Abstract
Benign enlargement of subarachnoid spaces (BESS) in the past had several synonyms like benign external hydrocephalus (BEH), extraventricular hydrocephalus, benign subdural effusion, benign extracellular fluid collection, extraventricular obstructive hydrocephalus, subdural hygroma, pseudo-hydrocephalus, benign extra-axial collections, subarachnomegaly, and subdural effusions of infancy. It is critical to differentiate between this entity and other CSF collections like arachnoid cysts which may be a mimic and warrant completely different line of management.
Subdural collections, in fact, are always looked at with suspicion from a perspective of pediatric non-accidental brain injury and therefore need to be clearly distinguished and some of them may need treatment while others may have complications in the long term. This review attempts to address the above issues and simplify their understanding.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Macrocephaly
- Benign enlargement of subarachnoid spaces
- Subdural effusion
- External hydrocephalus
- Arachnoid cyst
1 Introduction
Benign extracerebral collections are commonly found in infants, especially with the availability and growing use of various imaging modalities. Most of these collections, presenting as dilated subarachnoid spaces on imaging, are the most common cause of macrocephaly [1,2,3] in infancy. Though this disorder has been named as benign enlargement of subarachnoid space (BESS) in recent literature, there is great confusion surrounding the nature of this entity demonstrated by the various names used for its description like benign external hydrocephalus (BEH), extraventricular hydrocephalus, benign subdural effusion, benign extracellular fluid collection, extraventricular obstructive hydrocephalus, subdural hygroma, pseudo-hydrocephalus, benign extra-axial collections, subarachnomegaly, and subdural effusions of infancy [4, 5].
The variety in nomenclature reflects the differing views on their being considered one entity and difficult neuroimaging differentiation [3]. The anatomical substrate, whether subdural fluid or cerebrospinal fluid (CSF) in the subarachnoid space, has been a subject of disagreement amongst researchers [6, 7]. This review will try to address various issues about BESS viz. clinical manifestation, incidence and progression of macrocephaly, long-term prognosis, need for shunting, association with subdural collections, relation to non-accidental injury(NAI) and finally will discuss whether all cases are truly benign. The terms BESS and BEH will be used interchangeably throughout this review.
2 Definition
In 1918, external hydrocephalus was first defined by Dandy as a condition with increased intracranial pressure and dilated subarachnoid spaces in infancy. He had also subclassified hydrocephalus in several ways including division into internal and external hydrocephalus [8, 9].
In current literature, external hydrocephalus is commonly defined as a large or rapidly growing head circumference in infants combined with enlarged subarachnoid spaces and moderate to no ventricular enlargement on neuroimaging [10,11,12]. Kumar recommended additional criteria of the absence of “clinicoradiological features of raised intracranial pressure,” e.g., ventriculomegaly without periventricular lucency, and non-tense fontanels [4].
Macrocephaly, an essential component of BESS, is defined as head circumference more than two standard deviations above the population mean or above the 98th centile [13].
3 Epidemiology
A Norwegian retrospective population based study found an incidence of BESS to be 0.4 per 1000 live births [14]. In this study (86.4%) of the patients were male, and mean age at referral was 7.3 months.
A tertiary pediatric neurology center in Pennsylvania, USA, in a review of incidental findings, found that 0.6% of the children had external hydrocephalus [15]. Two thirds of these infants were found to be boys in another study [16]. BEH was associated with prematurity in many studies [10, 17].
Several studies have reported a familial predisposition in BEH [6, 18] with a 40% chance of a family member being macrocephalic while two reports have found this coherence as high as 80–90% [10, 19].
An autosomal dominant mode of transmission for the non-syndromic macrocephaly cases has been assumed by many [20,21,22], although a multifactorial model of inheritance with a polygenic genetic base was proposed by a 1996 study [23]. This group’s findings challenged the assumption of autosomal dominant inheritance for BESS since risk of recurrence appears to be much lower than it should have been if the assumption was true. There are many genetic conditions, on the other side of the spectrum, which are associated with dilatation of subarachnoid spaces like Mucopolysaccharidoses types I, II and III, Achondroplasia, Soto’s syndrome, Glutaric aciduria type I [24].
4 Etiology
Most cases are without a known cause and hence termed “idiopathic.” However, numerous conditions such as prematurity and intraventricular hemorrhage [16, 25, 26], meningitis [25, 27], metabolic disorder [28], steroid therapy [29], chemotherapy [30], neurosurgery [31], and trauma [25, 27] may be responsible for its causation. Many premature infants can have intraventricular hemorrhage and subarachnoid hemorrhage which can go undetected due to lack of symptoms, thereby rendering the idiopathic nature of BESS uncertain in those cases [32, 33].
5 Pathophysiology
5.1 Physiological Development of the Subarachnoid Spaces
The major seat of cerebrospinal fluid (CSF) production are the four choroid plexuses in the ventricles. After production, the CSF exits into the basal cisterns, entering the subarachnoid space over the surface of the cortex [34]. The secretory epithelium of the choroid plexus is formed by 6 weeks of gestation [35]. There is uncertainty around the time CSF production begins, but circulation is established from the ventricles to the subarachnoid space by 2 months of age [36]. The separation of the arachnoid membrane from the primitive dura mater leads to the formation of the subarachnoid space. This process then spreads from the ventral portion of the meso-rhombencephalon to the spinal cord caudally and to the prosencephalon cranially [37]. CSF is absorbed from the subarachnoid space into the cerebral venous system through herniations of the arachnoid membrane into the dural venous sinuses [38, 39]. Microscopic arachnoid villi are first formed by the microtubular invaginations of the subarachnoid space into the lumen of the dural venous sinuses in utero [34]. This embryological step probably correlates with the decrease in size of the arachnoid space after 32 weeks of gestation and demonstrable on fetal magnetic resonance imaging studies [40]. Arachnoid villi can be found in the fetus and newborn, and the granulations develop between 6 and 18 months of age [41, 42].
Infants can have varying functional maturity of the arachnoid villi which can result in absorption not keeping pace with CSF production for a period of time. CSF accumulates as a result, preferentially in the subarachnoid space until skull sutures are unfused. The ventricles remain undilated till the bulk of CSF absorption occurs through the subarachnoid channels that cover the cerebral hemispheres [43]. After 2 years of age, the capacity for CSF absorption exceeds the normal rate of production by two to four times [36]. Further growth of the arachnoid villi and granulations leads to the formation of macroscopic Pacchionian bodies which are visible by 3 years of age [34]. This process of evolution of a CSF absorption system can be variable in its timeline thereby leading to the variability in the size of the subarachnoid spaces in normal children [44] and providing a physiologic basis for benign enlargement of the subarachnoid spaces.
5.2 Pathogenesis
There are various theories that have been proposed to explain the pathogenesis of BESS.
-
1.
Delayed maturation of arachnoid villi: This is the most common theory which suggests that the defective CSF absorption due to immature arachnoid villi leads to CSF accumulation causing dilatation of the subarachnoid spaces and ventricles [43]. As the brain and skull are compliant, it does not lead to an increase in Intracranial pressure [25]. The maturation of villi around 18 months of age ends the CSF accumulation and consequent subarachnoid space dilatation.
-
2.
Arachnoid tear: This leads to a one way valve mechanism causing CSF accumulation [45]. This mechanism generally leads to subdural fluid collection but can also cause localized subarachnoid space dilatation.
-
3.
Loculation of CSF causing accumulation in the localized subarachnoid space [46].
-
4.
Subdural fluid impairing CSF absorption [47].
-
5.
Communicating hydrocephalus theory: Some believe external hydrocephalus is a step towards developing internal hydrocephalus [12]. If immature arachnoid villi are the cause, there may be restoration of CSF absorption around 18 months of age leading to resolution of BEH. However, children whose arachnoid villi are absent/grossly underdeveloped end up needing a shunt [48].
-
6.
Cranio-encephalic disproportion: When the skull grows faster than the brain, it leads to transient subarachnoid space enlargement [10, 49].
-
7.
Dural venous sinus patency and positional plagiocephaly: Cinalli et al. proposed that decreased patency of the venous sinuses and consequent increased venous outflow resistance contributes to the development of BEH in the first 3 years of life. The same authors found that positional plagiocephaly, found to be associated with BEH, contributed to the decreased patency of the homolateral dural sinus [50].
6 Clinical Manifestations
6.1 Macrocephaly
Hellbusch found 28 (71.8%) out 39 patients with BESS to have macrocephaly which meant that 28.2% of the cases had a normal head circumference [16]. The usual presentation of BESS is an otherwise normal infant presenting with increasing head circumference typically around 6 months of age which stabilizes around 18 months of age [51,52,53]. There might be marked frontal bossing as a result of the typical frontal subarachnoid space enlargement [54]. Head circumference measured after this period generally stays above but parallel to the 95th–98th centile [17, 18, 43]. On long-term follow-up, 11–87% of children with BESS end up with macrocephaly [6, 55, 56].
Most studies report no signs and symptoms of increased intracranial pressure (irritability, lethargy, vomiting, tense and bulging anterior fontanel) though they can be seen occasionally [1, 2, 4]. Rarely studies reported a tense anterior fontanel [18, 57], dilated scalp veins [58], hypotonia [59], ataxia [4], and seizures [6]. However, the sunset sign is not reported in any study [3]. The children generally achieve normal developmental milestones though mild motor delay has been reported attributable to the large head [4, 10].
7 Neuroimaging Findings
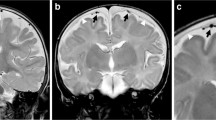
The classic Neuroimaging picture of BESS is that of enlarged frontal subarachnoid spaces beyond the upper limit with normal to moderately enlarged ventricles [3]. Concurrent findings include wide interhemispheric fissure, enlarged basal cisterns and third ventricle, no flattening of underlying gyri, CSF following gyral pattern and normal sulci posteriorly [4, 60, 61] (Fig. 1.1a, b).
A 1-month-old boy with incidental classical findings of enlarged subarachnoid spaces on axial T2W images. (a) The CSF spaces are seen following the gyral contour with no flattening of adjacent gyri. There is an increase in bifrontal subarachnoid spaces (green cross), while the lateral ventricles appear normal (yellow arrow). a distinctive feature of BESS, “cortical vein sign” is noted, comprising of elongated flow voids of bridging veins (red arrow). (b) In lower cuts, an enlargement of basal cisterns and fourth ventricle is noted (orange arrow) with conspicuous temporal subarachnoid spaces (green cross)
Some degree of ventricular enlargement is reported in most studies but there are no studies with exact measurements [62, 63]. There is a possible correlation between ventricular size and width of interhemispheric fissure [64]. Maytal et al. [65] found that the sequence of enlargement in BESS was the interhemispheric fissure followed by the frontoparietal convexity subarachnoid space with basal cistern and ventricular enlargement being a late radiological finding (Figs. 1.2a, b and 1.3).
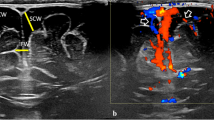
A 5-month-old male with incidental findings of BESS. (a) A small variation in imaging showing some prominence of lateral ventricles (yellow arrow) and enlarged subarachnoid spaces in bilateral fronto-temporal regions (green cross), as seen on axial T2W image. The flow voids of bridging cortical veins are prominent, ratifying the “cortical vein sign” (red arrow). there is a classical increase in the interhemispheric distance (yellow line) and cortico cranial width (blue line). (b) A coronal T2W view showing an increased sino-cortical width (SCW) (green line)
7.1 Normal Range [66,67,68,69]
The measurements used to quantify BESS are craniocortical width (CCW), interhemispheric fissure width (IFW) and sino-cortical width (SCW). Sinocortical width is defined as the distance from the lateral wall of the superior sagittal sinus to the cerebral cortex (Fig. 1.2a, b). There is no consensus on the cut-off values for any radiological measurement [5, 70]. The range of upper limits for the CCW is 4–10 mm (infants <1 year of age) and 3.3–5 mm (neonates). Upper limit ranges for IHW and SCW are 6–8.5 mm and 2–10 mm respectively. Tucker et al. suggested a grading system for BESS based on the depth of subarachnoid space as Grade 0 (<5 mm), Grade 1 (5–9 mm), Grade 2 (5–9 mm) and found association of incidental subdural collections with higher grades [71].
7.2 Imaging Modalities
-
1.
Cranial ultrasound (US)—Often the first procedure as it is easy to perform on an open fontanel. However limited posterior fossa visualization makes it difficult to rule out important causes of obstructive hydrocephalus in a macrocephalic child [72].
-
2.
Computerized tomography (CT)—Good visualization of neuroanatomy but risk of ionizing radiation leading to 0.07% increased lifetime risk of cancer mortality per scan [73, 74].
-
3.
Magnetic resonance imaging (MRI): Maximizes visualization and minimizes risk of radiation. Imaging modality of choice.
-
4.
CSF flow studies: Either by injection of an isotope or a contrast medium intrathecally. Studies have reported slow to no flow especially over cerebral convexities [18, 75].
7.3 Long-Term Neuroimaging Outcomes
Most studies show that the frontal subarachnoid space enlargement disappears within 2–3 years of age [4, 55]. Longest follow-up study of 19 years by Muenchberger showed that all patients eventually had a normal MRI [63].
8 Differential Diagnosis
There are some conditions that have to be differentiated from BESS on clinical and radiological grounds.
-
1.
Cerebral atrophy: It does not present with increasing head circumference in contrast to BESS. Radiologically, the presence of global widening of cerebral sulci points towards atrophy as BESS typically presents with enlargement of frontal subarachnoid spaces and interhemispheric fissure [76] (Fig. 1.4a, b).
-
2.
Subdural fluid collections: These can be differentiated from BESS by the “cortical vein sign” on MRI or US [77, 78]. A positive sign suggests that the fluid collection is caused by an enlarged subarachnoid space and not a subdural collection which would compress the subarachnoid space and the veins traversing it. On intrathecal injection of dye, the immediate influx of a contrast medium from CSF into a fluid collection suggests external hydrocephalus, whereas no influx indicates a subdural effusion [79]. Ment et al. observed that the enlargement of the basal cisterns often were seen in external hydrocephalus but not in subdural hygromas [54].
-
3.
Convexity and Galassi I arachnoid cyst: These can sometimes masquerade as a loculated extra-axial collection like BESS or subdural effusion as it follows CSF on all sequences [80] (Fig. 1.5). This may be more widespread over convexity in rare instances of ruptured arachnoid cyst [81].
Eight-month-old child with global developmental delay and macrocephaly. Bilateral incomplete opercularization of slyvian fissures and prominent CSF spaces in bitemporal regions, with a left subdural collection due to volume loss and signal changes in bilateral basal ganglia and thalami consistent with glutaric aciduria type 1 noted on T2W axial (a) and T1W axial imaging (b). (a) Cerebral atrophy with resultant temporal subarachnoid space enlargement with widening of Sylvian fissures (green cross) along with bilateral symmetrical hyperdensities in Globus Pallidus (red cross) and subthalamic nucleus (red star). There is the presence of a left subdural collection (blue arrow) showing displacement and compression of the traversing cortical veins (black arrowhead), in stark contrast to the right widened temporal and Sylvian subarachnoid spaces with prominent cortical vein flow voids (orange arrowhead). (b) A T1W axial image showing a thin left subdural collection due to volume loss (blue arrow) and enlargement of temporal subarachnoid spaces with widening of Sylvian fissures
9 Natural History
A developmental delay is commonly seen at some stage in infancy. Short-term outcomes have been reported by studies which generally found transient developmental delay, primarily gross motor delay and to a lesser extent delay in language development which decreased and corrected by 1–4 years [4, 58, 82]. A study by Muenchberger et al. [63] followed 15 children with BESS, nine of them had detailed neuropsychological assessment up to school with a mean final follow-up of 19 years. Though the final neurological assessment was normal and neuropsychological assessment found normal intellectual ability, several patients showed reduced performance on two tests associated with attention, and two patients with speech delay at 2 years of age performed at below-average levels in most psychological tests at long-term follow-up. Specific learning problems in reading and mathematics or a diagnosis of a psychiatric disease were found in 10 out of 15 patients and eight children had to repeat grades or go to special classes.
In another study, Laubscher et al. [58] did a long-term follow-up on 22 megalencephalic children with “dilated pericerebral subarachnoid spaces.” Twelve of them were developmentally delayed). Eleven of these 12 children who had reached school age at the time that the study ended had a normal school outcome. When compared with 22 children without BESS, looking at psychomotor development and school outcome, there was no significant difference between the two groups.
10 Treatment
There is no Class I evidence in literature comparing treatment (medical/surgical) versus no treatment, i.e., observation. As BESS is a condition which is by definition benign, it implicitly means that it does not require any treatment and resolves with time. Therefore, observation is the only form of treatment that is required for most cases. The reported modes of treatment, when required, are surgical and medical and the indications, though varied, are generally signs and symptoms of raised intracranial pressure like bulging fontanel, irritability, vomiting accompanied by a growing head circumference. The various forms of surgical treatment reported are direct shunting or burr hole drainage/prolonged external drainage followed by shunting if necessary.
Robertson and Gomez treated two out of six patients with shunts (one lumboperitoneal and one ventriculoperitoneal) because of excessive head growth, ventricular dilatation, and other signs of increased intracranial pressure [12]. One of them was followed for 7 years and developed normally. Ten out of the 14 patients reported by Tsubokawa et al. had macrocephaly and bulging fontanels [83]. All ten underwent surgery with temporary shunt insertion. At 4–6 months after surgery, neuroimaging normalization was seen, although the ventricle enlargement seemed to retract slower. Seven of the ten children operated had a developmental quotient (DQ) of more than 100 at follow-up, indicating normal development, while two of the four non-operated patients had a DQ of less than 39.
Temporary (48 h) bilateral burr hole drainage of the frontal subarachnoid spaces in a 6-month-old girl with external hydrocephalus and developmental delay was reported by Eidlitz-Markus et al. [84]. The head circumference and psychomotor development normalized within a few months and was sustained till the last follow-up at 2 years of age. However, only modest reduction in the size of the CSF spaces was noted. Similarly, Stroobandt et al. [85] suggested treatment with external drainage of pericerebral collections for a week followed by a shunt if the effusion did not resolve.
11 Benign Dilatation of Subdural Spaces
Benign enlargement of subdural spaces (BESDS) has been described by many authors using various terminologies like benign subdural effusion [86], benign subdural collection [47], subdural hygroma [87], etc. This entity has been used without clear differentiation to describe clinico-radiological features identical to BESS in many reviews thus adding to the confusion regarding its existence as a separate entity [47, 86].
Many of these studies were done primarily using CT and clinical findings with or without subdural taps to diagnose subdural collections. However, with the advances in imaging, the radiological differentiation between BESS and BESDS is more distinct. There are certain differentiating radiological criteria favoring BESS over BESDS which include (1) bi-hemispheric extracerebral fluid collections: anterior > posterior, (2) widening of the anterior interhemispheric fissure, (3) enlarged subarachnoid spaces, (4) no evidence of cortical atrophy, (5) enlarged or prominent basal cisterns, (6) mild to moderate ventriculomegaly without periventricular lucency, and (7) absence of restriction of blood flow in the cortex adjacent to fluid collection on diffusion-weighted MRI (DW MRI) [87].
There are various factors contributing to its causation like non-accidental injury (NAI), minor/major traumatic injury, meningitis, encephalitis, tumor, following a VP shunt, and without any specific cause (idiopathic) [88].
Interestingly, many studies have reported that BESS can be complicated by subdural hemorrhage (SDH) either spontaneously or following accidental injury [89,90,91,92]. The proposed theory is the stretching of bridging veins in the dilated subarachnoid space [92]. There is a mathematical model of the cranial vault suggested by Papasian and Frim [89] which explains the relationship between bridging vein stretching and width of the extra-axial spaces thereby supporting the above theory. The scenario of BESS predisposing to SDH also needs to be very clearly differentiated from SDH secondary to NAI due to obvious medicolegal implications. Clinically, infants with NAI have a very morbid neurological course and risks of mortality whereas those with SDH in pre-existing BESS have a benign course [92,93,94,95]. Radiologically, absence of associated intraparenchymal contusions and presence of features of BESS help differentiating from NAI. Caution should therefore be exercised while dealing with an infant with SDH in a scenario of BESS and presumptive diagnosis of NAI should be avoided when other evidence of NAI like long bone fractures, retinal hemorrhages, etc., are absent.
In their study of 20 patients with subdural effusions following minor head injury, Kumar et al. [87] reported that 55% had macrocephaly, 25% had tense AF, 83% presented with seizures, 30% with overt neurological findings like papilloedema, cranial nerve palsies, etc., and 70% with subtle neurological findings with irritability being the most common symptom. The infants with subtle features could mimic the features of BESS.
The various treatment options are observation, subdural needle aspirations, burr hole evacuation, subduro-peritoneal shunt (unilateral/bilateral), and craniotomy for drainage and excision of neomembranes [87]. It has been suggested that collections with thickness less than 7 mm on CT scan may have a better chance of resolving spontaneously, and hence non-operative approach may be sufficient [96]. Needle aspirations and burr hole drainage may need multiple procedures and are prone to infection. Subduroperitoneal shunts have a very high reported success rate in eliminating subdural collections between 80% and 100% [97,98,99]. Unilateral shunt is usually effective in controlling bilateral collections and an unvalved shunt is used in most reports [98, 100, 101]. Subdural shunts, though, have a reported obstruction rate of up to 14% and an infection rate of 5% [98, 99, 102]. Craniotomy is needed only in complex cases.
12 Is Benign Enlargement of Subarachnoid Spaces Really Benign?
The usual assumption about BESS is that of an infant presenting with macrocephaly and typical neuroimaging features who has some developmental delay transiently but finally catches up with its peers. This self-limiting nature of the disorder leads to it being perceived as “benign.” Many studies reporting a long-term normal outcome are based on clinical and neurological assessments.
However, studies using standardized neuropsychological tests have reported deficits in children with BEH on long-term follow-up. Alvarez et al. using the Denver Developmental Screening Test in 36 children found 14 children with delayed gross motor development, five with delayed language development, and one with global developmental delay at last follow-up at 30 months of age [10]. The same test used by Alper et al. revealed two out of 13 children with fine motor delay. The Peabody picture-vocabulary test used by the same authors showed expressive language delay in two out of seven children older than 2.5 years [6].
Zahl et al., in a retrospective population based study, reported that children and adolescents who were diagnosed with BEH during infancy generally do well. However, for some patients, there appear to be various developmental, social, and cognitive problems, and they seem to struggle more in school than their healthy peers [103]. In addition, various problems like mental retardation [58], epilepsy [104], social behavioral problems [103], autism spectrum disorders [105], and learning disabilities [58, 63] have been reported to be associated in children diagnosed with BEH on long-term follow-up.
In the light of the above evidence, it might be worth questioning the “benign” nature of this condition. However, this does not change the fact that probably most children presenting with this condition will show near resolution of imaging findings and halted progression of macrocephaly without any treatment and will achieve normal development in gross scores of assessment when done by neurologists and neurosurgeons. A more precise and specific outcome assessment by developmental pediatricians and neuropsychologists will be paramount to help establish subtle deficits and the actual impact of this disorder on the quality of life. The impact of timely intervention on long-term outcome is a subject which needs to be analyzed critically with prospective long-term studies. Prophylactic intervention in selected patients in this presumed self-limiting condition will also be a topic of interest and curiosity for future research.
13 Conclusion
Benign Enlargement of Subarachnoid spaces and Subdural collections are the most common forms of extracerebral collections found in infancy. Literature is abound with a variety of nomenclature describing these entities. Most cases of BESS present with typical neuroimaging findings and macrocephaly which is expected to settle down within the second year of life. The natural history of BESS favours grossly normal development though long term follow up and detailed neuropsychological tests have unveiled subtle or specific problems in various studies. Most cases do not require any treatment except few which may present with signs of raised intracranial pressure (ICP) warranting some form of surgical intervention. Subdural collections need to be differentiated from BESS. It is also essential to define the etiology of subdural collections as NAI is a significant cause and has profound medical and legal implications. Like BESS, subdural collections also warrant treatment when they present with raised ICP symptoms though many will be asymptomatic.
References
Haws ME, Linscott L, Thomas C, Orscheln E, Radhakrishnan R, Kline-Fath B. A retrospective analysis of the utility of head computed tomography and/or magnetic resonance imaging in the management of benign macrocrania. J Pediatr. 2017;182:283–289.e1 [cited 2022 Feb 22]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022347616312628.
Kuruvilla L. Benign enlargement of sub-arachnoid spaces in infancy. J Pediatr Neurosci. 2014;9(2):129 [cited 2022 Feb 22]. Available from: http://www.pediatricneurosciences.com/text.asp?2014/9/2/129/139309.
Zahl SM, Egge A, Helseth E, Wester K. Benign external hydrocephalus: a review, with emphasis on management. Neurosurg Rev. 2011;34(4):417–32 [cited 2022 Feb 22]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s10143-011-0327-4.
Kumar R. External hydrocephalus in small children. Childs Nerv Syst. 2006;22(10):1237–41 [cited 2022 Feb 22]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-006-0047-1.
Marino MA, Morabito R, Vinci S, Germanò A, Briguglio M, Alafaci C, et al. Benign external hydrocephalus in infants: a single centre experience and literature review. Neuroradiol J. 2014;27(2):245–50 [cited 2022 Feb 22]. Available from: http://journals.sagepub.com/doi/10.15274/NRJ-2014-10020.
Alper G, Ekinci G, Yilmaz Y, Ankan Ç, Telyar G, Erzen C. Magnetic resonance imaging characteristics of benign macrocephaly in children. J Child Neurol. 1999;14(10):678–82 [cited 2022 Feb 22]. Available from: http://journals.sagepub.com/doi/10.1177/088307389901401010.
Bodensteiner JB. Benign macrocephaly: a common cause of big heads in the first year. J Child Neurol. 2000;15(9):630–1 [cited 2022 Feb 22]. Available from: http://journals.sagepub.com/doi/10.1177/088307380001500913.
Dandy WE. An experimental, clinical and pathological study: part 1—experimental studies. Am J Dis Child. 1914;VIII(6):406 [cited 2022 Feb 22]. Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpedi.1914.02180010416002.
Dandy WE. Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg. 1918;68(6):569–79 [cited 2022 Feb 22]. Available from: http://journals.lww.com/00000658-191812000-00001.
Alvarez LA, Maytal J, Shinnar S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics. 1986;77(6):901–7.
Pettit RE. Macrocephaly with head growth parallel to normal growth pattern: neurological, developmental, and computerized tomography findings in full-term infants. Arch Neurol. 1980;37(8):518 [cited 2022 Feb 22]. Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneur.1980.00500570066011.
Robertson WC. External hydrocephalus: early finding in congenital communicating hydrocephalus. Arch Neurol. 1978;35(8):541 [cited 2022 Feb 22]. Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneur.1978.00500320061014.
DiLiberti JH. Inherited macrocephaly-hamartoma syndromes. Am J Med Genet. 1998;79(4):284–90 [cited 2022 Feb 23]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1096-8628(19981002)79:4<284::AID-AJMG10>3.0.CO;2-N.
Wiig US, Zahl SM, Egge A, Helseth E, Wester K. Epidemiology of benign external hydrocephalus in Norway—a population-based study. Pediatr Neurol. 2017;73:36–41 [cited 2022 Feb 23]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S088789941730070X.
Asch AJ, Myers GJ. Benign familial macrocephaly: report of a family and review of the literature. Pediatrics. 1976;57(4):535–9.
Hellbusch LC. Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J Neurosurg Pediatr. 2007;107(2):119–25 [cited 2022 Feb 23]. Available from: http://thejns.org/doi/abs/10.3171/PED-07/08/119.
Al-Saedi SA, Lemke RP, Debooy VD, Casiro O. Subarachnoid fluid collections: a cause of macrocrania in preterm infants. J Pediatr. 1996;128(2):234–6 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022347696703967.
Andersson H, Elfverson J, Svendsen P. External hydrocephalus in infants. Pediatr Neurosurg. 1984;11(6):398–402 [cited 2022 Feb 23]. Available from: https://www.karger.com/Article/FullText/120203.
Segal-Kuperschmit D, Cozacov C, Luder A. [Idiopathic external hydrocephalus]. Harefuah. 1995;128(3):150–2, 199.
Nogueira GJ, Zaglul HF. Hypodense extracerebral images on computed tomography in children: external hydrocephalus: a misnomer? Childs Nerv Syst. 1991;7(6):336–41 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00304833.
Palencia Luaces R, Aldana Gómez J, Tresierra Unzaga F. [Idiopathic external hydrocephaly and familial macrocephaly in infancy]. An Esp Pediatr. 1992;36(3):186–8.
Wilms G, Vanderschueren G, Demaerel PH, Smet MH, Van Calenbergh F, Plets C, et al. CT and MR in infants with pericerebral collections and macrocephaly: benign enlargement of the subarachnoid spaces versus subdural collections. AJNR Am J Neuroradiol. 1993;14(4):855–60.
Arbour L, Watters GV, Hall JG, Fraser FC. Multifactorial inheritance of non-syndromic macrocephaly. Clin Genet. 2008;50(2):57–62 [cited 2022 Feb 24]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1399-0004.1996.tb02349.x.
Paciorkowski AR, Greenstein RM. When is enlargement of the subarachnoid spaces not benign? A genetic perspective. Pediatr Neurol. 2007;37(1):1–7 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0887899407001518.
Kendall B, Holland I. Benign communicating hydrocephalus in children. Neuroradiology. 1981;21(2):93–6 [cited 2022 Feb 23]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00342987.
Lorber J, Bhat US. Posthaemorrhagic hydrocephalus: diagnosis, differential diagnosis, treatment, and long-term results. Arch Dis Child. 1974;49(10):751–62 [cited 2022 Feb 23]. Available from: https://adc.bmj.com/lookup/doi/10.1136/adc.49.10.751.
Rekate HL, Blitz AM. Hydrocephalus in children. In: Handbook of clinical neurology. Elsevier; 2016 [cited 2022 Feb 23]. p. 1261–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780444534866000648.
Bhasker B, Raghupathy P, Nair TM, Ahmed SR, deSilva V, Bhuyan BC, et al. External hydrocephalus in primary hypomagnesaemia: a new finding. Arch Dis Child. 1999;81:505–50.
Gordon N. Apparent cerebral atrophy in patients on treatment with steroids. Dev Med Child Neurol. 2008;22(4):502–6 [cited 2022 Feb 23]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1980.tb04355.x.
Enzmann DR, Lane B. Enlargement of subarachnoid spaces and lateral ventricles in pediatric patients undergoing chemotherapy. J Pediatr. 1978;92(4):535–9 [cited 2022 Feb 23]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022347678802832.
Hoppe-Hirsch E, Rose CS, Renier D, Hirsch J-F. Pericerebral collections after shunting. Childs Nerv Syst. 1987;3(2):97–102 [cited 2022 Feb 23]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00271133.
Burstein J, Papile L, Burstein R. Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. Am J Roentgenol. 1979;132(4):631–5 [cited 2022 Feb 23]. Available from: http://www.ajronline.org/doi/10.2214/ajr.132.4.631.
Govaert P, Oostra A, Matthys D, Vanhaesebrouck P, Leroy J. How idiopathic is idiopathic external hydrocephalus? Dev Med Child Neurol. 1991;33(3):274–6 [cited 2022 Feb 23]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1991.tb05121.x.
Davson H, Segal M. Introduction. In: Davson H, Segal MB, editors. Physiology of the CSF and blood-brain barriers. 1st ed. Boca Raton, FL: CRC Press; 1996. p. 3–7.
Volpe J. Neural tube formation and prosencephalic development. In: Volpe JJ, editor. Neurology of the newborn. 4th ed. Philadelphia: W.B. Saunders Co.; 2001. p. 3–44.
Sarnat H. Neuroembryology, genetic programming, and malformations of the nervous system. In: Menkes JH, Sarnat HB, Maria BL, editors. Child neurology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 330–47.
Girard NJ, Raybaud CA. Ventriculomegaly and pericerebral CSF collection in the fetus: early stage of benign external hydrocephalus? Childs Nerv Syst. 2001;17(4–5):239–45 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/PL00013727.
Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27(3):145–165 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s10143-004-0326-9.
Han CY, Backous DD. Basic principles of cerebrospinal fluid metabolism and intracranial pressure homeostasis. Otolaryngol Clin North Am. 2005;38(4):569–76 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S003066650500006X.
Watanabe Y, Abe S, Takagi K, Yamamoto T, Kato T. Evolution of subarachnoid space in normal fetuses using magnetic resonance imaging. Prenat Diagn. 2005;25(13):1217–22 [cited 2022 Feb 24]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/pd.1315.
Fishman R. Anatomical aspects of the cerebrospinal fluid. In: Fishman RA, editor. Cerebrospinal fluid in diseases of the nervous system. Philadelphia: W.B. Saunders Co; 1980. p. 6–18.
Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 1985;63(6):867–75 [cited 2022 Feb 24]. Available from: https://thejns.org/view/journals/j-neurosurg/63/6/article-p867.xml.
Barlow CF. CSF dynamics in hydrocephalus—with special attention to external hydrocephalus. Brain Dev. 1984;6(2):119–27 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0387760484800601.
Kleinman PK, Zito JL, Davidson RI, Raptopoulos V. The subarachnoid spaces in children: normal variations in size. Radiology. 1983;147(2):455–7 [cited 2022 Feb 24]. Available from: http://pubs.rsna.org/doi/10.1148/radiology.147.2.6601281.
Dandy WE. Treatment of an unusual subdural hydroma (external hydrocephalus). Arch Surg. 1946;52(4):421 [cited 2022 Feb 24]. Available from: http://archsurg.jamanetwork.com/article.aspx?doi=10.1001/archsurg.1946.01230050428003.
Snyder R, Snyder RD. Benign “subdural” collections. J Pediatr. 1979;95:499–500.
Robertson WC, Chun RWM, Orrison WW, Sackett JF. Benign subdural collections of infancy. J Pediatr. 1979;94(3):382–5 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022347679805752.
Gomez D, DiBenedetto A, Pavese A, Firpo A, Hershan D, Potts D. Development of arachnoid villi and granulations in man. Acta Anat Basel. 1982;111:247–58.
Girard N, Gire C, Sigaudy S, Porcu G, d’Ercole C, Figarella-Branger D, et al. MR imaging of acquired fetal brain disorders. Childs Nerv Syst. 2003;19(7–8):490–500 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-003-0761-x.
Cinalli G, di Martino G, Russo C, Mazio F, Nastro A, Mirone G, et al. Dural venous sinus anatomy in children with external hydrocephalus: analysis of a series of 97 patients. Childs Nerv Syst. 2021;37(10):3021–32 [cited 2022 Mar 7]. Available from: https://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-021-05322-5.
Carolan PL, McLaurin RL, Towbin RB, Towbin JA, Egelhoff JC. Benign extra-axial collections of infancy. Pediatr Neurosurg. 1985;12(3):140–4 [cited 2022 Feb 24]. Available from: https://www.karger.com/Article/FullText/120236.
Khosroshahi N, Nikkhah A. Benign enlargement of subarachnoid space in infancy: “a review with emphasis on diagnostic work-up”. Iran J Child Neurol. 2018;12(4):7–15.
Pascual-Castroviejo I, Pascual-Pascual SI, Velázquez-Fragua R. [A study and follow-up of ten cases of benign enlargement of the subarachnoid spaces]. Rev Neurol. 2004;39(8):701–6.
Ment LR, Duncan CC, Geehr R. Benign enlargement of the subarachnoid spaces in the infant. J Neurosurg. 1981;54(4):504–8 [cited 2022 Feb 24]. Available from: https://thejns.org/view/journals/j-neurosurg/54/4/article-p504.xml.
Castro-Gago M, Pérez-Gómez C, Novo-Rodríguez MI, Blanco-Barca O, Alonso-Martin A, Eirís-Puñal J. [Benign idiopathic external hydrocephalus (benign subdural collection) in 39 children: its natural history and relation to familial macrocephaly]. Rev Neurol. 2005;40(9):513–7.
Gherpelli JLD, Scaramuzzi V, Manreza MLG, Diament AJ. Follow-up study of macrocephalic children with enlargement of the subarachnoid space. Arq Neuropsiquiatr. 1992;50(2):156–62 [cited 2022 Feb 24]. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-282X1992000200004&lng=en&tlng=en.
Gooskens RH, Willemse J, Faber J, Verdonck AF. Macrocephalies—a differentiated approach. Neuropediatrics. 1989;20(3):164–9 [cited 2022 Feb 24]. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2008-1071284.
Laubscher B, Deonna T, Uske A, van Melle G. Primitive megalencephaly in children: natural history, medium term prognosis with special reference to external hydrocephalus. Eur J Pediatr. 1990;149(7):502–7 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF01959405.
Azais M, Echenne B. [Idiopathic pericerebral swelling (external hydrocephalus) of infants]. Ann Pediatr (Paris). 1992;39(9):550–8.
El-Feky M, Di Muzio B. Benign enlargement of the subarachnoid space in infancy. In: Radiopaedia.org. 2011 [cited 2022 Mar 6]. Available from: http://radiopaedia.org/articles/13118.
Kapila A, Trice J, Spies WG, Siegel BA, Gado MH. Enlarged cerebrospinal fluid spaces in infants with subdural hematomas. Radiology. 1982;142(3):669–72 [cited 2022 Feb 24]. Available from: http://pubs.rsna.org/doi/10.1148/radiology.142.3.6977789.
Medina LS, Frawley K, Zurakowski D, Buttros D, DeGrauw AJ, Crone KR. Children with macrocrania: clinical and imaging predictors of disorders requiring surgery. AJNR Am J Neuroradiol. 2001;22(3):564–70.
Muenchberger H, Assaad N, Joy P, Brunsdon R, Shores EA. Idiopathic macrocephaly in the infant: long-term neurological and neuropsychological outcome. Childs Nerv Syst. 2006;22(10):1242–8 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-006-0080-0.
Prassopoulos P, Cavouras D, Golfinopoulos S, Nezi M. The size of the intra- and extraventricular cerebrospinal fluid compartments in children with idiopathic benign widening of the frontal subarachnoid space. Neuroradiology. 1995;37(5):418–21 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00588027.
Maytal J, Alvarez L, Elkin C, Shinnar S. External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. Am J Roentgenol. 1987;148(6):1223–30[cited 2022 Feb 24]. Available from: http://www.ajronline.org/doi/10.2214/ajr.148.6.1223.
Fessell DP, Frankel DA, Wolfson WP. Sonography of extraaxial fluid in neurologically normal infants with head circumference greater than or equal to the 95th percentile for age. J Ultrasound Med. 2000;19(7):443–7 [cited 2022 Feb 24]. Available from: http://doi.wiley.com/10.7863/jum.2000.19.7.443.
Frankel DA, Fessell DP, Wolfson WP. High resolution sonographic determination of the normal dimensions of the intracranial extraaxial compartment in the newborn infant. J Ultrasound Med. 1998;17(7):411–5 [cited 2022 Feb 24]. Available from: http://doi.wiley.com/10.7863/jum.1998.17.7.411.
Fukuyama Y, Miyao M, Ishizu T, Maruyama H. Developmental changes in normal cranial measurements by computed tomography. Dev Med Child Neurol. 2008;21(4):425–32 [cited 2022 Feb 24]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1979.tb01645.x.
Libicher M, Tröger J. US measurement of the subarachnoid space in infants: normal values. Radiology. 1992;184(3):749–51 [cited 2022 Feb 24]. Available from: http://pubs.rsna.org/doi/10.1148/radiology.184.3.1509061.
Yew AY, Maher CO, Muraszko KM, Garton HJL. Long-term health status in benign external hydrocephalus. Pediatr Neurosurg. 2011;47(1):1–6 [cited 2022 Mar 6]. Available from: https://www.karger.com/Article/FullText/322357.
Tucker J, Choudhary AK, Piatt J. Macrocephaly in infancy: benign enlargement of the subarachnoid spaces and subdural collections. J Neurosurg Pediatr. 2016;18(1):16–20 [cited 2022 Feb 27]. Available from: https://thejns.org/view/journals/j-neurosurg-pediatr/18/1/article-p16.xml.
Enríquez G, Correa F, Lucaya J, Piqueras J, Aso C, Ortega A. Potential pitfalls in cranial sonography. Pediatr Radiol. 2003;33(2):110–7 [cited 2022 Feb 24]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00247-002-0836-y.
Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol. 2001;176(2):289–96 [cited 2022 Feb 24]. Available from: http://www.ajronline.org/doi/10.2214/ajr.176.2.1760289.
Martin DR, Semelka RC. Health effects of ionising radiation from diagnostic CT. Lancet. 2006;367(9524):1712–4 [cited 2022 Feb 24]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673606687485.
Briner S, Bodensteiner J. Benign subdural collections of infancy. Pediatrics. 1981;67(6):802–4 [cited 2022 Feb 24]. Available from: https://publications.aap.org/pediatrics/article/67/6/802/39072/Benign-Subdural-Collections-of-Infancy.
Lorber J, Priestley BL. Children with large heads: à practical approach to diagnosis in 557 children, with special reference to 109 children with megalencephaly. Dev Med Child Neurol. 2008;23(5):494–504 [cited 2022 Feb 27]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1981.tb02023.x.
Kuzma BB, Goodman JM. Differentiating external hydrocephalus from chronic subdural hematoma. Surg Neurol. 1998;50(1):86–8.
Chen CY, Chou TY, Zimmerman RA, Lee CC, Chen FH, Faro SH. Pericerebral fluid collection: differentiation of enlarged subarachnoid spaces from subdural collections with color Doppler US. Radiology. 1996;201(2):389–92 [cited 2022 Feb 27]. Available from: http://pubs.rsna.org/doi/10.1148/radiology.201.2.8888229.
Pietil TA, Palleske H, Distelmaier PM. Subdural effusions: determination of contrast medium influx from CSF to the fluid accumulation by computed tomography as an aid to the indications for management. Acta Neurochir (Wien). 1992;118(3–4):103–7 [cited 2022 Feb 27]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF01401294.
Botz B, Weerakkody Y. Subdural hygroma. In: Radiopaedia.org. 2012 [cited 2022 Mar 3]. Available from: http://radiopaedia.org/articles/18609.
Donaldson JW, Edwards-Brown M, Luerssen TG. Arachnoid cyst rupture with concurrent subdural hygroma. Pediatr Neurosurg. 2000;32(3):137–9 [cited 2022 Mar 8]. Available from: https://www.karger.com/Article/FullText/28918.
Nickel RE, Galtenstein JS. Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev Med Child Neurol. 2008;29(2):181–6 [cited 2022 Mar 1]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1987.tb02133.x.
Tsubokawa T, Nakamura S, Satoh K. Effect of temporary subdural-peritoneal shunt on subdural effusion with subarachnoid effusion. Pediatr Neurosurg. 1984;11(1):47–59 [cited 2022 Mar 2]. Available from: https://www.karger.com/Article/FullText/120159.
Eidlitz-Markus T, Shuper A, Constantini S. Short-term subarachnoid space drainage: a potential treatment for extraventricular hydrocephalus. Childs Nerv Syst. 2003;19(5–6):367–70 [cited 2022 Mar 2]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-003-0751-z.
Stroobandt G, Evrard P, Thauvoy C, Laterre C. [Subdural or sub-arachnoid pericerebral effusions in the infant with subdural or subarachnoid localisation (author’s transl)]. Neurochirurgie. 1981;27(1):49–57.
Mori K, Handa H, Itoh M, Okuno T. Benign subdural effusion in infants. J Comput Assist Tomogr. 1980;4(4):466–71 [cited 2022 Mar 3]. Available from: http://journals.lww.com/00004728-198008000-00009.
Kumar R, Singhal N, Mahapatra AK. Traumatic subdural effusions in children following minor head injury. Childs Nerv Syst. 2008;24(12):1391–6 [cited 2022 Mar 3]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-008-0645-1.
Tolias C, Sgouros S, Walsh AR, Hockley AD. Outcome of surgical treatment for subdural fluid collections in infants. Pediatr Neurosurg. 2000;33(4):194–7 [cited 2022 Mar 3]. Available from: https://www.karger.com/Article/FullText/55952.
Papasian NC, Frim DM. A theoretical model of benign external hydrocephalus that predicts a predisposition towards extra-axial hemorrhage after minor head trauma. Pediatr Neurosurg. 2000;33(4):188–93 [cited 2022 Mar 6]. Available from: https://www.karger.com/Article/FullText/55951.
Pittman T. Significance of a subdural hematoma in a child with external hydrocephalus. Pediatr Neurosurg. 2003;39(2):57–9 [cited 2022 Mar 6]. Available from: https://www.karger.com/Article/FullText/71315.
Ravid S, Maytal J. External hydrocephalus: a probable cause for subdural hematoma in infancy. Pediatr Neurol. 2003;28(2):139–41 [cited 2022 Mar 6]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0887899402005003.
McNeely PD, Atkinson JD, Saigal G, O’Gorman AM, Farmer J-P. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. AJNR Am J Neuroradiol. 2006;27(8):1725–8.
Hoskote A, Richards P, Anslow P, McShane T. Subdural haematoma and non-accidental head injury in children. Childs Nerv Syst. 2002;18(6–7):311–7.
Duhaime AC, Alario AJ, Lewander WJ, Schut L, Sutton LN, Seidl TS, et al. Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics. 1992;90(2 Pt 1):179–85.
Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P, et al. Subdural haemorrhages in infants: population based study. BMJ. 1998;317(7172):1558–61.
Rothenberger A, Brandl H. Subdural effusions in children under two years—clinical and computer-tomographical data. Neuropediatrics. 1980;11(02):139–50 [cited 2022 Mar 3]. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2008-1071384.
Morota N, Sakamoto K, Kobayashi N, Kitazawa K, Kobayashi S. Infantile subdural fluid collection: diagnosis and postoperative course. Childs Nerv Syst. 1995;11(8):459–66 [cited 2022 Mar 3]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00334966.
Litofsky NS, Raffel C, McComb JG. Management of symptomatic chronic extra-axial fluid collections in pediatric patients. Neurosurgery. 1992;31(3):445–50 [cited 2022 Mar 3]. Available from: https://academic.oup.com/neurosurgery/article/31/3/445/2752096.
Vinchon M, Noulé N, Soto-Ares G, Dhellemmes P. Subduroperitoneal drainage for subdural hematomas in infants: results in 244 cases. J Neurosurg. 2001;95(2):249–55 [cited 2022 Mar 3]. Available from: https://thejns.org/view/journals/j-neurosurg/95/2/article-p249.xml.
Aoki N, Masuzawa H. Bilateral chronic subdural hematomas without communication between the hematoma cavities: treatment with unilateral subdural-peritoneal shunt. Neurosurgery. 1988;22(5):911–3.
Aoki N, Mizutani H, Masuzawa H. Unilateral subdural-peritoneal shunting for bilateral chronic subdural hematomas in infancy. Report of three cases. J Neurosurg. 1985;63(1):134–7.
Mircevski M, Boyadziev I, Ruskov P, Mircevska D, Davkov S. Surgical treatment of acute subdural hygroma in children. Childs Nerv Syst. 1986;2(6):314–6 [cited 2022 Mar 3]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/BF00271946.
Zahl SM, Egge A, Helseth E, Skarbø A-B, Wester K. Quality of life and physician-reported developmental, cognitive, and social problems in children with benign external hydrocephalus—long-term follow-up. Childs Nerv Syst. 2019;35(2):245–50 [cited 2022 Mar 6]. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00381-018-4016-2.
Syvertsen M, Nakken KO, Edland A, Hansen G, Hellum MK, Koht J. Prevalence and etiology of epilepsy in a Norwegian county—a population based study. Epilepsia. 2015;56(5):699–706 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/epi.12972.
Surén P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012;130(1):e152–8 [cited 2022 Mar 6]. Available from: https://publications.aap.org/pediatrics/article/130/1/e152/29870/Autism-Spectrum-Disorder-ADHD-Epilepsy-and.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Samantray, S., Deopujari, C., Ali, S., Gala, F. (2023). Benign Enlargement of the Subarachnoid Spaces and Subdural Collections. In: Turgut, M., Guo, F., Turgut, A.T., Behari, S. (eds) Incidental Findings of the Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-031-42595-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-42595-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42594-3
Online ISBN: 978-3-031-42595-0
eBook Packages: MedicineMedicine (R0)