785–1798
Xie YJ, Lai DW, Mao Y, Zhang W, Shen WB, Guan RZ (2013a) Molecular cloning, characterization, and expression analysis of a novel gene encoding l-cysteine desulfhydrase from Brassica napus. Mol Biotechnol 54:737–746
Xie YJ, Zhang C, Lai DW, Sun Y, Samma MK, Zhang J, Shen WB (2013b) Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 171:53–62
Xu S, Liu Z, Liu P (2014) Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis. Int J Cardiol 172:313–317
Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18:1906–1919
Yoo KS, Ok SH, Jeong BC, Jung KW, Cui MH, Hyoung S, Lee MR, Song HK, Shin JS (2011) Single cystathionine β-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell 23:3577–3594
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, Wei ZJ, Liu J, Wang HL, Jiang ST (2011) Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol 60:251–257
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51:1086–1094
Zhang H, Wang MJ, Hu LY, Wang SH, Hu KD, Bao LJ, Luo JP (2010) Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ J Plant Physiol 57:532–539
Zhao WM, Zhang J, Lu YJ, Wang R (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20:6008–6016
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31200195), the Fundamental Research Funds for the Central Universities (KYZ201529), Natural Science Foundation of Jiangsu Province (BK2012364), Specialized Research Fund for the Doctoral Program of Higher Education (20120097120019), Youth Sci-Tech Innovation Fund, Nanjing Agricultural University (KJ2012022), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Wojtaszek.
Rights and permissions
About this article
Cite this article
Guo, H., Xiao, T., Zhou, H. et al. Hydrogen sulfide: a versatile regulator of environmental stress in plants. Acta Physiol Plant 38, 16 (2016). https://doi.org/10.1007/s11738-015-2038-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-2038-x