Abstract
Aim
There is considerable disparity in literature as regards to the presentation of subdural fluid collections in children. In this report, the authors have tried to establish the clinical and radiographic criteria to define the subdural effusions (SDEs) in children following minor head injury.
Methods
Twenty cases of traumatic SDEs following minor head injury were studied prospectively. The age of these children ranged from 1 month to 2 years with an average of 9 months. The duration from the onset of first symptom to presentation in our outpatient department varied from 1 month to 13 months with a mean of 4.2 months. The duration of follow-up was 6 months to 2 years with an average of 10 months.
Results
Fourteen out of 20 (70%) children presented with subtle findings. Six out of 20 (30%) children presented with overt neurological signs and symptoms. Seizures were the most common mode of presentation in this group. Bilaterality and ventriculomegaly were more common in the subtle group, each with an incidence of 43%. Seven out of 20 (35%) cases required operative management of traumatic SDEs. Recurrence was seen in two of 20 (10%) cases who had been conservatively managed previously. Only one child showed conversion of traumatic subdural hygroma to chronic subdural hematoma on conservative management.
Conclusion
Traumatic SDEs in children following minor head injury need to be differentiated from other causes of subdural fluid collections in children. The clinical and radiological criteria proposed by us helps to identify this subset of cases in most of the children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subdural effusion (SDE) or subdural hygroma (SDG) is a common post-traumatic lesion following both major and minor head injury. Despite its common occurrence, review of literature suggests that the pathogenesis, clinical significance, management and outcome following minor head injury are not well-established. A trivial trauma can cause a separation of the dura arachnoid interface, which is the basic requirement for the development of a SDG [9]. A child’s brain is characteristically different from that of an adult as a result of which they present with different clinical findings and outcome. Furthermore, a child’s brain is easily compressed so that it is easier to secure spaces for fluid collection due to brain atrophy after head trauma [3]. The treatment also differs as compared to adults because a child’s brain grows and shows fast recovery, plasticity. Most SDGs resolve spontaneously when the brain is well-expanded. However, a few SDGs become chronic subdural hematoma (SDH), when the necessary conditions persist over several weeks [13]. As the outcome is closely related to the primary head injury and not to the SDG itself, the outcome following minor head injury is usually favorable. We have tried to clarify the present knowledge on the pathogenic, diagnostic, and therapeutic aspects of this controversial lesion. Besides, various terms like subdural fluid collection, hygroma, effusion, chronic subdural hematoma, and external hydrocephalus have been interchangeably used in various reports in literature dealing with subdural fluid collections of varying etiology. An attempt is made to differentiate traumatic SDGs from other causes of benign extracerebral effusions in children, mainly external hydrocephalus.
Materials and methods
Twenty cases of traumatic SDGs following minor head injury were studied retrospectively and prospectively. The age of these children ranged from 1 month to 2 years with an average of 9 months. Six out of 20 were female children. The duration from the onset of first symptom to presentation in our outpatient department varied from 1 month to 13 months with a mean of 4.2 months. These cases were treated at Sanjay Gandhi Post Graduate Institute of Medical Sciences from January 1996 to December 2006. The clinical details of each case were recorded prospectively. The follow-up of each case was maintained by principal author on his personal computer. All the cases were managed by a single surgeon. The criteria for minor head injury was based on those proposed by an interdisciplinary special interest group (BISIG) of the American Congress of Rehabilitation Medicine (Handbook 16) [12] including:
-
1.
Loss of consciousness should not exceed 30 min.
-
2.
After 30 min the initial Glasgow coma scale score should be between 13 and 15.
-
3.
Post-traumatic amnesia should not exceed 24 h (assessment not possible in small children).
Diagnosis was chiefly based on computed tomography (CT) or magnetic resonance imaging (MRI) findings of a crescent-shaped hypodensity in the cerebral convexity. The follow-up of these cases was based on serial CT scans. The duration of follow-up was 6 months to 2 years with an average of 10 months. The cases with follow-up less than 5 months were excluded from this study. Other exclusion criteria included:
-
1.
H/O Meningitis
-
2.
Presence of intracranial hemorrhage
-
3.
Chronic SDH
-
4.
Intracranial/intraventricular surgery
-
5.
CSF diversion in hydrocephalus/endoscopic third ventriculostomy
-
6.
Cerebral palsy
-
7.
Cerebral atrophy
-
8.
Cerebral infarction
-
9.
External hydrocephalus
On the basis of clinical presentation, all the cases were classified into two categories:
-
Category A:
Subtle group
This category included children with no neurological deficits but with subtle signs and symptoms including delayed milestones, irritability, excessive salivation, occasional episodes of vomiting, and impaired neck holding.
-
Category B:
Overt group
This category included children with neurological features.
On the basis of management, these cases were divided into three categories:
-
Category I:
SDG resolved with conservative treatment
-
Category II:
SDG unresolved and under observation
-
Category III:
SDG resolved by operative drainage
Results
Eleven out of 20 (55%) children had a mild increase in head circumference (HC) but the anterior fontenalle (AF) was found to be tense in five of 20 (25%) children. Thus, only three children with increased HC had relatively tense AF. Fourteen out of 20 (70%) children presented with subtle findings with irritability being the most common mode of presentation. Six out of 20 (30%) children presented with overt neurological deficits, including presence of papilledema, cranial nerve palsies, seizures, and focal neurological deficits. Seizures were the most common mode of presentation being present in five of 6 (83%) children. When the presentation with seizures was analyzed in the entire group, only five of 20 (25%) children had seizures. The most common subtype of seizures was presentation with generalized tonic clonic seizures. The other subtypes included focal seizures, focal seizures with secondary generalization, and complex partial seizures of temporal origin in one child.
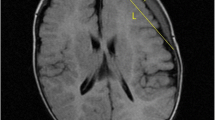
Radiographic evaluation with CT or MRI showed presence of unilateral effusions in 13 of 20 (65%) cases. Bilateral presentation was seen in only seven of 20 (35%) cases, although this is usually asymmetrical. When analyzed separately in the two categories, bilaterality was more common in the Subtle group, being present in six of 14 (43%) cases (Fig. 1a). Only one out of six children in the Overt group had bilateral SDG (16.5%). Seven out of 20 (35%) children presented with ventriculomegaly as defined by Evan’s criteria on radiographic analysis (Fig. 2a). Interestingly, all the seven children with ventriculomegaly had bilateral presentation and accordingly belonged to the Subtle group. The presence of mass effect as evidenced by ventricular effacement or midline shift was seen in six of 20 (30%) cases (Fig. 3). All these children were operated by burr hole drainage. One child without radiographic evidence of mass effect but significant unilateral effusion was also operated by burr hole drainage. The fluid drained was mainly dark colored CSF and rarely clear CSF. Thus, seven of 20 (35%) cases required operative management of traumatic SDG. Subdural pressure was monitored during operative drainage and was found to be elevated in all operated cases. Thus, subdural pressure monitoring is helpful in differentiating traumatic SDGs from external hydrocephalus [5]. Thirteen out of 20 (65%) children were treated conservatively with antiepileptic drugs (AEDs) and acetazolamide therapy for 4 weeks to 2 months. Eleven of 20 (55%) children improved clinically and had resolution of SDGs on CT or MRI scan—Category I (Fig. 1b). two out of 20 (10%) improved clinically following conservative therapy but CT scan failed to show resolution of the effusions—Category II (Fig. 2b).
Follow-up analysis showed a favorable outcome in all the cases. Seizures were defined to be controlled by either complete disappearance off AEDs or by significant reduction in previous dosages. The seizure control rate in the operated group was 100%, being achieved in all the seven cases. However, in the conservative group, the seizures could be controlled in 11 of 13 (85%) cases. Two children required adjunctive antiepileptic drugs. Recurrence was seen in two of 20 (10%) cases who had been conservatively managed previously. One of the child could be managed conservatively, while the other child had to be operated by burr hole drainage. Only one child showed conversion of traumatic SDG to chronic SDH on conservative management, yielding an incidence of only 5% (Fig. 4).
Discussion
Minor head injury is also called as ‘mild traumatic brain injury’ or ‘concussion’. Minor head injuries form the bulk of head injuries ranging from 80–90%. It amounts to 800,000 (minor) head injuries in India per year. It has been the subject of investigations in recent years, because it carries organic brain damage despite having normal CT of the brain [1]. Besides, minor head injury is also an important cause of SDE in children. Incidence varies from 0.81~13% regardless of the degree and site of brain injury [17]. History of trivial trauma could be obtained in nearly half of the children by asking leading questions in the cases where there had been no loss or alteration of sensorium following trauma.
Traumatic subdural effusion (TSE) refers to accumulation of CSF, xanthochromic or slightly blood-tinged fluid between the dura and the arachnoid membrane. It was first reported by Mayo in 1894 using the term “subdural hydroma” [10]. McConnell proposed the terms “subdural effusion” or “subdural fluid collection” [11]. A trivial trauma can cause a separation of the dura arachnoid interface, which is the basic requirement for the development of a SDE [9]. Arachnoid tear is likely to occur in the Sylvian fissure or chiasmatic region due to coup or countercoup injury because the arachnoid membrane adheres strongly to the sharp sphenoidal wing. For the development of SDE, it is necessary that the subarachnoid barrier is broken and a sufficient space is created to accumulate body fluid in the subdural area. Brain atrophy develops easily after trauma in children and in the elderly compared with adults since their brain is prone to be compressed. Another possibility proposed is that SDE results from injury due to deceleration or is an epiphenomenon with increased vascular permeability in the Sylvian veins or cortical capillaries [6]. Osmotic pressure mechanism has also been suggested as one of the causes or development of traumatic SDEs. Extension from a small SDH can also be a contributory factor in some cases [18, 19]. Presence of an associated structural lesion such as an arachnoid cyst can lead to traumatic SDEs in a small subset of cases [2], but this is very rare and we did not find any child with an associated structural lesion in the brain.
The clinical presentation in children with traumatic SDEs following minor head injury is distinct if clear history is available, which is not possible in most of the cases, particularly with subtle presentation. In our study, 11 of 20 (55%) children had an increase in head circumference but the anterior fontenalle was found to be tense in five of 20 (25%) children. Thus, only three children with increased HC had tense AF. This is an important finding as it signifies absence of significant mass effect and differentiates these cases from those with traumatic chronic SDH. However, children with external hydrocephalus also tend to present with macrocrania with lax AF [7]. Fourteen of 20 (70%) children presented with subtle findings such as delayed milestones, irritability, excessive salivation, occasional episodes of vomiting, and impaired neck holding. This pattern of clinical presentation is also seen in external hydrocephalus but rarely in chronic SDH. Six out of 20 (30%) children presented with overt neurological deficits such as papilledema, cranial nerve palsies, seizures, and focal neurological features. Seizures were the most common mode of presentation being present in five of six (83%) children. When the presentation with seizures was analyzed in the entire group, only five of 20 (25%) children had seizures. Presentation with seizures is more common with chronic SDH because of the highly epileptogenic effect of blood [8]. The high incidence of seizures in the overt group can be postulated to be a result of small component of SDH accompanying the traumatic SDEs in these cases. This is usually not appreciable on imaging as it becomes hypodense over a period of time.
Radiographic evaluation with CT or MRI reveals presence of unilateral crescent-shaped hypodensity over the cerebral convexity in most of the cases. A bilateral presentation is more common with external hydrocephalus, although the effusions tend to be symmetrical in most of these cases. Although there are few case reports which suggest that traumatic SDEs can also occur in posterior fossa [16], we did not encounter any such case. Children with subtle findings tend to present with bilateral effusion more frequently along with ventriculomegaly signifying the fact that ventricular enlargement in these cases can be a result of subarachnoid scarring. External hydrocephalus is characterized by certain radiological criteria which help to differentiate it from traumatic SDEs. These include:
-
Bi-hemispheric extracerebral fluid collections: anterior > posterior
-
Widening of the anterior interhemispheric fissure
-
Enlarged subarachnoid spaces
-
No evidence of cortical atrophy
-
Enlarged or prominent basal cisterns
-
Mild to moderate ventriculomegaly without periventricular lucency
-
Absence of restriction of blood flow in the cortex adjacent to fluid collection on diffusion-weighted MRI (DWMRI)
A characteristic “cortical vein sign” is present in external hydrocephalus which signifies absence of mass effect in these cases [7]. In traumatic SDEs, mass effect is evident in the form of effacement of sulci/gyri, effacement of basal cisterns/ventricles, and restriction of cortical blood flow on DWMRI. Differentiation of traumatic SDEs from chronic SDH can be difficult at times but in many cases with chronic SDH, membrane enhancement is seen on contrast scan which is a useful differentiating feature. Other conditions which need to be differentiated include effusions following meningitis, CSF diversion in hydrocephalus/endoscopic third ventriculostomy (ETV), cerebral palsy, cerebral atrophy, and cerebral infarction (Fig. 5). This is not difficult in most of the cases. A color-coded Doppler ultrasound is helpful in doubtful cases [15]. In benign subarachnoid space enlargement, color-coded Doppler sonography detects arachnoid vessels within the fluid collection. Besides, a high resolution ultrasound demonstrates the dural border of the arachnoid as an echogenic membrane which is a sign of the subarachnoid location of the fluid collection. In patients with traumatic SDEs and other subdural fluid collections, increased echogenity, absence of vascular structures and absence of surrounding border are seen.
Majority of the children with traumatic SDEs can be managed conservatively with antiepileptic drugs and acetazolamide therapy. The clinical symptoms improve, seizures get controlled and effusions tend to resolve with time. Thirty-five percent of cases required operative management of traumatic SDEs by burr hole drainage. Subdural pressure was found to be elevated in all the operated cases. Recurrences do occur in around 10% of the cases. An interesting finding is that conversion to chronic SDH is seen only in 5% of the cases, which is significantly less as compared to effusions following major head injury. The reported incidence in literature of conversion of traumatic SDEs to chronic SDH following major head injury is around 25–30% or even higher in few series [13]. Many other studies report the progression of traumatic SDEs to chronic SDH with incidence of 14% by Hong et al. [4], 25% by Yamada et al. [20], and 47% by Ohno et al. [14].
Conclusion
Conclusions from our study are:
-
(1)
Traumatic SDGs need to be differentiated from other causes of subdural effusions in children, mainly external hydrocephalus, chronic subdural hematoma, effusions secondary to meningitis, intracranial hemorrhage, cortical atrophy etc.
-
(2)
The clinical criteria include (a) absence of other etiological factors such as H/O meningitis, intracranial hemorrhage, intracranial/intraventricular surgery, CSF diversion in hydrocephalus/ETV, cerebral palsy etc.; (b) presentation with subtle findings such as delayed milestones, irritability, excessive salivation, occasional episodes of vomiting and impaired neck holding in most of cases; and (c) presentation with seizures in the overt group.
-
(3)
The radiological criteria include (a) bilateral effusions, although asymmetrical, in a subgroup of patients, (b) unilateral effusions in the overt group, and (c) mild ventriculomegaly may occur with bilateral effusions.
-
(4)
In approximately 55% of cases, traumatic SDGs get resolved by conservative treatment and in 10% cases they persist despite clinical improvement. Seizures get controlled in most of cases following treatment, seizure control rate being 100% in the operative group.
-
(5)
Recurrence of SDGs following minor head injury is uncommon and is reported in 10% of cases.
-
(6)
In 5% of cases, traumatic SDG may lead to chronic SDH.
References
Agarwal D, Mahapatra AK (2005) In: Mahapatra AK, Kamal R (eds) Minor head injury, 3rd edn. Modern, New Delhi, India, pp 123–128
Albuquerque FC, Giannotta SL (1997) Arachnoid cyst rupture producing subdural hygroma and intracranial hypertension: case report. Neurosurgery 41:951–956
Chugani HT, Muller RA, Dhugani DC (1996) Functional brain reorganization in children. Brain Dev 18:347–356
Hong DH, Lim HH, Bae WK, Kim PN, Kim IY, Lee BH et al (1994) Chronic subdural haematoma secondary to traumatic subdural hygroma. Korean J Radiol 30:219–224
Huh PW, Yoo DS, Cho KS, Park CK, Kang SG, Park YS et al (2006) Diagnostic method for differentiating external hydrocephalus from simple subdural hygroma. J Neurosurg 105:65–70
Kim BO, Kim SW, Lee SM (2005) Effectiveness of early surgery in children with traumatic subdural hygroma. J Korean Neurosurg Soc 37:432–435
Kumar R (2006) External hydrocephalus in small children. Childs Nerv Syst 22:1237–1241
Kumar R, Singhal N, Mahapatra AK (2007) Idiopathic chronic subdural haematoma, MCA infarct and cortical atrophy with status epilepticus in infants—report of two cases. Indian J Pediatr 74:1046–1048
Lee KS (1998) The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj 12:595–603
Mayo CH (1894) A brain cyst: the result of injury causing aphasia, hemiplegia, etc.; evacuation; complete recovery. NY Med J 59:434
McConnell AA (1941) Traumatic subdural effusion. J Neurol Neurosurg Psychiat 4:237–256
Mild head injury interdisciplinary special interest group of the American Congress of Rehabilitation Medicine (1993) Definition of mild traumatic brain injury. J Head Trauma Rehabil 8:86–88
Murata K (1993) chronic subdural hematoma may be preceded by persistent traumatic subdural effusion. Neurol Med Chir (Tokyo) 33:691–696
Ohno K, Suzuki R, Masaoka H (1987) chronic subdural haematoma preceded by persistent traumatic subdural fluid collection. J Neurol Neurosurg Psychiatry 50:1694–1697
Rupprecht T, Lauffer K, Storr U, Hofbeck M, Wenzel D, Böwing B (1996) Extra-cerebral intracranial fluid collections in childhood: differentiation between benign subarachnoid space enlargement and subdural effusion using color-coded duplex ultrasound. Klin Padiatr 208:97–100
Schaeybroeck PV, Vanlommel E, Lagae L, Calenbergh FV, Casaer P, Plets C (1999) Treatment of a symptomatic posterior fossa subdural effusion in a child. Childs Nerv Syst 15:2
Sohn IT, Lee KS, Doh JW, Bae HG, Yun IG, Byun BJ (1997) A Prospective study on the incidence, patterns and premorbid conditions of traumatic subdural hygroma. J Korean Neurosurg Soc 26:87–93
Stone JL, Lang RG, Sugar O, Moody RA (1981) Traumatic subdural hygroma. Neurosurgery 8:542–550
Yamada H, Kageyama N, Nakajima M, Nakamura S (1979) Acute post-traumatic subdural hematoma in infancy: special reference to the mechanism of collection of subdural fluid. No Shinkei Geka 7:55–62
Yamada H, Nihei H, Watanabe T, Shibui S, Murata S (1979) Chronic subdural hematoma occurring consequently to the posttraumatic subdural hygroma—on the pathogenesis of the chronic subdural hematoma. No To Shinkei 31:115–121
Acknowledgements
The author is grateful to Mr. A. P. Dhar Dwivedi, Department of Neurosurgery, SGPGIMS, Lucknow, for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Singhal, N. & Mahapatra, A.K. Traumatic subdural effusions in children following minor head injury. Childs Nerv Syst 24, 1391–1396 (2008). https://doi.org/10.1007/s00381-008-0645-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-008-0645-1