Abstract
As a result of more than 30 of experience studying cholesteatoma, this chapter proposes to discuss all aspects related to this disease in a contemporary view but without leaving aside the historical aspects. We begin with the definitions, classifications, and histological aspects, thus defining cholesteatoma from the basic science to the principles guiding clinical and surgical management. Histopathology, biology, and animal models proposed for the study of cholesteatoma will also be analyzed. From the concepts of etiopathogenesis, we will also address the theories for the formation of cholesteatoma and the growth patterns. From the clinical point of view, we also describe the routine evaluation in the office (otoendoscopy, audiology, and radiology) and the surgical principles since the surgical technique will be covered in specific chapters in this book.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Middle ear cholesteatomas are one of the most intriguing otologic diseases, and their treatment demands a meticulous and complete analysis of many different facets. As a rule, the main discussion always narrows down (mistakenly) to the surgical technique. Over the years, countless congresses, symposiums, and meetings have been organized on five continents with the aim of discussing advances in the understanding of this disease. As we mentioned earlier, the focus of these debates emphasizes the following hierarchy:
-
1.
THE SURGICAL TECHNIQUE or how do we do it?
-
2.
THE SURGICAL TIMING or when to do it?
-
3.
THE SURGICAL REATIONALE or why do we do it?
-
4.
WHAT DOES ORIENT OUR DECISIONS or the theoretical basis!
We believe that the best and most complete way to approach this condition is to reverse this order. Thus, in our opinion, the priority should be rearranged as follows:
-
1.
WHAT DOES ORIENT OUR DECISIONS or the theoretical basis!
-
2.
THE SURGICAL REATIONALE or why do we do it?
-
3.
THE SURGICAL TIMING or when to do it?
-
4.
THE SURGICAL TECHNIQUE or how do we do it?
Thus, as at the base of a pyramid, the broad theoretical base will support our therapeutic decisions, defining more securely why, when, and how to treat. Not infrequently and in very special situations, surgery may not be the best option at a given time. Likewise, it is necessary to better and more thoroughly understand the natural history of the disease and its pathogenesis (masterfully defined by MM Paparella as the “journey between the etiology and the established pathology”). This is the best way to propose a set of rational strategic actions throughout the process that may abort its evolution and the potential risks of complications. We always like to paraphrase the famous British neurosurgeon Henry Marsh:
It is often said it takes three months to learn how to do an operation, three years to learn when to do it, and 30 years to learn when not to do it.
The Expression
The German anatomist Johannes Mueller used the word “cholesteatoma” for the first time, in 1838 [1]. The root of this word means chole (from cholesterol); steato from fatty tissue), and oma (ending meaning tumor in biology), that is, a tumor in which fatty tissue and cholesterol crystals are present. Etymologically, this term is completely incorrect since cholesteatomas originates from the keratinized stratified squamous epithelium of the tympanic membrane (TM) and/or external auditory canal (EAC) and does not have cholesterol crystals or fat in its composition and, in addition to its tumor nature being largely debatable.
Other names have been suggested through the years such as pearly tumor, by Cruveilhier (1829), margaritoma (Craigie 1891), epidermal cholesteatoma (Cushing 1922), epidermoid by Critchley and Ferguson (1928), and keratoma by Shuknecht, in 1974 (apud [1]). All these terms, although more adequate and descriptive, have not become popular in the medical literature and the word cholesteatoma definitely has been enshrined among otologists.
The Definition
Friedmann defined cholesteatomas in 1959 [2], as cystic structures covered by stratified squamous epithelium, resting on a fibrous stroma of variable thickness, which may contain some elements of the original mucous lining.
More simply, Schuknecht, in 1974 [3], defines them as accumulation of exfoliated keratin inside the middle ear or any pneumatized area of the temporal bone, arising from a keratinized stratified squamous epithelium.
Ferlito et al. [4] describes the cholesteatoma as an epidermoid cyst, of independent and progressive growth, with destruction of the adjacent tissues, especially the bone tissue, with a tendency to recur (Fig. 42.1).
Informally, we tend to simplify by referring to cholesteatomas as “skin in the wrong place!” Macroscopically, it is a round or oval cystic lesion with variable shape and size; histologically, it may be broken into three main components: (1) the matrix—keratinized stratified squamous epithelium; (2) the perimatrix—a rich network of connective tissue, collagen fibers, and inflammatory cells; and (3) the cystic content—keratin and epithelial rests (Fig. 42.2).
Epidemiology and Risk Factors
It is estimated that over 20 million people worldwide are afflicted with chronic otitis media (COM). Of these, one-fourth (about five million) have a cholesteatoma [5], although the overall number of cases of acquired cholesteatoma seems to be in decline [6, 7]. The annual incidence of cholesteatoma is reported as 3 per 100,000 in children and 9.2 per 100,000 adults. Males slightly outnumber females in a ratio of 1.4:1, and cholesteatomas that present in the middle ear are more frequently found in persons younger than 50 years of age [8, 9]. Caucasian persons show the highest prevalence, but cholesteatoma is infrequently found in Inuit, Native American, and Asian populations [10]. Several reports reviewed by Jennings et al. evaluated familial clustering and inheritability of cholesteatoma and found that incomplete penetrance exists and may depend on a combination of environmental and genetic factors for the formation of an acquired cholesteatoma. Jennings also states that evidence from syndromic cases suggests genes controlling ear morphology may be risk factors for congenital or acquired cholesteatoma formation [11]. Syndromes where a diagnosis of cholesteatoma has been reported finding include Turner syndrome [12,13,14], Treacher Collins syndrome[15], Down syndrome [16,17,18,19], and focal dermal hypoplasia [20]. Numerous reports of patients presenting with cleft palate and cholesteatoma [21,22,23,24,25,26,27] have been presented with the rate of incidence approaching 6% in that population [26]. When compared to children who did not develop a cleft palate, those with a cleft palate face a 100–200 times greater likelihood of developing a cholesteatoma [22, 26]. Additionally, a link between allergic rhinitis and the development of cholesteatoma was recently discovered in that patients with allergic rhinitis presented with a significantly lower 10-year cholesteatoma disease-free rate [28].
At the Chronic Otitis Media Outpatient Clinic at Hospital de Clínicas de Porto Alegre (AOMC-HCPA), 2603 patients were diagnosed with COM in follow-up. Of those, 638 (24.51%) presented cholesteatoma, which was bilateral in 17.1%. The mean age was 34, 49 years, and 53.5% were female. Also, most patients were 18 years or older (63.8%). Concerning the cholesteatoma growth patterns, the anterior epitympanic was 1.9%, the posterior epitympanic (PEC) was 32.9%, the posterior mesotympanic (PMC) corresponded to 33.7%, two-route cholesteatoma was 14.8%, and open cholesteatoma (or indeterminate) was 16.7%.
Classification
Cholesteatomas are classified as congenital or acquired. The former can be found in five temporal bone regions; in turn, the acquired can be subdivided into primary or secondary (Table 42.1).
Congenital cholesteatomas (CCs) were defined by Derlacki and Clemis [29] as a conglomerate of epithelial remnants found in ears with intact tympanic membranes and usually without a previous history of infections. According to Valvassori [30], they can be found in four regions of the temporal bone: tympanic-mastoid, petrous apex, cerebellopontine angle, and jugular foramen. There is still a fifth location, described by Sobol [31], who reported the existence of small epithelial pearls between the layers of the tympanic membrane.
If the congenital nature of some cholesteatoma is quite clear, there is much debate about the origin of acquired cholesteatomas. Conceptually, they have been dichotomized into two groups: primary and secondary. The former would result from progressive tympanic retraction, which, at some point, loses its self-cleaning properties and starts to accumulate desquamated epithelium and keratin. On other hand, secondary cholesteatomas would arise from the migration (invasion) of the external auditory canal epithelium into the middle ear through a marginal perforation of the tympanic membrane. Once inside the middle ear, a standard biological behavior would follow: it would encyst and start producing keratin [32]. Today, our understanding that the division of cholesteatomas into these two models, although being quite didactic and easy to understand, is vastly operationally incomplete. We will expand on this discussion in specific sections of this chapter.
Meyerhoff and Truelson [33] tried to classify cholesteatomas according to their pathophysiology, location, ossicular defects, and presence of complications, also dividing them into congenital and acquired, the latter being primary, secondary, or tertiary.
Tos [34] proposed an otoscopic classification, dividing cholesteatomas into:
-
1—Attic.
-
2—Pars Tensa I (marginal disease).
-
3—Pars Tensa II (central disease).
In 1993, the same author proposed another classification, based on the site of origin of the cholesteatoma, which he considers an important factor for the surgical procedure and for the prognosis. This taxonomy presents three categories:
-
1—Attic cholesteatoma—a retraction of the pars flaccida or Shrapnell’s membrane, extending from the attic, passing through the aditus, and eventually reaching the antrum, mastoid, or tympanic cavity.
-
2—Cholesteatoma of the sinus tympani—posterosuperior retraction or perforation of the pars tensa, extending to the tympanic sinus and posterior recesses.
-
3—Cholesteatoma of the pars tensa—retraction and total adhesion of the pars tensa of the tympanic membrane (TM) involving the tympanic orifice of the eustachian tube (ET).
Saleh and Mills [35] proposed another classification, according to the sites affected by cholesteatoma, characterized as follows:
-
S1—If the cholesteatoma is restricted to the place where it started.
-
S2—when the disease extends to another location.
-
S3—if it affects three locations.
-
S4—if installed in four locations.
-
S5—for cases in which the first affected site and, in addition to this, four or more are involved.
These same authors distinguish seven locations used for this classification: attic and antrum, middle ear, mastoid, ET, labyrinth, and middle fossa.
Saleh and Mills [35] also present a classification of the condition of the ossicular chain, based on the descriptions of Wullstein [36] and [37], through the following score:
-
0—intact ossicular chain.
-
1—Incus is eroded and with discontinuity of the ossicular chain.
-
2—Incus and stapes superstructure are eroded.
-
3—Malleus head and incus are absent, and the stapes superstructure is eroded.
As for preoperative complications, Saleh and Mills [35] classified cholesteatoma as:
-
C0—when there are no complications.
-
C1—for the occurrence of one complication.
-
C2—for the existence of two or more.
As complications, the authors consider lateral semicircular canal (LSC) fistula, facial paralysis, total sensorineural hearing loss (SHL), sinus thrombosis, and intracranial invasion.
Finally, in 2017, a task force of international researchers was assembled with the aim of standardizing these classifications and proposing pathogenesis models. The conclusions were published in the Journal of International Advances in Otolaryngology—EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma [38]. The clinical classification suggested in the final consensus of this group contemplates the division of congenital and acquired cholesteatomas and ratifies pathogenesis models (Fig. 42.3).
Among the conclusions of this consensus and in order to simplify the extent of cholesteatoma, they propose the so-called STAM system dividing the middle ear and mastoid space into four sites: difficult access sites (S), tympanic cavity (T), attic (A), and mastoid (M). The difficult access sites (S) include S1, the supratubal recess (also called the anterior epitympanum or protympanum), and S2, the sinus tympani. The posterior border of the attic is the posterior end of the incus short process or the fossa incudis. The mastoid includes the antrum and mastoid cells (Fig. 42.4).
The EAONO/JOS also proposed a very encompassing staging system that applies to four types of middle ear cholesteatoma (pars flaccida cholesteatoma, pars tensa cholesteatoma, congenital cholesteatoma, and cholesteatoma secondary to a tensa perforation). This system is summarized in Table 42.2 [38].
Histopathology
Cholesteatoma, macroscopically, is a round or oval cystic lesion with variable configuration and size. Ferlito et al. [4] characterized cholesteatoma as an epidermoid cyst, with independent and progressive growth, with destruction of the adjacent tissues, especially bone, with a tendency to recur.
The advent of transmission electron microscopy made possible many advances in the knowledge of cellular structure. Using this instrument, in 1972, Lim and Saunders [39] presented a detailed histological description of cholesteatomas. They described that cholesteatoma has a keratinized stratified squamous epithelium, with four layers identical to those of normal epidermis (basal, spinous, granulosa, and cornea), Langerhans cells (in greater numbers than in normal epidermis), and keratohyaline granules. They called this epithelium the matrix of the cholesteatoma. They also observed the presence of a connective tissue, containing collagen fibers, fibrocytes, and inflammatory cells, which was called perimatrix, which was in contact, in most cases, with a layer of scaly or cylindrical ciliated cells, remnants of the original mucosa of the middle ear. In some cases, although the perimatrix was absent at optical microscopy, it was present when studied with the transmission electronic microscope, showing itself to be extremely thin, with practically absent collagen fibers and containing crystals of calcium carbonate. The cystic content was formed by accumulated keratin, epithelial debris, and inflammatory compounds.
This tripartite structure, matrix, perimatrix, and cystic content, is well illustrated in Fig. 42.5 and will be detailed below:
(a) Stratified squamous epithelium, keratinized, with an average of three layers of cells. Missing perimatrix. (b) Stratified squamous epithelium, keratinized, with an average of six layers of cells. Narrow, fibrotic perimatrix, with rare lymphocytes. Absence of granulomas. (c) Stratified squamous epithelium, keratinized, with an average of four layers of cells. Very narrow perimatrix, without fibrosis and without inflammatory infiltrate. (d) Stratified squamous epithelium, keratinized, with an average of six layers of cells. Narrow and delicate perimatrix, without fibrosis and with a very discreet inflammatory infiltrate. (e) Stratified squamous epithelium, keratinized, with an average of 12 layers of epithelial cells. The perimatrix exhibits dense fibrosis, accentuated chronic inflammatory infiltrate, and is delimited in its deep plane by simple cuboidal epithelium. (f) Stratified squamous epithelium, keratinized, with an average of 13 layers of epithelial cells showing epithelial hyperplasia. Perimatrix shows discreet fibrosis with accentuated inflammatory infiltrate and neutrocytic exudation, being delimited in its deep plane by simple cuboidal epithelium. Absence of granulomas
Perimatrix
Paludetti et al. [40] described the perimatrix as a mass of granulation tissue or inflamed subepithelial connective tissue. According to Milewski et al. [41], the growth of a cholesteatoma would require angiogenesis in the connective tissue of the perimatrix, and that cells and substances of the healing cascade could have an important role in the development and growth of cholesteatomas. These processes would involve the fibroblastic growth factor b (b-FGF), which, according to these authors, could stimulate the production of collagenase. They also suggested that the persistence of inflammation would cause a permanent healing process in the perimatrix, the proliferation of fibroblasts (granulation tissue), and epithelium (matrix).
Ferlito et al. [4] describe the perimatrix as the most peripheral portion of the cholesteatoma, consisting of granulation tissue or inflammatory subepithelial connective tissue, with lymphocytes, histiocytes, and neutrophils. Sprekelsen et al. [42] state that the matrix and perimatrix, in normal or pathological tissues, are formed by type IV collagen, tenascin, fibronectin, b-FGF, and metalloproteinases (MMP). According to Jacob et al. [43], the increase in the proliferation of the cholesteatoma matrix would be the result of the inflammation process, suggesting that the perimatrix would be the main factor in the development of cholesteatomas.
Hamzei et al. [44] analyzed 21 cholesteatomas, through polymerase chain reaction (PCR), immunohistochemistry, and histology, with the aim of investigating the factors of stimulation and differentiation of osteoclasts present in cholesteatomas, using the skin of the external acoustic meatus as a control. Immunohistochemical analysis demonstrated an increase in osteoclast precursor cells and macrophages in cholesteatomas. The perimatrix analysis demonstrated that, in this region of the cholesteatoma, there are all the necessary factors for osteoclastogenesis and for the stimulation of bone reabsorption.
Briefly, we like to define the perimatrix as a rich inflammatory network that surrounds the cholesteatoma. It represents an authentic “battlefield” which may play interesting and antagonistic roles in this dynamic process: at times smoothing the paths for the aggression of the highly proliferative epithelium and sometimes helping the native mucosa to wedge the invasion.
Matrix
The cholesteatoma matrix consists of keratinized stratified squamous epithelium characterized by the presence of intercellular bridges and by a regular arrangement of the various cell layers. It is made up of five components:
-
Basal layer or stratum germinative—composed of columnar epithelium, formed of cubic cells, which exhibit an enlarged nucleus with a hyperchromatic and basophilic appearance.
-
Stratum spinosum or malpighian layer—composed of larger cells, still relatively cylindrical in shape, which become polyhedral in the more superficial layers.
-
Stratum granulosum or granular layer—cells become progressively flattened, containing keratohyaline and hyperchromatic granules in the cytoplasm.
-
Stratum lucidon—often goes undetected.
-
Stratum corneum—with a hyperkeratotic and scaly appearance, in which the keratin lamellae form the cystic content.
Acquired cholesteatomas are sometimes accompanied by glandular metaplasia. If the cholesteatoma sac ruptures, keratin is released in the subepithelial layer, resulting in a foreign body-type reaction. Osteoclasts are frequently observed at the interface of the cholesteatoma matrix and the subjacent bone tissue [4].
Cystic Content
The cystic contentis composed of well-differentiated anucleate keratinocytes and laminar keratin masses [45, 46].
All cholesteatomas, whether acquired or congenital, present practically the same morphological characteristics. However, due to their different formation mechanism, some of them may present particularities. According to Ferlito et al. [4], the matrix would be thicker in the acquired form than in the congenital form, and this would generally be composed of 15 cell layers, while the congenital form would only present five cell layers. However, Dornelles et al. [45], studying acquired cholesteatomas, found an average number of eight cell layers in the matrix. Another histopathological variation observed by Ferlito et al. [4] was that in congenital cholesteatoma, contrary to the acquired one, normally it presents few inflammatory signs, being generally absent the glandular structures of the perimatrix. Another difference would be the dendritic cells, identified in greater numbers in the squamous epithelium of congenital cholesteatomas. The method used to identify dendritic cells was immunohistochemistry, using the S-100 protein as a specific marker. This marker can be useful in the histopathological diagnosis of cholesteatoma [47].
In short, the histological diagnosis of cholesteatoma is performed through the identification of its components: perimatrix, matrix, and cystic component. The ultrastructural characteristics of cholesteatomas are similar to those of normal epidermis. In particular, Langerhans cells were found in the spinosum layer, between the keratinocytes, and Merkel cells in the germinal layer. Scanning electron microscopy showed the presence of corneocytes in the form of hexagonal disks, organized in regular columns, with each column surrounded by six others [4].
In Fig. 42.5, some cross-sectional images of acquired cholesteatomas can be seen, showing the great variability in the thickness of the perimatrix, as well as its histological components.
Biology of Cholesteatoma
The study of cells, in optical and electron microscopy, can give the misleading impression that these are static structures. However, on the contrary, many processes and movements are constantly happening in the cellular intimacy, occurring more quickly in some tissues, slower in others. It is easy to understand that, as cells differentiate, they simultaneously acquire certain structural and physiological characteristics.
Epithelia are tissues with limited life, with constant renewal, due to continuous mitotic activity. The speed of this cell replacement is variable, ranging from two to 50 days, depending on the tissue considered [48].
The connective tissue, which constitutes the cholesteatoma perimatrix, presents a more complex growth process, since it is formed by several types of cells— fibroblasts, macrophages, mast cells, plasmocytes, and leukocytes—separated by abundant intercellular material. The richness of this material is one of its most important characteristics. It consists of two parts: one with a defined microscopic structure—connective fibers—and the other unstructured— fundamental amorphous substance [48]. For all this complexity, it is to be expected that the process of renewing this tissue is quite elaborated.
There are hypotheses that chronic otitis media with cholesteatoma (CCOM) could be the result of uncontrolled cell proliferation [4] comprising a series of complex and dynamic events involving cellular and extracellular components with alterations in their biological behavior, such as dysregulation of keratinocytes [49], which show hyperproliferative growth and alterations in cell differentiation. However, it is not known for sure whether this lack of control is caused by defects in genes that control proliferation, by cytokines released from inflammatory cells, or by other yet unknown mechanisms [1]. Therefore, determining the existence of defects in its biology, biochemistry, and genetics is critical to understanding its pathogenesis.
As previously described, the capacity for invasion, migration, change in differentiation, proliferation, and recurrence of cholesteatomas is very similar to neoplasms; however, there is reluctance among researchers to accept the inclusion of cholesteatomas in this category [50, 51]. For cholesteatomas to be considered a neoplastic lesion, evidence of genetic instability is required; this can be manifested through changes in the DNA or specific chromosomal abnormalities. In 1995, Shinoda and Huang [52] detected the p53 protein in cholesteatomas, suggesting that these could be tumors. However, Desloge et al. [51] demonstrated that there were no alterations in the DNA, thus discarding this hypothesis.
As the cited researches do not indicate any genetic instability of these lesions, we must investigate another possible reason for the development of the cholesteatoma, being necessary to ask about the origin of its characteristic keratinized squamous epithelium. To study this issue, many investigations using immunohistochemical analysis have been carried out to compare the location of differentiating markers in cholesteatomas and in the skin of the external auditory canal. Due to the properties presented by cytokeratins, they have been considered by many investigators as one of the best instruments for this purpose [49, 53, 54].
Cytokeratins are proteins that constitute one of the two categories of intermediate filaments, located in the cytoplasm of epithelial cells; they have 20 subclasses, and their expression depends on the type of epithelium and its stage of differentiation [55]. Pereira [56], Albino et al. [50], and Kim and Chung [57] reported that the matrix of cholesteatomas expresses cytokeratin 16 (CK16) in the suprabasal layers, and the expression of this protein filament is characteristic of hyperproliferative epithelia. Leperque et al. [58] describe that CK16 does not appear in the normal epithelium, except in areas under pressure and friction, or in the epithelium lining the hair follicles. According to Broekaert et al. [59], CK16 is expressed in specific regions, such as the tympanic annulus and the medial and inferior regions of the external acoustic meatus. Pereira et al. [60] state that the cholesteatoma has a cytokeratin pattern similar to that of the external acoustic meatus and the epithelial layer of the tympanic membrane. The presence of CK16 in the cholesteatoma matrix could indicate its hyperproliferative behavior, similar to that of hyperproliferative epidermal diseases, even if the histological aspect of the cholesteatoma is the same as that of the normal epidermis. According to Albino et al. [50], the cholesteatoma is formed as a result of the attempt to repair an injury, which could explain the presence of CK16, characterizing this epithelium as immature with a predominance of cell proliferation. Kujipers et al. [54] analyzed the pattern of cytokeratins and suggested that the cholesteatoma matrix is not the result of a metaplastic change. In their study, they found an epithelium similar to that of the tympanic membrane and the skin of the external auditory canal, but in different stages of proliferation, depending on the degree of inflammation present.
A characteristic sign of cholesteatomas is infiltration of the perimatrix by cells of the immune system. Piltcher [61], in his thesis on cytokines in chronic otitis media with effusion, states that, in addition to the already known risk factors, such as tubal dysfunction and infections, much research on otitis media has been directed to the study of the different components of the inflammatory response. The key issue is whether inflammation should be considered only as a defense process or whether it plays a role in the perpetuation of CCOM. Milewski [62]suggested that inflammatory cytokines, fibroblasts, and macrophages would be responsible for the origin, growth, and bone destruction of cholesteatomas. Several cytokines and growth factors could be involved in the mechanism of proliferation and development of the cholesteatoma epithelium [63, 64]. Tomita [65] states that there are several hypotheses that growth factors and cytokines, present in cholesteatomas, induce the activation of genes, such as c-myc, causing the deregulation of cell proliferation. Sudhoff et al. [66] investigated the distribution and expression of tumor growth factor (TGF-alpha), epithelial growth factor (EGF-R), and c-myc oncogenein normal middle ear epithelial cells and in cholesteatomas. These factors were found in the matrix of cholesteatomas, but not in normal cells. In addition to the autocrine regulation of the epithelium, through the production of epithelial growth factor (EGF), cholesteatoma hyperproliferation could depend on the interaction of the subepithelial tissue and the inflammatory changes that occur in this pathology. Sudhoff et al. [67] investigated the expression and location of growth factors in angiogenesis in 22 cholesteatomas, in comparison with the normal epidermis of the external auditory canal and the normal middle ear mucosa to identify some growth factors involved in the pathogenesis. of cholesteatomas. These researchers found, in normal skin and mucosa, 5.3 ± 1.2 vessels/mm2, while in the cholesteatoma group there were 21.1 ± 11.7 vessels/mm2, and this average varied according to the degree of inflammation in the perimatrix: 9.0 ± 3.5 vessels/mm2 in grade I, 19.2 ± 3.6 vessels/mm2 in grade II and 31.7 ± 9.4 vessels/mm2 in grade III. They also reported a decrease in type IV collagen and laminin in the basement membrane in cholesteatoma compared to controls.
A common feature in the pathogenesis of various types of cholesteatomas is the presence of bacteria which could promote a critical bond between the cholesteatoma and the host, preventing the newly formed epithelium from completing its differentiation process, which would leave it in a quiescent state, minimally proliferative, without being migratory or invasive at this stage [68]. Interactions between inflammatory cells and the cholesteatoma epithelium could be responsible for inducing the aberrant biological characteristics of this pathology.
Chole and Faddis [68] studied, by transmission electronic microscopy, 24 human cholesteatomas and 22 Mongolian squirel (gerbil). Of the samples from humans, 16 showed histological findings consistent with biofilm bacteria, while in the gerbil material, 21 showed evidence of this bacteria. This finding could be related to the activity of cholesteatomas, mainly with persistent or recurrent infections and their resistance to topical and systemic antimicrobials. The authors suggested that the cholesteatoma matrix is an ideal medium for the development of a mixed microbiological biofilm. These authors also state that bacteria with biofilm are resistant to antibiotics by mechanisms different from those used by planktonic bacteria; however, the exact mechanism of resistance of bacterial colonies in biofilm is unknown.
The studies published so far present many data regarding the biology of cholesteatomas, but many doubts persist. As previously mentioned, cholesteatomas present neoplastic characteristics (invasion, migration, and change in differentiation), but, so far, no indication of genetic instabilities in their structure has been found, a fact that rules out the possibility of classifying them as neoplasms. Another property that seems constant in cholesteatomas is their hyperproliferative activity, perhaps residing here as a possible answer to their characteristics of aggressiveness and uncontrolled growth. In addition to this fact, the stimuli of the immune response, represented by cytokines related to the inflammatory cells of the perimatrix, represent a strong candidate for the role of main actor in this intricate network of mechanisms. All these hypotheses lead us to consider the complexity involved in the biology of cholesteatomas and, consequently, the tangle of events related to their pathogenesis.
Animal Models
Several animal models have already been used for the experimental study of acquired cholesteatoma, among which we can mention rabbits, chinchillas, guinea pigs, rats, and Mongolian gerbils [69]. However, the gerbil seems to be the currently preferred animal model since it spontaneously develops aural cholesteatomas [70]. Also, those cholesteatomas are similar to the humans, concerning the epithelial and subepithelial linings of the middle ear and the destructive characteristics of the gerbilline cholesteatoma [71].
In addition to the spontaneous cholesteatomas, numerous methods were tried to develop the disease in animal models. Numerous substances, such as talcum powder and fibrina, dimethyl-benzanthrancene (a chemical carcinogen widely used for experimental purposes), propylene glycol, have been used [69]. Schmidt and Hellstrom [72, 73] performed a perforation in the posterosuperior quadrant of the tympanic membrane of rats and weekly introduced dimethylbenzanthracene for four consecutive weeks. After survival from 1 to 8 weeks, the authors observed the invagination of highly desquamative stratified squamous epithelium through the perforation in 89.5% of the animals, and in 47% of these there was complete epithelization of the middle ear. The authors observed the permanence of the inflammatory process and the presence of purulent secretion throughout the experiment.
In other animal models, where cholesteatomas were also induced, different methods were employed ranging from the inoculation of bacteria and upper respiratory infections [74, 75], obstruction of the eustachian tube (ET) [76, 77], skin grafts placed in the middle ear [78], and external auditory canal (EAC) ligation [79, 80].
The rate which experimentally induced cholesteatomas was high, varying from 39% [81] to 100% [82], with a wide range of results between them, depending on the animals and the protocol used. Bauer et al. [83] introduced the use of otoendoscopy to the gerbilline model, through which the authors evaluated the development and the characteristics of pars flaccida retraction pocket and cholesteatoma in Mongolian gerbils after the obliteration of the eustachian tube, compared to a control group. At the end of the 16-week follow-up, cholesteatoma was present in 34.2% of the ears in the intervention and in 20.6% in the control group (p = 0.197).
The lowest positivity rates, regardless of protocol, were generally obtained in cases where the survival period was short (e.g., 2 weeks, 0 positivity; [81]) or the irritant agent concentration was low (e.g., propylene—10% glycol, 12.5% positivity; [84]). In cases where the survival period was longer (e.g., 5 months), the positivity obtained reached 87.5% [81]; in parallel, in cases where the concentration of the stimulating agent was high (e.g., 90% propylene glycol), the positivity obtained reached 100% [85].
Furthermore, in animal models where the eustachian tube was obstructed, the tendency toward greater positivity in relation to the presence of cholesteatomas, with greater survival, was clearly observed [76, 77]. Wolfman and Chole [76, 77] obtained the formation of cholesteatomas with an increasing percentage with a greater survival, in up to 75% of the animals, in a model in the Mongolian gerbil with obstruction of the eustachian tube and consequent retraction of the tympanic membrane. Huve et al. [82] compared the incidence and the histopathological aspects of spontaneous and the two most used induced Mongolian gerbils’ models of cholesteatoma (EAC obliteration and the ET cauterization). It was observed that the incidence of cholesteatoma in Mongolian gerbils after EAC obliteration was significantly higher than that observed after ET cauterization, which in turn was significantly higher than the spontaneous occurrence of the disease in the control group (100%, 52.9% and 16.7%, respectively, p < 0.0001).
Despite this apparent relationship between the development of cholesteatomas and middle ear ventilation deficiency, Meyerhoff et al. [86], in an animal model in chinchillas, using 60% propylene glycol and placing a ventilation tube (VT) in the bulla, demonstrated that, despite ventilation of the middle ear, cholesteatomas developed in 66.6% of the animals. They concluded that negative pressure is not necessarily a primordial factor for the development of cholesteatomas, following this protocol.
Some animal models were presented, where an attempt was made to inhibit the formation of cholesteatomas induced by the use of propylene glycol. This attempt was unsucccesful when cyclophosphamide [87], isotretinoin [88], and hyaluronic acid [89] were used. Wright et al. [90] used 60% propylene glycol in the bula of chinchillas that had ventilation tubes, and the application was repeated two more times. In these animals, after the third application of propylene glycol, 5% 5-fluorouracil was placed on the lateral surface of the tympanic membrane, and this application was repeated twice more. After 4 weeks of survival, it was observed that the use of 5-fluorouracil prevented the formation of cholesteatomatous cysts in all 16 temporal bones studied. However, microscopic invasions of epithelium on the medial surface of the tympanic membrane were observed in four studied ears, and three of them had tympanic membrane perforation.
The same methodology employed in the study resulted in intense hyperplasia of the epithelial and connective layers of the tympanic membrane and cholesteatomas in 60–70% of the animals, when 5-fluorouracil was not used [86, 91]. The antimetabolic action of 5-fluorouracil apparently has an effect on reducing epidermal proliferation, and retinoic acid also seems to have an effect on epidermal keratinization and on glandular secretory activity. Metaplasia and keratinization of the middle ear epithelium were observed by Chole and Frush [92], in rats fed with a diet low in vitamin A, and its supplementation led to a normalization of these epithelial alterations. Furthermore, a study carried out in cell culture of specimens of human cholesteatomas, obtained in surgeries, demonstrated the inhibitory effect of retinoic acid on their proliferation [93], different from the experimental results obtained by Jove et al. [88] with isotretinoin, which is a synthetic analog of retinol (vitamin A). White et al. [89] used hyaluronic acid with the aim of reducing connective tissue hyperproliferation, which, together with the inflammatory process, is believed to be one of the main factors involved in the pathogenesis of acquired cholesteatomas. Sixty propylene glycol was placed in the chinchillas’ bula and, after the third injection, a viscous solution of 1.5% hyaluronic acid was placed laterally to the tympanic membrane, and the application was repeated six more times. After a four-week survival, the presence of cholesteatomas was observed in approximately 70% of the animals that received propylene glycol, regardless of the use of hyaluronic acid or not. Pownell et al. [87] used cyclophosphamide systemically in chinchillas for 14 days, and on days 5, 8, and 11 the animals received bilateral applications of propylene glycol. After a four-week survival, the presence of cholesteatomas was observed in 50% of the animals. In this animal model, cyclophosphamide, which has an anti- inflammatory and immunosuppressive action, did not seem to inhibit the formation of cholesteatomas.
Studies related to epithelial migration in the pathogenesis of acquired cholesteatomas were mostly related to the invagination of the squamous epithelium, through discontinuities of the basement membrane in the pars flaccida of the TM or to the migration of squamous cells from the external auditory canal and/or tympanic membrane, through perforations secondary to chemical necrosis.
Hueb [94] performed an experimental study on epithelial migration patterns and acquired cholesteatomas in chinchillas. The objective was to establish a new animal model, based on the pathogenesis of epithelial migration by intentional tympanic perforation, in the presence of a tissue irritant, with evaluation of the presence and remission of the effects of the inflammatory process. Through mechanical perforation of the tympanic membrane, a modified collagen membrane was introduced into the left middle ear, without occluding the eustachian tube or introducing infectious agents (15 animals and 3 subgroups). This membrane consisted of type B bovine collagen, succinyl chloride, and butyl isocyanate. After sacrifice (8, 10, and 12 weeks of life) and proper preparation and staining of the sections with hematoxylin and eosin, migration of squamous cells through the perforation and formation of cholesteatoma was observed in 53.5% of the animals. Migration occurred through tympanic perforation, collagen membrane, organized effusion, and granulation tissue, all functioning as a “facilitating bridges.” In some animals, there was closure of the tympanic perforation through the migration of squamous epithelium cells over the organized effusion. Cholesteatomas formed most frequently in the region of the anterior tympanic bulla, occasionally in the epitympanic region. The mean thickness of the squamous epithelium and keratin layer in the cholesteatoma region was greater than that observed for the anterior and posterior regions of the external auditory canal and tympanic membrane.
The demonstration of these data suggested that, in the region of cholesteatomas, the squamous epithelium proliferated and produced keratin more intensely than in its regions of origin, certainly stimulated by the irritating factor of the membrane components, in addition to not being subject to structural limitations and functional aspects of the regions of origin. Epithelial migration, occurring in areas of granulation tissue and/or inflammatory process, suggested a direct relationship between cause/stimulus and cholesteatoma. In addition to the importance of the inflammatory process in stimulating and “bridging” the migration of the epithelium, it was observed that the granulation tissue is responsible for bone erosion in otitis. It was also observed that the formation of granulation tissue occurred due to an intense cellular infiltration of the inflammatory fluid in the middle ear (immature granulation tissue) with secondary epithelialization of the mucosa in these areas (mature granulation tissue). These findings corroborate epithelial migration as being involved in the genesis of acquired cholesteatomas and also demonstrate the fundamental participation of the inflammatory fluid in the origin of the granulation tissue and its importance in the association between the inflammatory process/granulation tissue/cholesteatomas.
Etiopathogenesis
Ferlito [4] describes that three predisposing conditions would be necessary for the development of a cholesteatoma: (a) the meeting of two different epithelia in the middle ear cleft; (b) chronic destruction of the submucosal layer of the middle ear by infectious and inflammatory processes; and (c) the healing process or proliferation phase.
The mechanisms underlying the etiopathogenesis of acquired cholesteatoma remain a subject of competing hypotheses with, basically, six main theories (Table 42.3), which have generated controversy for over 100 years.
Congenital
The theory of congenital cholesteatomas (Fig. 42.6), according to which they would arise from nests of epithelial cells, which over the years would multiply until the formation of an epithelial tumor, was proposed by Korner and Virchow (apud Eggston 1959), in the nineteenth century, and supported by Cushing and McKenzie, as reported in Jackler [95], during the first half of the twentieth century.
Cawthorne [96] reported a series of nine temporal bone cholesteatomas associated with facial paralysis. In 1963, the same author reported a case of a young man affected by a cholesteatoma in the middle ear, behind an intact tympanic membrane, suggesting that it would be a form of cholesteatoma originating from embryonic remains. Delarcki and Clemis [29] presented 10 cases of patients with this type of cholesteatoma. The diagnostic criteria postulated by these authors were:
-
presence of intact tympanic membrane,
-
the absence of a history of otitis media, otorrhea, or otologic procedure.
Levenson [97] altered the definition by Clemis and Derlacki by admitting the congenital origin in some selected cases with a positive history for the presence of otitis. In fact, the relationship between congenital cholesteatoma and otitis media is controversial. Some hypotheses were formulated:
-
Congenital cholesteatoma develops regardless of whether there is otitis media or not. The finding by Derlacki and Clemis of the relationship between congenital cholesteatoma and the absence of a history of otitis media could be due to chance due to the small number of patients included in the study. There is no concrete evidence that previously reported cases of congenital cholesteatomas did not have a past history of otitis media in childhood.
-
The origin of congenital cholesteatoma is independent of otitis media, but when cholesteatomas are large or occupy a strategical location blocking the aeration of the middle ear or mastoid, they can cause otitis media.
-
Congenital cholesteatoma would be the result of a perforation of the tympanic membrane or otitis media. All patients with congenital cholesteatoma would have to present a previous history of otitis media or tympanic membrane perforation. If this hypothesis were true, the so-called congenital cholesteatoma would not be congenital but acquired. This hypothesis is the least likely.
Levenson et al. [97], studying 37 children with congenital cholesteatomas of the middle ear, suggested that they could have originated from an epithelioid formation (EF), identified in the latero-supero-anterior portion of the tympanic cavity. These embryonic structures are derived from the first branchial arch, at the junction of the eustachian tube and the middle ear. According to this theory, the epithelioid formation, which is always present during embryonic development, should regress from the 33rd week of gestation. The persistence of EF would shelter the niche that formed the congenital cholesteatoma. Karmody [98] performed a systematic analysis on children’s temporal bones in order to histologically document the origin of congenital cholesteatomas (CCs). Characteristic histological findings of this form of cholesteatoma were found in two patients. In both cases, the masses were asymptomatic, located in the anterosuperior quadrant of the tympanic cavity. Current studies relate the persistence of epidermoid formation with the development of congenital cholesteatomas [99,100,101].
Regarding other locations in the temporal bone, Gacek [102] proposed that cholesteatomas of the petrosal apex would be born from the foramen lacerum, which would be a favorable area for the persistence of epithelial remains, which would later trigger the formation of epidermoid cysts. Reeves [103] indicated previous trauma as a possible seeding element of ectopic epithelium in these situations. Souza and Costa [104], reviewing 30 cases of epidermoid lesions of the cerebellopontine angle, found no history of significant trauma in any of them.
Implantation
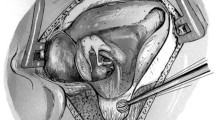
The introduction of stratified squamous epithelium in the middle ear can give rise to the so-called post-traumatic cholesteatoma. The triggering event of this condition can be classified as traumatic or iatrogenic. Indeed, Sheehy [33] reports the formation of small pearls of cholesteatomas on the tympanic membrane as one of the frequent complications of tympanoplasties that use the placement of the graft above the perforation (overlay). Regarding myringotomy with placement of a ventilation tube (VT), the cholesteatoma originating from this procedure is a relatively rare complication (Fig. 42.7). One of the hypotheses refers to the development of a cholesteatoma due to the implantation of epithelial cells in the middle ear, resulting from cell migration through the myringotomy, or from the displacement of a small TM flap to the middle ear during the procedure. Another hypothesis refers to the induction of cholesteatoma after VT removal, with a retracted tympanic membrane, due to the persistence of negative pressure in the middle ear.
McKennan and Chole [105] point out some singularities of post-traumatic cholesteatomas:
-
1.
Late onset—patients usually develop cholesteatomas years after the original trauma.
-
2.
Atypical development.
-
3.
Large proportions—as these patients usually have a negative history of otitis media, the mastoids are well pneumatized, which apparently would allow extensive growth of the cholesteatoma, before it manifests itself clinically.
-
4.
Open surgical techniques must be used in the treatment of these pathologies due to their size.
-
5.
There is an increased risk of CSF leaks.
Metaplasia
Wendt, in 1873 [106], was the first proponent of the theory related to epithelial metaplasia as a possible causative agent of cholesteatomas. His theory was based on the observation that the epithelium of the respiratory tree can undergo squamous metaplasia when exposed to chronic infection and trauma. This hypothesis received new impetus after some works carried out years later by Birrel [107] and Sadé [108] which pointed in this direction. Sadé [109] performed biopsies of the middle ear mucosa of children with otitis, finding islands of keratinized squamous epithelium. Chole and Frush [92] observed that vitamin A deprivation in rats led to keratinization of the tympanic mucosa.
Still, although Friedmann [2], Birrel (1958), Schechter [110], and others agree that the middle ear epithelium can undergo metaplastic transformation to stratified squamous epithelium, there is little evidence that it will become keratinized [111].
Van Blitterswijk [112] suggested the metaplastic origin of cholesteatomas from observations on the pattern of keratinocyte differentiation and expression of cytokeratins. However, Broekaert [113] and Vennix [49] related this differentiation and expression of “ectopic” cytokeratins to the hyperproliferative characteristic associated with the modulator effect of the middle ear mesenchyme, and not to a metaplastic process.
Migration
Epithelial migration, whether originating from the external auditory canal or the tympanic membrane, is considered by numerous investigators as the most common cause involved in the pathogenesis of acquired middle ear cholesteatomas [94]. This theory was initially postulated by Habermann [114] and Bezold [115] simultaneously, being based on a well-known pathological phenomenon, namely the epithelization suffered by sinuses and fistulous tracts. Thus, the cholesteatoma would be produced by the migration of squamous epithelium originating from the EAC into the middle ear cleft, which would arrive there through a breach in the TM (Figs. 42.8 and 42.9). These considerations are based on clinical observations of cholesteatomas in the presence of tympanic membrane retractions and/or perforations and on studies on the greater similarity between the patterns of cytokeratin found in cholesteatomas with those found in the external auditory canal and tympanic membrane [78, 112, [116, 117].
This would occur despite the migratory direction of the EAC epithelium in humans in the opposite direction to the middle ear, that is, from the malleus umbo to the external acoustic meatus. The factors responsible for the inversion of this migratory flow, as well as those that would lead to the appearance of a cholesteatoma and not just the pure and simple epithelialization of the middle ear, have not yet been well determined.
Data obtained from studies in humans are not suggestive of hyperproliferation of the normal epidermis in patients with cholesteatoma [118] or even of changes in the pattern of epithelial migration in patients with unilateral cholesteatoma (Mori arty et al., 1991). Following this line of reasoning, it is assumed that, for cells to migrate from the external auditory canal and/or tympanic membrane and epithelialize the middle ear, a stimulus and a bridge are needed. Additionally, for the development of cholesteatoma, changes in the tympanic membrane (perforation or retraction) would be prerequisites.
Hyperplasia
Initially, it was believed that migration of the epithelium to the middle ear would develop in the presence of an associated tympanic perforation. This is what basically happened following the so-called necrotizing acute otitis media, in which the cholesteatoma appeared years later. In these cases, the gates for epithelial invasion would be perforations known as “marginal,” that is, with the absence of tympanic remnant in a given segment. In “central” perforations (with a residual tympanic rim around 360 degrees from the perforation), the ring of fibrosis created around the perforation would impose an obstacle (not completely insuperable) to the migration of the epithelium.
Despite being ingenious, this theory is not able to justify the presence of cholesteatomas in other situations. The incidence of necrotizing otitis seen in daily practice does not equal the number of new cases of detected cholesteatomas. Furthermore, as Tos [119] argued well, very rarely necrotizing otitis is observed causing tympanic perforations in the region of Schrapnell’s membrane. The need for tympanic rupture as an obligatory prerequisite for the development of cholesteatomas began to be questioned and theories trying to prove the exact opposite began to be formulated.
The EAC skin close to the tympanic membrane is extremely active. Acanthosis and hyperkeratosis are particularly prevalent in the vicinity of the attic, with cellular activity occurring primarily in the basal cell layer, being intensified by middle ear infections [110]. Ruedi [120] demonstrated this fact by experimentally irritating the middle ear mucosa and stimulating basal cell hyperreactivity. As a result, he obtained the formation of streams of squamous cells toward the middle ear from the EAC and, subsequently, cholesteatoma.
Although the evidence for basal cell proliferation and subepithelial tissue invasion is unequivocal, it would require the basement membrane or lamina to either invaginate with the invading epithelial cells or undergo microruptures to allow the epithelial cells to proliferate into adjacent tissues and subsequently reconstruct itself. For Chole and Tingling [121], this last hypothesis would be the most likely. According to these authors, the basement membrane is made up of glycoproteins and collagen. To provoke its rupture, specific collagenases would be necessary. Apparently, not only inflammatory processes but also the epithelial cells themselves can secrete these enzymes. Loss of the basal lamina leads to the emergence of the contact guidance phenomenon, originally described by Giacometti [122] and demonstrated in the ear by Lim et al. [39]. Due to this phenomenon, the loss of the basement membrane would stimulate the basal cells to form pseudopodia toward the subepithelial tissue, which in turn would originate epithelial cones and finally cholesteatomas. However, there are studies that do not confirm the relationship between cholesteatoma expansion and distortions in the basal lamina [49]. The hyperproliferative phenotype is not homogeneous across all cholesteatomas. An increased expression of non-epidermal cytokeratins was observed in the peripheral portions of the cholesteatoma, in the region where there is direct contact with the inflammatory process of the middle ear mesenchyme. Lim [123] reported the presence of inflammatory cells in the mucous–cutaneous junction of the cholesteatoma, relating the invasive phenotype of the cholesteatoma to the inflammatory stroma, and not to characteristics inherent to the squamous epithelium. On the other hand, in the more central regions of the cholesteatoma matrix, the hyperproliferative condition is less marked. This indicates that, after the matrix develops, there is a tendency for the return of the original, non-hyperplastic phenotype.
Perforations in the tympanic membrane in dry, uninfected ears can remain for years without any sign of epidermal growth in the middle ear. Vennix [49] evaluated, through histological sections, the transition between the epidermis and the epithelium of the middle ear. In this study, smooth transitions were found between the two epithelia, suggesting the existence of stable mucosal–cutaneous junctions. The disorganization of these junctions, concomitant with hyperproliferation of keratinocytes and formation of inflammatory tissue, would be related to the pathogenesis of cholesteatomas. Thus, the development of an underlying inflammatory and/or infectious process would be necessary for the tympanic perforation to evolve into a middle ear cholesteatoma.
More studies are still needed in order to satisfactorily evaluate, at the molecular level, the middle ear cholesteatoma–mesenchyme interface.
Invagination
The relative frequency of cholesteatomas located in the attic and aditus ad antrum associated with defects in Shrapnel’s membrane stimulated interest in the emergence of a theory for its pathogenesis that could justify the preference of this pathology to occupy such regions.
Bezold [124] described a theory relating ET dysfunction to cholesteatoma formation, called the invagination theory. Malfunction of the tube function would generate a negative pressure inside the middle ear, effusion, and retraction of the TM, mainly in the pars flacida, which would result, after an infectious and inflammatory stimulus, in the development of cholesteatoma. Wolfman and Chole [76, 77] obtained experimental evidence of cholesteatomas secondary to tympanic retractions. When using guinea pigs whose auditory tubes had been blocked with electrocautery, they found cholesteatomas in 75% of the animals sacrificed 16 weeks after the initial insult. Cassano et al. [125] 52, in a study that included 40 ears of children with tympanic retractions not submitted to any treatment, observed, after 2 years of follow-up, the progression of severe retractions to cholesteatoma in 20% of the cases.
Sadé et al. [126], in a cohort involving 215 ears with tympanic membrane retractions, observed the incidence of cholesteatoma in only one ear with pars tensa retraction pocket (2%) and in only two ears with moderate and severe retraction in the pars flaccida (2%). This study, however, analyzed retractions of different degrees of severity and with very variable follow-up times. Some clinical studies, however, have failed to demonstrate this evolution accurately. This is probably due to the low incidence of this pathology and the difficulty in guaranteeing the follow-up of these patients for long periods. Thus, other alternatives designed to test this appealing hypothesis are needed.
The invagination theory can be conceptually summarized in the following steps (Figs. 42.10 and 42.11):
-
Middle ear negative pressure.
-
Retraction and invagination of the pars flaccida or segments of the pars tensa.
-
Stage of simple retractions (the diameter of its external opening remains larger than its bottom).
-
Stage of “bottle-shaped” retraction pockets (the diameter of the bottom of the pocket is wider than its opening).
-
Loss of the self-cleaning properties of the pocket.
-
Keratin accumulation, infection, and expansion.
Pathophysiology
Since 2008, we have been indirectly studying the pathogenesis of chronic otitis media by examining the contralateral ear (CLE). Our observations have systematically showed a high prevalence of alterations in the CLE in clinical (Costa et al.), histopathological (Rosito et al.), functional (Silveira Netto et al.), and radiological (Noschang et al.) studies. Moreover, our results demonstrate that the frequency of alterations in the CLE was even higher in patients with COM with cholesteatoma. All our findings point to the same direction or the disease’s tendency to affect both ears. Costa et al. stressed the importance of studying the diseased ears in pairs to understand the dynamic pathological process at presentation. Therefore, the maxim “you will be in my shoes tomorrow” was used by those authors to emphasize that the ears should be analyzed as an intrinsically related pair and not as an isolated unit. In doing so, frequently the first affected ear might predict the future status of the CLE. Regardless of the presence of cholesteatoma, the astute analysis of both ears may shed light into three key aspects of the disease process: where did it come from? (etiology), what is the current condition? (established pathology), and, more importantly, how fast and in which direction is the disease progressing? (natural history). Precise and critical analysis of both ears plays a key role in the prognostic assessment of each case, since the ear established with COM may predict the likely evolution of the CLE. One of our studies [127] changed our perspective, and the focus was redirected from the main ear (with cholesteatoma) to the CLE in an attempt to better understand the earlier steps of the condition. Only about one-third of the CLEs were considered normal. Moderate-to-severe TM retraction and cholesteatoma were undoubtedly the most prevalent pathological changes. Analyzing only the group of subjects with alterations in the CLE, we observed that 95.8% of them presented with retraction or signs of previous retraction (outside-in perforations), or progression of these retractions (cholesteatoma) in the CLE (Fig. 42.12).
Interestingly, our results showed that there was a strong association between growth patterns of cholesteatomas in the main ear and the location of TM retractions in the CLE (Fig. 42.12). Therefore, it seems plausible to infer that these retractions retrospectively represent the initial phases of cholesteatoma formation in the main ear.
The mechanisms responsible for progressive TM retraction leading to cholesteatoma formation are still debated. ET dysfunction resulting in impaired middle ear ventilation has been indicated as an important factor. Cauterization of the ET in gerbils resulted in retraction of the PF and cholesteatoma in 75% of the animals [76, 77]. Paradoxically, studies have shown that a patent ET can also result in middle ear alterations. This finding can be easily picked up during the clinical exam under magnification and the use of dynamic otoscopy (Toynbee and Valsalva maneuvers, swallowing and sniffing). In our experience, patulous ET as a main cause of middle ear cholesteatomais much more common than one could expect, but, still, receives very little attention from the literature. One interesting feature of this type of cholesteatoma is the association with well-developed mastoids suggesting that the middle ear has been aerated during childhood [128].
Middle ear inflammation, leading to changes in the mucosa and subepithelial space, also may explain the increased gas loss rate—Ars et al. [129]—(Fig. 42.13). Whatever the causative mechanism, negative pressure seems to play, at least, an initial role in TM retractions since it brings in closer contact the TM and middle ear structures (especially those projecting more laterally into the middle ear: neck of the malleus; long process of the incus and the dome of the promontory).
Besides the proximity to these structures, why do the retractions develop preferentially in the pars flaccida and the posterosuperior aspect of the pars tensa? As its name suggests, the pars flaccida possess higher elastic properties, allowing it to be drawn in more easily. It is composed of three layers, with epithelial layers similar to the pars tensa but a thicker and less organized connective tissue layer in-between. Furthermore, the pars flaccida is the only part of the TM that has been shown to contain mast cells. Mast cells are known to secrete a number of pro-inflammatory cytokines and proteinases. Mast cell migration into epithelium is seen in cholesteatoma but has not been observed in normal skin from any other anatomic site [95]. Additionally, the lateral aspect of the Prussak space is represented by the Shrapnel membrane (PF), and its medial and inferior aspects are formed respectively by the neck and the short process of the malleus. The superior limit is the fold of the lateral malleolar ligament, which also represents the floor of the lateral malleolar space; this ligament inserts laterally on the medial wall of the scutum. The anterior aspect of the Prussak space is bounded by a thin, membranous fold among the tympanic membrane and the anterior malleolar ligament fold, which inserts laterally on the tympanic membrane and medially on the neck and long process of the malleus. The posterior wall is represented by a large posterior pocket (the posterior pocket of von Tröltsch), which is the main route of ventilation. This posterior pocket is bounded laterally by the pars tensa and pars flaccida of the TM and medially by the posterior malleolar ligament fold. This posterior pocket develops in a posterior–inferior direction and opens at the most cranial portion of mesotympanum, so, in most people, ventilation of the Prussak space occurs through the communication with the mesotympanum (the only ventilation route that is separated from the epitympanic upper unit). This ventilation route is narrow, especially compared with the ventilation routes through tympanic isthmus, which aerates the upper epitympanic compartment and is wider. For these reasons, the possibility of anatomic reduction of the passage until the closing of the posterior pocket is plausible, especially the presence of thick and viscous secretions within the Prussak space that could cause a chronic sectorial dysventilation associated with a retraction of the Sharpnell membrane and its adhesion with the malleus neck [130].
Regarding the posterosuperior quadrant retraction of the TM, some extra considerations are needed. The tympanic annulus (that is absent in the pars flaccida) consists of a thickening of the TM periphery, and it is firmly inserted in the tympanic sulcus. In the PT, this combination between annulus and sulcus confers firmness and consistency to the region. However, as the tympanic annulus detaches superiorly from the sulcus, it goes toward the lateral process of the malleus, forming the anterior and posterior malleolar ligaments. Consequently, in the PF, there is no tympanic annulus. Thus, the TM is more malleable, filling the notch of Rivinus and being attached directly to the scutum. The tympanic sulcus, in its posterior region, is divided into two portions, separated, in most cases, by the emergence of the chorda tympani nerve. Inferior to the nerve, the sulcus maintains its characteristics identical to the inferior and anterior quadrants. It is well defined, with a depth between 0.5 and 0.9 mm, evident borders, and a stable surface. Above the nerve, the tympanic ring is no longer located within the sulcus but passes along the medial face of the posterior bone wall in 93% of the temporal bones studied by Paço et al. [131]. From that point on (the emergence of the chorda tympani nerve), the tympanic ring progressively becomes detached from the sulcus, which, in turn, progressively becomes shallower until it disappears.
Topographically, the emergence of the chorda tympani nerve marks the boundaries ofthe posterosuperior quadrant. These characteristics bring less tension on the TM in the PSquadrant compared to the other quadrants.
Another issue that we deem essential to highlight about the PS quadrant concerns the histology of the TM in this region. The middle layer of the PT (the lamina propria) consists of collagen types II and IV and is connected to the malleus handle and the tympanic bone. It consists of two layers, one radially oriented and the other circular in shape. The radial fibers (stratum radiatum) are attached to the manubrium of the malleus and run radially to the annulus. Meanwhile, the circular fibers (stratum circulare) are arranged concentrically with insertion into the manubrium. The latter are located medially in relation to the former [132, 133].
In turn, the PS quadrant presents some peculiarities compared to the other portions of the PT, which would give it a greater chance of atrophy and consequent retraction in this region in case of negative pressure in the middle ear. First, the region does not have a developed circular fibrous layer. In addition, its vascularization is more abundant, allowing greater penetration of collagenase-producing inflammatory cells, which have a more significant potential for destroying collagen fibers, which are already less dense by nature.
Besides the composition of the TM in these two segments, we postulate that the site of the obstruction is related to the creation of hypo-ventilated micro-spots within the middle ear cleft.
As we have mentioned before, regarding the posterosuperior quadrant of the PT a decreased middle ear pressure leads to a medial displacement of the TM and the handle of the malleus toward the dome of the promontory [134]. The medialization of the malleus handle, the prominence of the dome and subiculum of the promontory, and a less than firm attachment to the tympanic annulus reduce the distances and spaces in the PS quadrant creating a theoretically hypo-ventilated micro-spot isolated from the aeration routes. Furthermore, the presence of the ossicular chain (with attached tendons and mucosal folds) completes a scenario of multiples structures competing for space [82].
In relation to PF retraction, the tympanic isthmus seems to have a crucial role. We believe that, once created, these micro-spots may become stable through tight fibrous adhesions between the inner mucosal layer of the TM and the mucoperiosteum of the ossicles and middle ear (which may become the precursor of the future cholesteatoma perimatrix), regardless of the reestablishment of ME ventilation.
As pointed out by Jackler [95], although a middle ear vacuum could initiate TM retraction, it cannot credibly be the sustaining force for progressive growth of the cholesteatoma pouch. The epitympanum, aditus, and antrum become blocked early in the course of the disease and subsequently fill with mucous and/or inflammatory tissues; creation of a vacuum due to gas reabsorption is impossible under these circumstances. We still argue whether the TM retraction per se is enough to cause cholesteatoma formation. We believe that other factors that can disrupt the stability of the retraction are essential. Sudhoff and Tos [135], after observing the retraction of both the PT and the PF in some children, proposed a four-step concept for the pathogenesis of cholesteatoma that combines the retraction and proliferation theories: (i) the retraction pocket stage; (ii) proliferation of the retraction pocket, subdivided into cone formation and cone fusion; (iii) expansion of cholesteatoma; and (iv) bone resorption. On the other hand, Jackler et al. [136] proposed the theory of mucosal traction, which is based on the premise that the squamous pouch is drawn inward by the interaction of opposing motile surfaces of middle ear mucosa.
After observing thousands of tympanic retractions through powerful microscope lenses and with the use of endoscopes at various angles, we found that the existence of typical retraction pockets (the base larger than the external opening) in addition of being rare, were found almost exclusively in the region of the PF. Even so, through the serial follow-up of several patients, we were able to clearly observe the transition of many retractions (without the bottleneck appearance) into cholesteatomas (Fig. 42.14). Without exception, in all these cases the accumulation of epithelial debris and keratin was always associated with an inflammatory (infectious) process in frank activity. We conclude that TM retractions can become unstable through two mechanisms: either by spontaneous and natural accumulation of epithelium (true retraction pocket and natural accumulation), or, more commonly, by epithelial hyperproliferation triggered by an acute inflammatory process (hyperactive retraction and inflammation-hyperplasia) (Figs. 42.14 and 42.15).
Clinical example of possible mechanisms causing instability in a retracted TM: (1) spontaneous and natural accumulation of epithelium (true retraction pocket and natural accumulation), and (2) epithelial hyperproliferation triggered by an acute inflammatory process (hyperactive retraction and inflammation-hyperplasia)
We have followed patients with gross tympanic retractions who, after spending several years practically asymptomatic, suddenly present a drastic destabilization in their clinical picture with the appearance of drainage typical of cholesteatoma. It is clear under these circumstances that the catalyst for this change was an acute inflammatory trigger (Fig. 42.16).
Finally, through serial observations over time, we began also started to notice the presence in the external auditory canal of currents of epithelial desquamation that systematically march toward areas of previous TM retraction or perforation (Figs. 42.17 and 42.18). During the careful removal and under microscopy of these sheets of epithelial rests, we can notice that they systematically extend around the tympanic annulus and invade the middle ear and its posterior recesses. It is difficult to know if this epithelial migratory flow is made in one direction or the other (EAC-middle ear or middle ear-EAC), but it seems very plausible to us that it is toward the middle ear in a potential attempt to repair a damage inflicted on the tympanic membrane (perforation or retraction).
In short, the existence of congenital and implantation cholesteatomas is indisputable. Regarding the mechanism of pathogenesis of other acquired cholesteatomas, the only point of convergence in all theories is that TM retractions were almost universally implied in the first stages of its development. We do not exclude the role of cellular hyperplasia or even epithelial migration in the process, but our thousands of observations and experiments endorse the essential role of TM retraction at least in the earlier phases of cholesteatoma pathogenesis. In the intimacy of this tiny nutshell space, a whole universe of biological events is set into motion frequently, driving the retraction to a self-determining outcome. It also seems clear that the transition from a previously stable retraction to an active cholesteatoma always requires the presence of an acute inflammatory trigger.
Cholesteatoma Growth Pathways
Most cholesteatomas assume typical growth patterns, according to their site of origin and related anatomical structures and, when in expansion, they follow sinuous paths, between mucous folds, ligaments, and ossicles. Migration routes of cholesteatomas tend to follow vestigial planes created in embryogenesis. It is not uncommon for multiple cholesteatoma sacs to occur in the same ear, involving two, even three growth routes simultaneously. While the vast majority of cholesteatomas follow one or more routes, others assume a different growth pattern. This probably occurs due to anatomical variations of the mucous folds and ligaments, which tend to channel and guide the growth of cholesteatomas.
Jackler [95] proposed a widely accepted classification with three main routes followed by the disease:
-
(a)
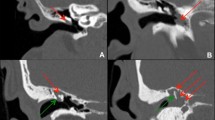
Posterior epitympanic (PEC): this is the most common route. It starts from an invagination of Shrapnell’s membrane penetrating posteriorly through Prussak’s space, following the embryological path of the saccule medius. This route passes through the superior incundal space, lateral to the body of the incus, crossing the aditus ad antrum, and entering the mastoid. These cholesteatomas can reach the mesotympanum by dipping through the floor of Prussak’s space into the posterior space of Von Trölscht (Fig. 42.19).
-
(b)
Posterior mesotympanic (PMC): the posterosuperior portion of the pars tensa retracts toward the mesotympanum, forming a sac extending to the antrum via the posterior tympanic isthmus and inferior incundal space. The surgical inaccessibility of the posterior tympanic recess makes it difficult to completely remove cholesteatomas located in this area. Unlike the posterior epitympanic pathway, the extension of these cholesteatomas to the mastoid passes medial to the malleus and incus, following the embryological course of the posterior and superior pouchs (Fig. 42.20).
-
(c)
Anterior epitympanic pathway (AEC): the anterior epitympanic pathway arises from a retraction of the tympanic membrane anterior to the malleus head, following the path of the saccule anticus. Cholesteatomas in this area may go unnoticed during surgical exploration if the region anterior to the malleus head is not adequately explored. Since the lower limit of the epitympanum is related to the horizontal portion of the facial nerve (FN) and the geniculate ganglion, facial nerve dysfunction may occur in these lesions. Anteroinferior extension into the supratubal recess is common and the mesotympanum is reached via the anterior space of Von Tröltsch (Fig. 42.21).
Depending on the route followed by the cholesteatoma, the associated hearing loss will be early or late. The most commonly encountered growth patterns are summarized in Table 42.4.
A few years ago, with the aim of making an inventory of our cases we carried out a cross-sectional comparative study of 638 ears with middle ear cholesteatoma and no history of ear surgery treated at our institution searching for the prevalence of each traditional growth patterns (flaccida or PEC; tensa or PMC; anterior epitympanic). In our study, we observed a similar prevalence of 34.3% for PEC and 33.8% for PMC. These findings were in agreement with those of a previous report that found a prevalence of 45% for pars tensa and 41% for attic cholesteatomas [138].
However, 124 of the cholesteatomas (30.0%) could not be classified as PEC, PMC, or anterior epitympanic. We observed that, in 57 ears (13.8%), both the pars flaccida and the pars tensa were involved, so we termed them 2-route cholesteatomas. Finally, in 67 ears (16.2%), no precise growth pattern could be identified by videotoscopy. We classified these cholesteatomas as undetermined [139]. In view of these findings, we have changed our classification, currently including the three traditional routes described by Jackler [137] incorporating two new elements: (1) two routes; (2) open or undetermined. Figure 42.22 shows our classification and the prevalence of each route in our series.
Regarding age groups, PEC was more prevalent in adults, whereas AEC and PMC were more prevalent in children (in fact, AEC was exclusively found in this age group). The prevalence of two routes and undetermined cholesteatoma was similar between both age groups [139].
When we analyze the cholesteatomas which predominantly involved the pars tensa, the classification among authors is a bit different. While Jackler considered only PMC as originated from the posterior sector of the pars tensa, Tos divided the pars tensa cholesteatoma into two variants:
-
Sinus (which is the equivalent to PMC);
-
Tensa (open according to our classification).
We agree with the posterior mesotympanic concept because it refers to a typical route of extension in which, in contrast to posterior epitympanic, the mastoid progression of cholesteatoma typically passes medial to the malleus and incus, and the sinus tympani and facial recess are generally involved. What is the clinical relevance of this progression? First, it grows over the most delicate part of the ossicular chain (long process of incus and incudostapedial joint) causing early conductive hearing loss. Second, the extension for the posterior recesses makes the complete surgical removal a very laborious and complicated task.
When the entire area of the middle ear is affected by the cholesteatoma, however, it is difficult to determine precisely whether the disease is the result of a complete pars tensa atelectasis or whether it is a posterior mesotympanic or even a posterior epitympanic cholesteatoma that has advanced to other compartments. For such reasons, we preferred to classify unknown cases as undetermined or open.
One may ask: What’s the importance of tracking the routes of middle ear cholesteatomas formation? We firmly believe that the correct knowledge of the paths followed by cholesteatomas extremely helpful in the pathophysiological understanding of the disease and also during the preoperative planning and selection of most appropriated surgical approach.
One last issue regarding the classification of cholesteatomas is that their sites of origin and progression routes that remain to be addressed are regarding the AEC. The classification of cholesteatomas into congenital and acquired is useful since it separates two types of cholesteatoma with distinct pathogenesis and biological behavior. However, sometimes it is difficult to clinically determine whether the cholesteatoma is congenital or acquired. First, congenital cholesteatoma is rare, accounting for approximately 4% of childhood cholesteatomas and 2–5% of all cholesteatomas [140]. Second, the classic definition by Derlacki and Clemis [29] of congenital cholesteatomas as a pearly mass medial to an intact tympanic membrane and no history of otorrhea, tympanic membrane perforation, or previous otologic procedures have been questioned mainly because middle ear infection in children is almost universal [97]. In our study, we found a prevalence of 1.9% of AEC and all of them in children. Furthermore, all the contralateral ears were healthy [139]. For these reasons, we hypothesize that the few EAC in our series could be, indeed, not acquired but congenital (even when associated with retraction and drainage). Corroborating this hypothesis, it is well known that the most prevalent location of congenital cholesteatomas is the anterosuperior quadrant of the mesotympanum [141] where they arise from epidermoid residues of the fetal middle ear [142]. The growing of a cholesteatoma in this specific location may lead to obliteration of the anterior segment of the tympanic isthmus (which in healthy ears is an open structure [130]) followed by pars flaccida retraction.
Bone Erosion
As we have pointed out before, ossicular erosion is one of the most frequent consequences of the progression of cholesteatoma, and the pattern and impact of this damage depend on its origin and the pathways in which cholesteatoma develops. Partial or total ossicular erosion is observed in approximately 80% of patients with cholesteatoma [143]. The two main factors probably involved in cholesteatoma-related ossicular erosion are chronic inflammation, which leads to cytokine release and osteoclast activation, and pressure necrosis, caused by the cholesteatoma mass [144].
While the PEC arises in the pars flaccida and progresses laterally to the head of the malleus and body of incus, the PMC grows directly over the fragile long process of the incus, erodes serially the incudostapedial joint, the suprastructure of the stapes reaching the footplate and oval window. These distinctive growth patterns can lead to different levels of hearing impairments, even including inner ear damage and sensorineural loss.
In order to verify whether the hearing impairment caused by PEC differed from that caused by PMC, we conducted a cross-sectional study including 264 ears of patients with cholesteatoma, who had not been subjected to ear surgery [139]. When the air–bone gaps (ABG) were compared, the mesotympanic group had greater thresholds at 500, 2000 Hz, and a greater pure-tone average (p = 0.003, p = 0.03, and p = 0.02, respectively). PMC showed greater air–bone gaps thresholds at the speech frequencies than posterior epitympanic cholesteatoma. Moreover, the two growth patterns were very similar with regard to all other audiometric parameters analyzed in this study [139].
Our results agree with those of several other studies that showed that incus was the most affected ossicle [143,144,145]. This may be due to the incus mass, its prominent bone marrow, and mainly, due to exposure and fragility of the long process. Martins et al. [146] showed that the erosion of each ossicle contributes to the increase in ABG in a graded and independent manner. The same authors also showed that the status of incus has the most statistically significant association with ABG [146]. Maresh et al. [147] compared primary and secondary acquired cholesteatomas (according to the authors, attic and mesotympanic cholesteatomas, respectively) and found that malleus erosion is more prevalent in the former and stapes erosion in the latter. The prevalence of incus erosion did not differ between the groups. Our results, however, showed a greater prevalence of incus erosion in MPC. This was expected since the PMC cholesteatoma grows just over the incus and its erosion can also explain the greater ABG in this group of patients. We must consider, however, that the ABG differences between PEC and PMC could be underestimated since sometimes the cholesteatoma cyst may itself serves as a bridge to transmit the soundwave from the remnants of the TM to the footplate the so-called columellar effect of the cholesteatoma a phenomenon that could underestimate the potential size of the ABG, especially when the disease is located in the posterior mesotympanum.
Bone absorption is stimulated by a variety of factors, including inflammation, local pressure, specific cytokeratin, and keratin [8, 9]. The enzymatic concept, in which enzymes of epithelial origin are considered responsible for bone destruction, was defined by Abramson [148,149,150], who demonstrated the presence of collagenases and hydrolases in cholesteatomas, a hypothesis later confirmed by Thompsen [151]. Ken and Gordon (1972), indicated that collagenase may be involved in bone resorption, but not as an isolated factor. Ferlito et al. [4] suggested that the destructive property of cholesteatomas, bone erosion, is caused by collagenase production by components of squamous and fibrous epithelial tissue. It has not yet been well demonstrated whether mineralized bone can be absorbed by collagenase. To the hypothesis of bone resorption by biochemical action, exclusively exercised by collagenolytic enzymes, other agents were later incorporated, such as tumor necrosis factor (TNF), interleukins (IL-1 α), and prostaglandins (PGE2) [93, 152, 153].
Imai et al.[154] found that a significantly larger number of osteoclasts were observed on the eroded bone adjacent to cholesteatomas than in unaffected areas, and that fibroblasts in the cholesteatoma perimatrix expressed RANKL. Also, the concentrations of interleukin-1β, interleukin-6, tumor necrosis factor α, and prostaglandin E2 were increased in cholesteatomas compared with normal skin. Furthermore, interleukin-1β was expressed in infiltrating inflammatory cells in the cholesteatoma perimatrix [154].
Sangal et al. [155] explored the hypothesis that genetic predisposition for inflammation can influence the development and severity of cholesteatoma. Patients with cholesteatoma exhibited a homozygous CARD8 C10X mutation status 1.95 times higher (29.41% vs. 9.52%) than control population. Although this does not prove that homozygous CARD8 mutation status is causal, it can suggest it as a predisposing factor for cholesteatoma. This result remains an intriguing finding that warrants further investigation. In addition, the potential effect of these mutations on the progression of cholesteatoma (considered by those authors as the severity of bone erosion) was also studied. Mutant CARD8 genotypes exhibited significantly greater levels of bone erosion compared to patients without CARD8 mutations. These results suggest that the host inflammatory state exerts a significant influence on the progression of cholesteatoma bone destruction [155].
Bacteriology
Inherent to the formation and progression of cholesteatoma is the colonization by bacteria within the middle ear and the formation of biofilm that aids in the persistence of inflammation. Numerous pathogens, including Gram-positive, Gram-negative, and various fungal elements, have been identified in the middle ear in association with cholesteatoma tissues. Significant difficulty exists when attempting to treat these elements as delivery of systemic antibiotics is hampered by the lack of blood flow to the lesion and the fact that topical antibiotics may not penetrate as deep as necessary to eradicate pathogens that may be the root cause of the general inflammatory stimulus that leads to the progression of cholesteatomas. Advanced infection can progress to significant complications such as cavernous sinus thrombosis, meningitis, brain abscess, and mastoiditis.
The microbial flora of chronic otitis media is different from that found in acute otitis media. The bacterial agent, which causes the initial process of acute otitis media with perforation of the tympanic membrane and otorrhea, is generally not the same as that isolated in the chronic infection of the middle ear and mastoid that accompanies cholesteatoma. Thus, the recommended antibiotic therapy for acute otitis media may not be effective in cases of chronic suppurative otitis media with cholesteatoma.
The aerobic bacteria most commonly isolated in cases of chronic suppurative otitis media with cholesteatoma are P. aeruginosa, S. aureus, Proteus sp., K. pneumoniae, and E. coli. Among the anaerobic organisms found are Bacteroides, Peptostreptococcus, Clostridium, and Bacteroides sp. [156]. In many cases, mixed floras are found. Kenna and Bluestone [157] evaluated the bacteriology of CCOM in 36 children. Pseudomonas aeruginosa was the most common bacteria, present as the sole pathogen in 31% of cases and in 67% of polymicrobial cultures. Most recently, Fujikawa et al. [158] found a large difference in the colonization of the COM with and without cholesteatoma, also suggesting that S. aureus infection is involved in cholesteatoma progression.
Acquired cholesteatoma frequently becomes chronically infected, and the biofilm colonization of the middle ear seems to be responsible for resistance to topical and systemic antimicrobial agents [68]. Increased bacterial retaining and biofilm formation are histologically found in association with massive entrapment of keratin and keratinocyte proliferation resulting in an expanding matrix with osteoclasts recruitment and bone erosion [68]. Galli et al. [159] found a high rate of bacterial biofilm evidence (81.3%) in cholesteatoma, even though the causal relationship remains unclear. The authors hypothesize the keratinized matrix of cholesteatoma and the destruction of the ciliated epithelium of the respiratory tract may represent an ideal substrate for biofilm colonization and survival.
In clinical practice, cultures of middle ear secretions are rarely performed in uncomplicated cases, as the treatment of CCOM is essentially surgical. Identification of the germ is only clinically important in cases with complications, such as abscesses or meningitis. However, antibiotic therapy is useful as an adjuvant treatment, both preoperatively and perioperatively, in order to reduce the risks of postoperative infection.
Clinical Picture and Preoperative Workup
A careful analysis of the symptoms and signs allows physicians to determine the need for surgery, its urgency, and the anticipated results. We always discuss with our fellows, residents, and medical students that the art of taking a good medical history can never be overemphasized. Especially when dealing with a disease that has the possibility to recur as one of its trademarks (like the cholesteatoma). It is mandatory to conduct a careful interview with the patient and/or his family focusing three key periods along the timeline:
-
1.
Today (the present).
-
2.
Yesterday (the past).
-
3.
Tomorrow (the future).
The ideal preoperative workup encompasses the art of history taking, a complete ENT physical examination and important ancillary tests (audiology, imaging, etc.). The workup should guide the physician to three distinct temporal moments equally important (Fig. 42.23):
-
1.
Today: The diagnosis.
-
2.
Yesterday: The etiology.
-
3.
Tomorrow: The prognosis.
This information will permit the clinician to design a rational therapeutic plan which will make possible:
-
1.
Today: To treat established disease.
-
2.
Yesterday: To manage etiologic factors trying to avoid recurrences.
-
3.
Tomorrow: To abort the natural history and future complications.
Signs and Symptons
General Information
In 2016, we carried out a study aimed to determine the prevalence of cholesteatoma in patients with chronic otitis media and describe clinical, audiological, and surgical characteristics. For such, we designed a cross-sectional and prospective cohort analysis including 1710 patients with chronic otitis media, treated between August 2000 and June 2015, without prior surgery. Detailed clinical history, videotoscopy, and audiometry were performed, in addition to review of medical records to search for surgical data. Of the 1710 patients with COM evaluated in the study, cholesteatoma was present in 419 (24.5%). The mean age of patients was 34.49, standard deviation (SD) 19.8, and 53.5% were female. White patients accounted for 86.5% of the study population and 5.9% were Black. Adults corresponded to 63.8%. Cholesteatoma was identified in the right ear was in 234 patients (55.8%). The prevalence of cleft palatects in this population was 4.3%.
In the contralateral ear evaluation, only 150 of them (36.1%) were normal and cholesteatoma was identified in 71 (17.1%). The duration of symptoms was longer in patients with changes in the contralateral ear than in those with normal contralateral ear (mean of 14.99 and 11.69, respectively; p = 0.007). There was no difference in the prevalence of cholesteatoma in the contralateral ear between children and adults (p = 0.20) and between patients with and without palate malformation (p = 0.19).
The main complaints of patients at the time of the first evaluation in this service are shown in Fig. 42.24. Hypoacusis, with or without otorrhea, was the main complaint of 84.4% of the study population, and otorrhea was observed in 87%. There were no differences regarding the main complaint when we compared cholesteatomas classified by the route of formation (p = 0.27).
Otorrhea
This is the most common manifestation of chronic otitis media, especially cholesteatoma. It is important to ask about its duration, frequency, character, and bad smell. Long-lasting, constant, purulent, or bloody malodorous otorrhea is always associated with significant disease in the middle ear and mastoid. Mucoid discharge of short duration, on-and-off drainage, may denote a simple perforation with no major pathologic findings. It is not infrequent, though, that some cholesteatomas may evolve silently without typical drainage.
Hearing Loss
This second most common complaint is closely related to the amount of damage in the middle ear; thus, in the absence of cholesteatoma, a conductive loss of 20 dB usually indicates integrity of the ossicular chain. Erosion of the ossicles (particularly the long process of the incus) or fixation raises the hearing loss to 30 dB or more. Interestingly, some cholesteatomas can destroy the ossicular chain but still preserve good hearing. In these situations, the cholesteatoma can transmit the sound from the external ear to the oval window in a so-called columellar effect (Fig. 42.25).
Another perplexing situation is the so-called myringostapediopexy (which is a pre-cholesteatoma finding defined by Costa et al. as a retraction limited to the posterosuperior region, in which there is erosion of the long process of the incus and fixation of the affected TM to the head of the stapes). A study conducted by our group found that 53% of the patients with this condition presented with an air–bone gap ≤25 dB at all frequencies. This finding suggests that such condition may mimic a surgical type III tympanoplasty in which the TM is repaired and advanced to the head of the stapes (Fig. 42.26a, b).
In the group of patients included in our cohort study [139], 92.41% underwent audiometry. Regarding bone conduction and air conduction thresholds, the tritonal average was 17.08 dB (SD 16.16 dB) and 46.35 db (SD 22.34 dB), respectively. Regarding gap sizes, the tritonal average is 29.84 db (SD 13.61 dB). The size of the air–bone (grouped in intervals of 20/20 dB) in tritonal average is shown in Fig. 42.27. There was no correlation between gap size and age of the patient at the time of evaluation (R = 0.03; p = 0.55) or duration of symptoms (R = 0.08; p = 0.13).
In another study of our group, we aimed to investigate the potential differences in the hearing impairment caused by PEC and PMC [139]. Our rationale was based on the assumption that while the PEC arises in the pars flaccida and progresses over the head of the malleus and body of incus, the PMC develops on the fragile incus long process, erodes the incudostapedialjoint, and may compromise the oval window niche more easily. These distinct growth patterns could lead to different hearing impairments, even including sensorineural damage.
A total of 264 ears were analyzed in this study. Patient distribution according to the cholesteatoma growth pattern was similar: 50.4% had PEC and 49.6% had PMC.
The prevalence of BC PTA thresholds above 25 dB was low and similar between the two groups. According to the AC PTA, only 20% of the ears had normal hearing. The majority of them had at least moderate hearing loss. No difference was found between the PEC and PMC groups for degree of hearing loss, and the means of the AC PTA and the medians of the BC PTA were similar between the PEC and PMC groups. The results of ABG PTA, however, showed that the thresholds were greater in the PMC group (Figs.42.28 and 42.29).
An issue that has recently gained attention is additional sensorineural hearing loss due to chronic otitis media. While the conductive loss can be minimized through surgery, sensorineural hearing loss constitutes a permanent after effect, attenuated only through the use of a hearing aid. However, a few groups have reported a decrease in sensorineural function in these patients as well. Some years ago, we evaluated the occurrence of sensorineural hearing loss in patients with this disease. We reviewed the files of patients with unilateral chronic otitis media. One hundred and fifty patients met the inclusion criteria: normal otoscopy and normal hearing in the contralateral ear. The main outcome measure were bone conduction threshold averages calculated for frequencies of 500, 1000, 2000, 3000, and 4000 Hz, with comparison between the normal ear and the ear with chronic otitis media. Thresholds were examined separately for each frequency. The bone conduction threshold averages for the normal side were lower than those for the ear with chronic otitis media. The threshold shift was statistically significant for each frequency (p < 0.0001, Student’s t test). There were differences between the groups when analyzed for age (500 and 1000 Hz) or the presence of cholesteatoma (1000 Hz). This study shows that chronic otitis media is associated with a decrease in cochlear function. The association was even greater when cholesteatoma was present. A variety of studies show the connection between COM and cochlear damages. Paparella et al. [160, 161], English et al. [162], Dumich and Harner [163], and Walby et al. [164] have demonstrated an association of the COM with cochlear damage, pointing to the round window as the structure responsible for the transmission of the pathological process to the labyrinth (Fig. 42.30). The anatomy and localization of the round and oval window niches predispose to pathology in the presence of otitis media. This way, they could behave as true entrance doors for toxins coming from the ME to arrive in the inner ear, configuring a legitimism interaction between these two compartments.
Vertigo
Continuous true vertigo in the presence of a cholesteatoma represents a labyrinthine irritation or a fistula in a semicircular canal until proven otherwise.
Labyrinthine fistula (LF) is one of the most common complications associated with cholesteatoma and represents an erosive loss of the endochondral bone overlying the labyrinth. The loss of the overlying protective bone allows pressure or mass-induced motion of the underlying endosteum, perilymph, and by contiguity, the endolymphatic compartment, evoking vestibular and sometimes auditory symptoms. The etiology of cholesteatoma-induced LF in some patients is still poorly understood. Cholesteatoma growth pattern, age of the patient, and time of the disease are possible factors that influence the site of bone erosion and aggressiveness of the disease, which may lead to development of LF. Preoperative detection of LF is of great importance to ear surgeons. LF may not be associated with any specific symptom or sensorineural hearing loss (SHL) before surgery; nevertheless, proper management is essential to prevent poor outcome. [165].
In 2018, our group conducted a study to evaluate patients with cholesteatoma in order to identify possible risk factors or clinical findings associated with labyrinthine fistula [166]. Secondary objectives were to determine the prevalence of labyrinthine fistula in the study cohort to analyze the role of computed tomography and to describe the hearing results after surgery of those patients where a PF was identified. For such, we carried out a retrospective cohort study including patients with an acquired middle ear cholesteatoma in at least one ear with no prior surgery, who underwent audiometry and tomographic examination of the ears or surgery at our institution. As a result, we analyzed a total of 333 patients, of which 9 (2.7%) had labyrinthine fistula in the lateral semicircular canal (LSC) (Fig. 42.31). As in our service, most patients with LF are routinely submitted to open tympanomastoidectomy, so, whenever identified during the surgery, they were classified according to the degree of labyrinth involvement, as described in Table 42.5 (modified from [167]). We only considered as LF the Stages II or III.
In eight patients, the fistula was first identified on image studies and confirmed at surgery (Fig. 42.31).
Interestingly, in patients with posterior epitympanic and two-route cholesteatomas, the prevalence was 5.0%; and in cases with remaining cholesteatoma growth patterns, the prevalence was 0.6% (p = 0.16). We think that this big difference can be explained by the fact that whenever the attic is primarily involved in the growth of a cholesteatoma, its natural expansion is toward the antrum, where it comes in contact with the most projecting limb of the posterior labyrinth or the LSC. The prevalence ratio for labyrinthine fistula between patients who reported vertigo before the surgery was twice as many as those who did not report.
CT is important in the preoperative identification of LF. In previous studies, CT showed 50% sensitivity in the diagnosis of LF. However, more recent studies have reported sensitivity between 85% and 100%, owing to high-resolution CT and thinner cuts (0.5 mm slices) [168,169,170].
CT is also able to predict the presence of a membranous fistula versus a bone fistula with sensitivity of 66% and specificity of 71% [170].
In our study population [166], high-resolution CT identified LF in all patients, which was confirmed at surgery. The high sensitivity and specificity in our study can be explained by the selection criteria, since we only included patients with LF Type II or Type III with evident bone erosion.
Currently, it has been our policy that a CT scan is mandatory for all patients prior to cholesteatoma surgery for the analyses of anatomic landmarks, disease extension, and mastoid pneumatization, and subsequently, selection of the optimal surgical technique.
Paralysis or Paresis of the Facial Nerve
Currently, paralysis or even paresis of the facial nerve (FN) are uncommon complications of cholesteatoma with an incidence ranging from 1% to 3.4% [171,172,173] but whenever present (partial or complete) it demands immediate attention and prompt treatment since any delay can lead to irreversible tragic sequelae and, also, other differential diagnosis must also be considered (tumor or an acute aggressive infection).
As pointed out by Psillas and Constantinidis [174], FP to cholesteatoma may have several causes and mechanisms injuring the nerve alone or in association: osteitis, bony erosion, direct pressure, or compression resulting from edema and inflammation of the nerve caused by bacteria or neurotoxic substances, secreted from the cholesteatoma matrix.
It has been shown that the facial nerve fills 35–65% of the fallopian canal (Fig. 42.32); the remaining portion is filled with extra neural blood vessels and connective tissue, without leaving any empty space [175]; thus, edema secondary to infection can easily affect the neural transmission.
Temporal bone sections with a normal anatomy. The facial nerve is found inside the fallopian canal (arrows) which is completely surrounded by bone. (a) Facial nerve inside the fallopian canal (b) Facial nerve and its relationship with the cochleariform process (c) Facial nerve and its relationship with the incus (d) Facial nerve in a view of the mesotympanum
It is very plausible that the structure of the fallopian canal could also play an important role in facilitating earlier neural damage. Thus, channels that present marked dehiscence are likely to render the nerve more vulnerable exposing the neural tunnel to toxins and potential compression from its periphery (Fig. 42.33).
Direct pressure on the facial nerve due to cholesteatoma has also been reported; however, FP was expected only after blockage of more than 50% of facial nerve fibers. In slowly evolving facial palsy, the most likely etiology is erosion of the fallopian canal with compression of the facial nerve (Fig. 42.34) (Psillas and Constantinidis) [174].
(a) A cholesteatoma occupying the posterior recess of the mesotympanum destroying the incus and suprastructure of the stapes but set apart from the facial nerve by a dense layer of bone (red dashed arrows). (b) A massive cholesteatoma eroding the facial canal, but the nerve is not yet compressed (red dashed arrows). (c) A massive cholesteatoma eroding the facial canal and compressing the facial nerve (red dashed arrows).
Pain
Pain is not a common complaint, so, when present, it should be approached carefully. An associated external otitis, an expanding mass of cholesteatoma, or a suppurative complication must be ruled out.
Polyps
The finding of a polyp in the external auditory canal (EAC) is often thought to be a manifestation of inflammatory middle ear disease. Also known as aural polyp (AP), it is usually characterized as a soft to rubbery reddish mass (1) within the external auditory canal, therefore, lateral to the tympanic membrane (Figs. 42.30, 42.35, and 42.36).
The type of chronic otitis media (COM) that first comes to mind for most otologists as the underlying cause for AP is cholesteatoma. Previous studies in the last decades estimated a great variability of cholesteatoma as the final diagnosis for polyps, with prevalence ranging from 25% to 88% [176].
We have carried out a cross-sectional study with patients with unilateral or bilateral aural polyps. A total of 2432 patients were evaluated, and 133 (5.4%) showed a polyp in the external auditory canal. CCOM (58%) and NCCOM (28.3%) were responsible for 86.3% of all polyps evaluated, and the majority of diagnoses were established through surgery (76.5%). Furthermore, we have found that symptoms associated with polyps and their aspect do not reveal the most probable etiologies making imaging exams, biopsy, and surgery necessary steps in aural polyp investigation. Concerning MEC, the frequency of the routes of formation was: 12 PEC, 12 PMC, 7 two-routes (both the pars flaccida and the pars tensa are involved), and 10 undetermined. Another case was classified as congenital, and five patients had not undergone surgery.
Otologic Evaluation
Careful inspection of the ear includes at least an examination under magnification with the surgical microscope. An otoendoscope can be helpful in reaching hidden recesses of the temporal bone in follow-up of an open cavity. This tool is of particular importance in cases presenting a pars flaccida cholesteatoma. Sometimes, even the otomicroscopy may overlook a silent erosion of the scutum due to the straight field of vision of the microscope. The endoscope penetrating deeper in the ear canal will never miss a finding like this (Fig. 42.37a, b).
The whole 360° of the TM should be inspected, but special care is dedicated to the pars flaccida, the area near the attic, and the posterosuperior quadrant. Besides discharge (discussed earlier), one should note the pattern of perforation and the status of mucosa in the middle ear. A test for fistula should be used when vertigo or dizziness is present. A patch test and a test of the ET, with rare exceptions, are excluded from the authors’ workup.
During the otomicroscopy, the canal should be meticulously cleansed all secretions, debris, and cerume. Special attention should be paid to the pars flacida area where not rarely small perforations may be covered by dried crusts. In this regard, one of the authors (SSC) has an aphorism which incites to “never trust in an attic cerume” (Figs. 42.38 and 42.39a, b).
Hearing Evaluation
Careful audiometric tests should be performed routinely. These findings must be compared with those of the basic tests with tuning forks performed by the otologist. The audiometric battery should include pure-tone bone/air conduction thresholds, speech-reception levels, and speech discrimination scores. Results of these more sophisticated tests must always coincide with results of the tuning fork test. Because the authors rarely order a test of ET function in their routine, tympanometry and tests of acoustic reflex are performed only in the presence of an intact TM. Erosion of the ossicles (particularly the long process of the incus) or fixation raises the hearing loss to 30 dB or more. Interestingly, some cholesteatomas can destroy the ossicular chain but still preserve good hearing. In these situations, the cholesteatoma can transmit the sound from the external ear to the oval window in a so-called columellar effect (Fig. 42.25).
Radiologic Evaluation
The imaging evaluation of patients with COM is not always necessary and should be adapted according to clinical findings. In the authors’ routine, they dispense with further investigations in patients without cholesteatoma and who present with dry, central perforation and good hearing (small air–bone gap).
A CT scan is mandatory for all with marginal perforations, retraction pockets, an air–bone gap of >25 dB, and all cholesteatomas, regardless its size. Of course, also there’s no doubt about ordering CT when we are facing large cholesteatomas, gross bony erosions, exuberant granulation tissue filling the external canal and preventing visualization of structures within the middle ear, chronic silent otitis media, unilateral marked hearing loss, suspicion of malignancy of the temporal bone, or presence of complications.
For cholesteatoma evaluation and planning of the surgery, including the surgical technique, the authors developed a checklist for the CT scan, which will be analyzed both in the axial and coronal plans. Also, the plane of Pöschl (in the same plane as the superior semicircular canal) must be available for the surgeon in the planning. Our checklist is detailed in Table 42.6.
Our group published an interesting case report, which emphasizes the role of the CT in the surgical planning [177]. Figure 42.40 shows a typical posterior mesotympanic cholesteatoma, with an air–bone gap compatible with the disease. The authors firmly believe that, at the present time, there is no reason to perform cholesteatoma surgery without ordering a CT scan. This way the images of the same patient (Fig. 42.41) show an ectopic carotid artery, which leads to an anatomical obstruction of the eustachian tube. We can conclude that there was a clear relationship between the displacement of the carotid artery, the obstruction of the ET, middle ear gas deprivation, and the further retraction of the TM leading to cholesteatoma formation. It appears unequivocal that the sealing of the protympanum by the artery led to TM invagination, keratin accumulation, and infection.
In conclusion, through the analysis of one single case, two important concepts emerge:
-
1.
ET dysfunction may play a decisive role in the pathogenesis of COM, at least in the earlier phases of the process;
-
2.
Temporal bone CT scan is affirmed as an extremely important step in surgical planning. It has the ability to show the extent of the disease, to influence the surgical technique employed, and it can help an informed surgeon anticipate intraoperative difficulties.
Magnetic resonance imaging (MRI) has improved cholesteatoma detection rates considerably in the past decade and can be ordered in specific situations. Accurately predicting disease location and extension is essential for staging, planning, and preoperative counseling. Also, considering the second-look for wall-up tympanomastoidectomies, MRI improved sensitivity and specificity and may allow the surgeon to confidently monitor patients, therefore avoiding unnecessary surgery. While the CT is not able to differentiate granulation tissue from cholesteatoma, in the MRI the former enhances intensely with gadolinium on T1-weighted images, while the last does not enhance with gadolinium.
Regardless of the site of origin or etiologic basis, cholesteatomas do not enhance with gadolinium unless infiltrated by granulation tissue. This is a helpful differential diagnostic point that distinguishes these pathologic conditions from other retrotympanic masses, such as paragangliomas or schwannomas.
Cholesterol granuloma on CT scan is nonspecific and similar to that of typical granulation tissue. Magnetic resonance imaging is diagnostic because extracellular methemoglobin within the lesion results in a bright signal in all spin-echo pulse sequence.
There is a specific chapter about the Radiologic Evaluation in COM later in this book, where this issue will be larged explored.
Complications
Complications of otitis media can be divided into intratemporal and extratemporal and are processed by three basic mechanisms:
-
(a)
extension by preformed spaces,
-
(b)
bone erosion;
-
(c)
osteothrombophlebitis.
These complications will be studied in a specific chapter.
Treatment
The treatment of chronic cholesteatomatous otitis media is essentially surgical. The primary objective is the complete eradication of the disease, providing the patient with a dry ear and safe from complications. The secondary objective, but no less important, is the preservation or improvement of the function of the tympanossicular system, when this is possible [178, 179].
This subject will be extensively explored in later chapters of this book.
References
Cruz OL, Costa SS. Clinical and surgical otology. EdRevinter; 1999.
Friedmann I. Epidermoid cholesteatoma and granuloma. Ann OtolRhinolLaryngol. 1959;68:57–9.
Schuknecht HF. The pathology of the ear. Cambridge: Harvard University; 1974.
Ferlito O, Devaney KO, Rinaldo A, Milroy C, Wenig B, Iurato S, Mccabe BF. Clinicopathological consultation ear cholesteatoma versus cholesterol granuloma. Ann OtholRhinolLaryngol. 1997;106:79–85.
Aquino JE, Cruz Filho NA, de Aquino JN. Epidemiology of middle ear and mastoid cholesteatomas: study of 1146 cases. Braz J Otorhinolaryngol. 2011;77:341–7. https://doi.org/10.1590/S1808-86942011000300012.
Bennett M, Warren F, Jackson GC. Congenital cholesteatoma: theories, facts and 53 patients. Otolaryngol Clin N Am. 2006;39:1081–94. https://doi.org/10.1016/j.otc.2006.08.001.
Shohet JA, De Jong AI. The management of pediatric cholesteatoms. Otolaryngol Clin N Am. 2002;35:841–51. https://doi.org/10.1016/S0030-6665(02)00052-X.
Olszewska E, Wagner M, Bernal-Sprekelsen M, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004a;261:6–24. https://doi.org/10.1007/s00405-003-0623-x.
Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, Sudhoff H. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004b;261(1):6–24.
Barath K, Huber AM, Stampfil P, et al. Neuroradiology of cholesteatomas. AJNR Am J Neuroradiol. 2011;32:221–9. https://doi.org/10.3174/ajnr.A2052.
Jennings BA, Prinsley P, Philpott C, et al. The genetics of cholesteatoma. A systematic review using narrative synthesis. Clin Otolaryngol. 2017;43:55–67. https://doi.org/10.1111/coa.12900.
Bois E, Nassar M, Zenaty D, et al. Otologic disorders in Turner syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;135:21–4. https://doi.org/10.1016/j.anorl.2017.08.006.
Hall JE, Richter GT, Choo DI. Surgical management of otologic disease in pediatric patients with Turner syndrome. Int J PediatrOtorhinolaryngol. 2009;73:57–65. https://doi.org/10.1016/j.ijporl.2008.09.022.
Lim DBN, Gault EJ, Kubba H, et al. Cholesteatoma has a high prevalence in Turner syndrome, highlighting the need for earlier diagnosis and the potential benefits of otoscopy training for paediatricians. Acta Paediatr. 2014;103:e282–7. https://doi.org/10.1111/apa.12450.
Mann W, Al-Nawas B, Wriedt S. Cholesteatoma of the hypotympanum in a patient with Treacher Collins syndrome. Auris Nasus Larynx. 2014;41:101–4. https://doi.org/10.1016/j.anl.2013.05.005.
Bacciu A, Pasanisi E, Vincenti V, et al. Surgical treatment of middle ear cholesteatoma in children with Down syndrome. OtolNeurotol. 2005;26:1007–10. https://doi.org/10.1097/01.mao.0000185042.46523.9b.
Nash R, Possamai V, Maskell S, et al. Canal wall reconstruction and preservation in the surgical management of cholesteatoma in children with Down’s syndrome. Int J PediatrOtorhinolaryngol. 2014;78:1747–51. https://doi.org/10.1016/j.ijporl.2014.07.038.
Paulson LM, Weaver TS, Macarthur CJ. Outcomes of tympanostomy tube placement in children with Down syndrome—a retrospective review. Int J PediatrOtorhinolaryngol. 2014;78:223–6. https://doi.org/10.1016/j.ijporl.2013.10.062.
Suzuki C, Ohtani I. Bone destruction resulting from rupture of a cholesteatoma sac: temporal bone pathology. OtolNeurotol. 2004;25:674–7. https://doi.org/10.1097/00129492-200409000-00005.
Büchner SA, Itin P. Focal dermal hypoplasia syndrome in a male patient. Report of a case and histologic and immunohistochemical studies. Arch Dermatol. 1992;128:1078–82. https://doi.org/10.1001/archderm.1992.01680180072008.
Djurhuus BD, Skytthe A, Faber CE, Christensen K. Cholesteatoma risk in 8593 orofacial cleft cases and 6989 siblings: a nationwide study. Laryngoscope. 2015;125:1225–9. https://doi.org/10.1002/lary.25022.
Harris L, Cushing SL, Hubbard B, et al. Impact of cleft palate type on the incidence of acquired cholesteatoma. Int J PediatrOtorhinolaryngol. 2013;77:695–8. https://doi.org/10.1016/j.ijporl.2013.01.020.
Imbery TE, Sobin LB, Commesso E, et al. Long-term otologic and audiometric outcomes in patients with cleft palate. Otolaryngol Head Neck Surg. 2017;157:676–82. https://doi.org/10.1177/0194599817707514.
James AL, Papsin BC. Some considerations in congenital cholesteatoma. CurrOpinOtolaryngol Head Neck Surg. 2013;21:431–9. https://doi.org/10.1097/MOO.0b013e328364b457.
Kopcsányi G, Vincze O, Bagdán V, et al. Retrospective analysis of tympanoplasty in children with cleft palate: a 24-year experience. II. Cholesteatomatous cases. Int J PediatrOtorhinolaryngol. 2015;79:698–706. https://doi.org/10.1016/j.ijporl.2015.02.020.
Kuo CL, Lien CF, Chu CH, et al. Otitis media with effusion in children with cleft lip and palate: a narrative review. Int J PediatrOtorhinolaryngol. 2013;77:1403–9. https://doi.org/10.1016/j.ijporl.2013.07.015.
Lau CC, Loh KK, Kunaratnam N. Middle ear diseases in cleft palate patients in Singapore. Ann Acad Med Singap. 1988;17:372–4.
Kuo CL, Shiao AS, Wen HC, et al. Increased risk of cholesteatoma among patients with allergic rhinitis: a nationwide investigation. Laryngoscope. 2017;128:547–53. https://doi.org/10.1002/lary.26220.
Derlacki EL, Clemis JD. Congenital cholesteatoma of the middle ear and mastoid. Ann OtholRhinolLaryngol. 1965;74:706–27.
Valvassori GE. Benign tumors of the temporal bone. Radiol Clin N Am. 1974;12:533–42.
Sobol SM. Intramembranous and mesotympanic cholesteatomas associated with an intact tympanic membrane in children. Ann Othol. 1980;98:312–7.
Costa SS, Hueb MM, Ruschel C. Chronic cholesteatomatous otitis media. In: Cruz OL, Costa SS, editors. Clinical and surgical otology. Ed. Revinter; 1999.
Meyerhoff WL, Truelson J. Cholesteatoma staging. Laryngoscope. 1986;96:935–9.
Tos M. Incidence, etiology and pathogenesis of cholesteatoma in children: a long-term study of results. OtholRhinolLaryngol. 1988;40:110–7.
Saleh HA, Mills RP. Classification and staging of cholesteatoma. Clin Otolaryngol. 1999;24:355–9.
Wullstein H. Theory and practice of tympanoplasty. Laryngoscope. 1956;66(8):1076–93. https://doi.org/10.1288/00005537-195608000-00008.
Austin DF. Types and indications of staging. Arch Otolaryngol. 1969;89(2):235–42. https://doi.org/10.1001/archotol.1969.00770020237005.
Yung M, Tono T, Olszewska E, Yamamoto Y, Sudhoff H, Sakagami M, Mulder J, Kojima H, İncesulu A, Trabalzini F, Özgirgin N. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J Int Adv Otol. 2017;13(1):1–8. https://doi.org/10.5152/iao.2017.3363.
Lim DJ, Saunders WE. A cquired cholesteatoma: light and electron microscopic observations. Ann Otol. 1972;81:2–12.
Paludetti G, Alamadori G, Ottaviani F, Rosignoli M, Rossodivita M, D’Alatri L. Ultrastructural aspects of cholesteatoma of the middle ear. Acta Otorhinolaryngol Ital. 1989;9(2):169–80.
Milewski C, Fedorowski A, Stan AC, Walter GF. Basic fibroblast growth factor (b-FGF) in the perimatrix of cholesteatoma. HNO. 1998;46(9):804–8.
Sprekelsen BM, Ebmeyer J, Anonopoulos A, Sudhoff H. Basement membrane alterations in the middle ear cholesteatoma. Acta OtorEsp. 2001;52:330–5.
Jacob R, Welkoborsky HJ, Mann W. Epithelium-stroma interaction in cholesteatoma of the middle ear. Laryngorhinootologie. 2001;80(1):11–7.
Hamzei M, Ventriglia G, Hagnia M, Antonopolus A, Bernal-Sprekelson M, Dazert S, Hildmann H, Sudhoff H. Osteoclast stimulating and differentiating factors in human cholesteatoma. Laryngoscope. 2003;113(3):436–42.
DornellesC, CostaSS, MeurerL, CoelhoA, Cursino, A. Correlation between clinical inflammation and perimatrix thickness of acquired cholesteatomas. HCPA On-Line Magazine, 2004. www.hcpa.ufrgs.br.
Cristina Dornelles, Sady S. da Costa, Luíse Meurer, Cláudia Schweiger. Some considerations about acquired adult and pediatric cholesteatomas. Rev. Bras. Otorrinolaringol. 2005;71(4). https://doi.org/10.1590/S0034-72992005000400023.
Frankel S, Berson S, Godwin T, Han JC, Parisier SC. Differences in dendritic cells in congenital and acquired cholesteatomas. Laryngoscope. 1993;103(11 Pt 1):1214–7. doi: https://doi.org/10.1288/00005537-199311000-00002.
Junqueira LC, Carneiro J. Basic histology. Rio de Janeiro: Publisher Guanabara; 1985.
Vennix PP, Kuijpers W, Peters TA, Tonnaer EL, Ramaekers FC. Keratinocyte differentiation in acquired cholesteatoma and perforated tympanic membranes. Arch Otolaryngol Head Neck Surg. 1996;122:825–32.
Albino AP, Reed JA, Bogdany JK, Sassoon J, Desloge RB, Parisier SC. Expression of p53 protein in human middle ear cholesteatomas: pathogenetic implications. Am J Otol. 1998a;19(1):30–6.
Desloge RB, Carew JF, Finstad CL, Steiner MG, Sassoon J, Levenson MJ, Staiano-Coico L, Parisier SC, Albino AP. DNA analysis of human cholesteatomas. Am J Otol. 1997;18(2):155–9.
Shinoda H, Huang CC. Expressions of c- jun and p53 proteins in human middle ear cholesteatoma: relationship to keratinocyte proliferation, differentiation and programmed cell death. Laryngoscope. 1995;105(11):1232–7.
Bujia J, Schiling V, Holly A, Stammberger M, Kastenbauer E. Hyperproliferation-associated keratin expression in human middle ear cholesteatoma. Acta Otolaryngol. 1993;113:364–8.
Kuijpers W, Vernnix PP, Peters TA, Ramaekers FC. Squamous metaplasia of the middle ear epithelium. Acta Otolaryngol. 1996;116:293–8.
Moll R, Franke VW, Schiller DL. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24.
Pereira CSB. Analysis of studies on the expression of cytokeratins in acquired cholesteatoma. Masters dissertation. Faculty of Medical Sciences of Santa Casa de São Paulo; 1997.
Kim CS, Chung JW. Morphologic and biologic changes of experimentally induced cholesteatoma in Mongolian gerbils with anticytokeratin and lectin study. Am J Otol. 1999;20(1):13–8.
Lepercque S, Broekaert D, Van Cowwenberge P. Cytokeratin expression patterns in the human tympanic membrane and external ear canal. Eur Arch Otorhinolaryngol. 1993;250:78–81.
Broekaert D, Coucke P, Lepercque S, Ramaekers F, Van Muijen G, Boedts D, Leigh I, Lane B. Immunohistochemical analysis of the Cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann OtholRhinolLaryngol. 1992;101:931–8.
Pereira CSB, Almeida CIR, Viana MR. Immunoexpression of cytokeratin 16 and nuclear antigen Ki-67 in acquired middle ear cholesteatoma. Rev Bras Otorhinolaryngol. 2002;68(4):453–60.
Piltcher O. A new experimental model for the investigation of otitis media with effusion and its application in the study of cytokines during the different phases of this disease. Doctoral thesis. Faculty of Medical Sciences of Santa Casa de São Paulo, 2000.
Milewski C. Role of perimatrix fibroblast in development of acquired middle ear cholesteatoma. The hypothesis. HNO. 1998;46(5):494–501.
Aumente PO, Bujía J, Kim C, Jiménez Giménez J, López VP. Estudiocuantitativo de la presencia de interleucina-1 e interleucina-6 en elcolesteatoma de oído medio [A quantitative study of the presence of interleukin-1 and interleukin-6 in cholesteatoma of the middle ear]. Acta OtorrinolaringolEsp. 1996;47(4):259–62.
Bujia J, Kim C, Ostos P, Kastenbauer E, Hultner L. Role of interleukin 6 in epithelial hyper proliferation and bone resorption in middle ear cholesteatomas. Eur Arch Otorhinolaryngol. 1996b;253(3):152–7.
Tomita S. Molecular aspects of cholesteatoma—immunoexpression of cell cycle controlled proteins: p53, BAX and BCL-2. SP Doctoral Thesis. School Paulista de Medicina, 2000.
Sudhoff H, Borkowski G, Bujia J, Hildmann H. ImmunhistochemistryUntersuchungen von MittelohrschleimhautrestenimCholesteatom. HNO. 1997;45(8):630–5.
Sudhoff H, Dazert S, Gonzales AM, Borkowski G, Park SY, Baird A, Hildmann H, Ryan AF. Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am J Otol. 2000;21:793–8.
Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg. 2002;128:1129–33.
Yamamoto-Fukuda T, Takahashi H, Koji T. Animal models of middle ear cholesteatoma. J Biomed Biotechnol. 2011;2011:394241. https://doi.org/10.1155/2011/394241.
Chole RA, Henry KR, McGinn MD. Cholesteatoma: spontaneous occurrence in the Mongolian gerbil Merionesunguiculatis. Am J Otol. 1981;2(3):204–10.
Chole RA, Chiu M. Ultrastructure of middle earmucosa in the mongolian gerbil. MerionesunguiculatusActa Oto-Laryngol. 1985;100(3–4):273–88.
Schmidt SH, Hellstrom S. Experimental cholesteatoma in the rat. Acta Otolaryngol. 1994a;114(4):430–4.
Schmidt SH, Hellstrom S. Experimental cholesteatoma in the rat. Acta Otolaryngol (Stockh). 1994b;114:430–4.
Friedmann I. The comparative pathology of otitis media,experimental and human—II. The histopathology of experimental otitis of the guinea-pig with particular reference to experimental cholesteatoma. J LaryngolOtol. 1955a;69(9):588–601.
Friedmann I. Comparative pathology of otitis media: experimental and human. II. The histopathology of experimental otitis with particular references to experimental cholesteatoma. J LaryngolOthol. 1955b;69:588–601.
Wolfman DE, Chole RA. Experimental retractionpocket cholesteatoma. Ann OtolRhinolLaryngol. 1986a;95(6I):639–44.
Wolfman DE, Chole RA. Experimental retraction pocket cholesteatoma. Ann OtholRhinolLaryngol. 1986b;95:639–44.
Vennix PPCA, Kuijpers W, Peters TA, Tonnaer ELGM, Ramaekers FCS. Growth and differentiation of meatal skin grafts in the middle ear of the rat. Arch Otolaryngol—Head Neck Surg. 1994;120(10):1102–11.
McGinn MD, Chole RA, Henry KR. Cholesteatoma.Experimental Induction in the Mongolian Gerbil, MeirionesUnguiculaus. Acta Otolaryngol (Stockh). 1982a;93:61–7.
McGinn MD, Chole RA, Henry KR. Cholesteatona. Experimental induction in the Mongolian gerbil, Merionesunguiculatus. Acta Otolaryngol. 1982b;93:61–7.
Wright CG, Meyerhoff WL, Burns DK. Middle ear cholesteatoma: an animal model. Am J Otolaryngol. 1985a;6(5):327–41. https://doi.org/10.1016/s0196-0709(85)80010-7.
Huve FDC, Bauer JA, Selaimen FA, Silva MNLD, Costa SSD. Experimental cholesteatoma: a comparison between spontaneous and induced models. Braz J Otorhinolaryngol. 2023;89(1):73–8. https://doi.org/10.1016/j.bjorl.2021.09.003.
Bauer JA, Huve FDC, Oliveira FH, Silva MNLD, Sperling N, Costa SSD. Mongolian Gerbils as a model for the study of cholesteatoma: otoendoscopic as a diagnostic tool. Int Arch Otorhinolaryngol. 2022;26(4):e643–8. https://doi.org/10.1055/s-0041-1740159.
Vassalli L, Harris DM, Gradini R, Applebaum EL. Propylene glycol-induced cholesteatoma in chinchilla middle ears. Am J Otolaryngol. 1988;9(4):180–8. https://doi.org/10.1016/s0196-0709(88)80026-7.
Huang CC, Shi GS, Yi ZX. Experimental inductionof middle ear cholesteatoma in rats. Am J Otolaryngol. 1988;9(4):165–72. https://doi.org/10.1016/s0196-0709(88)80024-3.
Meyerhoff WL, Wright CG, Gerken GM. Effects of middle ear ventilation on Cholesteatoma development in experimental animals. Acta Otolaryngol (Stockh). 1990;110:1990.
Pownell PH, Wright CG, Robinson KS, Meyerhoff WL. The effect of cyclophosphamide on development of experimental cholesteatoma. Arch Otolaryngol Head Neck Surg. 1994;120(10):1114–6.
Jove MA, Vassalli L, Raslan W, Applebaum EL. The effect of isotretinoin on propylene glycol-induced cholesteatoma in chinchilla middle ears. Am J Otolaryngol. 1990;11:5–9.
White SJ, Wright CG, Robinson KS, Meyerhoff WL. Effect of topical hyaluronic acid on experimental cholesteatoma. Am J Otolaryngol. 1995;16:312–8.
Wright CG, Bird LL, Meyerhoff WL. Effect of 5-fluorouracil in cholesteatoma development in an animal model. Am J Otolaryngol. 1991;12:133–8.
Masaki M, Wright CG, Lee DH, Meyerhoff WL. Experimental cholesteatoma. Epidermal ingrowth through Tympanic membrane following middle ear application of propylene. Glycol Acta Otolaryngol (Stockh). 1989;108:113–21.
Chole RA, Frush DP. Quantitative aspects of Eustachian tube epithelium during vitamin A deprivation and reversal. In: Sadé, editor. Cholesteatoma and mastoid surgery, 1982. Proceedings of the 2nd International Conference. Amsterdam: Kluger; 1982.
Minotti AM, Kountakis SE, Leighton WR, Cabral FR. Effects of extracellular calcium on cholesteatoma migration and adhesion in vitro. Otolaryngol Head Neck Surg. 1996;115(5):458–63.
Hueb MM. Acquired cholesteatoma: experimental advances in understanding its pathogenesis. Doctoral Thesis, Faculty of Medicine of the University of São Paulo, 1997.
Jackler RX. The surgical anatomy of cholesteatoma. Otolaryngol Clin N Am. 1989;22(5):883–96.
Cawthorne T, Griffith A. Primary cholesteatomata of the temporal bone Arch Otolaryngol. 1961;73:252–61. https://doi.org/10.1001/archotol.1961.00740020260002. PMID: 13691781.
Levenson MJ, Michaels L, Parisier SC. Congenital cholesteatomas in children and embryologic correlations. Laryngoscope. 1988;98:949–55.
Karmody CS, Byahatti SV, Blevins N, Valtonen H, Northrop C. The origin of congenital cholesteatoma. Am J Otol. 1998;19(3):292–7.
Huang J, Yi Z, Lin G. Altered cytokeratin expression of the epidermoid formation in the tympanic cavity of human fetuses. Chung Hua Erh Pi Yen Hou Ko TsaChih. 1996;31(2):72–4.
Jaisinghani VJ, Paparella MM, Schachern PA. Silent intratympanic membrane cholesteatoma. Laryngoscope. 1998;108:1185–9.
Kayhan FT, Mutlu C, Schachern PA, Le CT, Paparella MM. Significance of epidermoid formations in the middle ear in fetuses and children. Arch Otolaryngol Head Neck Surg. 1997;123(12):1293–7.
Gacek RR. Diagnosis and management of primary tumors of the petrous apex. Ann OtholRhinolLaryngol. 1975;84(Suppl. 18):1–20.
Reeves DL. Epidermoid [mixed] tumors of the central nervous system. J Neurosurg. 1976;26:21–4.
Souza CE, Souza R, Costa SS, et al. Cerebellopontine angle epidermoid cysts: a report on 30 cases. J Neurol Neurosurg Psychiatry. 1989;52:986–90.
MacKennan KX, Chole RA. Post-traumatic cholesteatoma. Laryngoscope. 1989;99:779–82.
Wendt H. Desquamative Entzündung des Mittelors Arch Heilk. 1873;14:428.
Birrell JF. Cholesteatosis of the attic. J LaryngolOthol. 1958;72:620–5.
Sadé J. The metaplastic and congenital origin of cholesteatoma. Acta Otolaryngol. 1983;96:119–29.
Sadé J, editor. Secretory otitis media and its sequelae. New York, Edinburgh, London: Churchill Livingstone; 1979.
Schechter G. A review of cholesteatoma pathology. Laryngoscopy. 1969;79:1907–20.
Ruedi L. Cholesteatosis of the attic. J LaryngolOtol. 1958;72:593.
Blitterswijk CA, Grote JJ, Lutgert RW, Hesse Ling SC, Out CJ, Muijen GNP, Fransen JAM. Cytokera tin patterns of tissues related to cholesteatoma pathogenesis. Ann OtholRhinolLaryngol. 1989;98:635–40.
Broekaert D, Van Oostveldt P, Coucke P, Reyniers P, Kluyskens P, Gillis E. Differentiation of nuclei during keratinization in middle ear cholesteatoma. DNA cytophotometry completed by computerized image analysis. Acta Otolaryngol. 1988;105(1–2):90–9. https://doi.org/10.3109/00016488809119450.
Haberman J. ZurEntstehung des Cholesteatoms des Mittelohrs. Arch Ohrenheilk. 1889;27:42–50.
Bezold FC. Perforation of the membrane saggy Shrapnell und Tubernverschluss. Zeitschr für Ohrenheilk. 1980;20:5–28.
Chao WY, Huang CC. An immunocytochemical study of cytokeratin expression in human middle ear cholesteatoma. Arch Otorhinolaryngol. 1989;246(1):37–42. https://doi.org/10.1007/BF00454132.
Youngs R, Rowles P. The Spatial Organization of Keratinocytes in acquired middle ear cholesteatoma resembles that of external auditory canal skin and Pars Flacci da. Acta Otolaryngol (Stockh). 1990;1990(110):115–9.
Aberg B, Edström S, Johannesson A, Larko O. Evaluation of epidermal hyperproliferation in patients with cholesteatoma. Am J Otol. 1991;12:93–6.
Tos M. Upon the relationship between secretory otitis in childhood and its sequelae in adults. J LaryngolOthol. 1981;98:1011–22.
Ruedi L. Cholesteatoma formation in the middle in animal experiments. Acta Otolaryngol. 1959;50:233–42.
Chole RA, Tingling SP. Basal lamina breaks in the histogenesis of cholesteatoma. Laryngoscope. 1985;95:270–5.
Giacometti L. Cells in motion. Primate Netus. 1968;6:3–9.
Lim DJ, DeMaria TF. Panel discussion: pathogenesis of otitis media. Bacteriology and immunology Laryngoscope. 1982;92(3):278–86. https://doi.org/10.1288/00005537-198203000-00011.
BezoldF.Textbook of otology; 1908.
Cassano M, Cassano P. Retraction pockets of pars tensa in pediatric patients: clinical evolution and treatment. Int J Pediatr Otorhinolaryngol 2010;74(2):178–182. doi: https://doi.org/10.1016/j.ijporl.2009.11.004. Epub 2009 Dec 3. PMID: 19962200.
Sadé J, Avraham S, Brown M. Atelectasis, retraction pockets and cholesteatoma. Acta Otolaryngol. 1981;92(5–6):501–12. https://doi.org/10.3109/00016488109133289.
Rosito LP, da Silva MN, Selaimen FA, Jung YP, Pauletti MG, Jung LP, Freitas LA, da Costa SS. Characteristics of 419 patients with acquired middle ear cholesteatoma. Braz J Otorhinolaryngol. 2017;83(2):126–31. https://doi.org/10.1016/j.bjorl.2016.02.013.
Monsell EM, Harley RE. Eustachian tube dysfunction. Otolaryngol Clin N Am. 1996;29(3):437–44.
Ars B, Wuyts F, Van de Heyning P, Miled I, Bogers J, Van Marck E. Histomorphometric study of the normal middle ear mucosa. Preliminary results supporting the gas-exchange function in the postero-superior part of the middle ear cleft. Acta Otolaryngol. 1997;117(5):704–7. https://doi.org/10.3109/00016489709113463.
Marchioni D, Piccinini A, Alicandri-Ciufelli M, Presutti L. Endoscopic anatomy and ventilation of the epitympanum. Otolaryngol Clin N Am. 2013;46(2):165–78. https://doi.org/10.1016/j.otc.2012.10.002.
Paço J, Branco C, Estibeiro H, Oliveira E, Carmo D. The posterosuperior quadrant of the tympanic membrane. Otolaryngol Head Neck Surg. 2009;140(6):884–8. https://doi.org/10.1016/j.otohns.2009.01.009.
Lim DJ. Structure and function of the tympanic membrane: a review. Acta OtorhinolaryngolBelg. 1995;49:101–15.
Lim DJ. Tympanic membrane: electron microscopic observations, part II: Pars flaccida. Acta Otolaryngol. 1968;66:515–32.
Bhide A. Etiology of the retraction pocket in the posterosuperior quadrant of the eardrum. Arch Otolaryngol. 1977;103(12):707–11. https://doi.org/10.1001/archotol.1977.00780290043004.
Sudhoff H, Tos M. Pathogenesis of sinus cholesteatoma. Eur Arch Otorhinolaryngol. 2007;264(10):1137–43. https://doi.org/10.1007/s00405-007-0340-y. Epub 2007 May 30
Jackler RK, Santa Maria PL, Varsak YK, Nguyen A, Blevins NH. A new theory on the pathogenesis of acquired cholesteatoma: mucosal traction. Laryngoscope. 2015;125(Suppl 4):S1–S14. https://doi.org/10.1002/lary.25261. Epub 2015 May 25
Jackler RK. Experimental evidence against middle ear oxygen absorption. Laryngoscope. 1985;95(10):1281–2. https://doi.org/10.1288/00005537-198510000-00031.
Black B, Gutteridge I. Acquired cholesteatoma: classification and outcomes. OtolNeurotol. 2011;32(6):992–5. https://doi.org/10.1097/MAO.0b013e3182255874.
Rosito LP, Teixeira AR, Netto LS, Selaimen FA, da Costa SS. Cholesteatoma growth patterns: are there audiometric differences between posterior epitympanic and posterior mesotympanic cholesteatoma? Eur Arch Otorhinolaryngol. 2016;273(10):3093–9. https://doi.org/10.1007/s00405-016-3918-4.
Potsic WP, Korman SB, Samadi DS, Wetmore RF. Congenital cholesteatoma: 20 years’ experience at the Children’s Hospital of Philadelphia. Otolaryngol Head Neck Surg. 2002;126(4):409–14. https://doi.org/10.1067/mhn.2002.123446. PMID: 11997782
Kazahaya K, Potsic WP. Congenital cholesteatoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):398–403. https://doi.org/10.1097/01.moo.0000136875.41630.d6.
Nevoux J, Lenoir M, Roger G, Denoyelle F. Ducou Le pointe H, Garabédian EN. Childhood cholesteatoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(4):143–50. https://doi.org/10.1016/j.anorl.2010.07.001. Epub 2010 Aug 11
Chole RA. The molecular biology of bone resorption due to chronic otitis media. Ann N Y Acad Sci. 1997;830:95–109.
Dornelles C, Schmidt Rosito LP, Meurer L, da Costa SS, Argenta A, Alves SL. Hystology findings’ correlation between the ossicular chain in the transoperative and cholesteatomas. Braz J Otorhinolaryngol. 2007;73(6):738–43. https://doi.org/10.1016/S1808-8694(15)31169-1.
Durko M, Kaczmarczyk D. Proliferation activity and apoptosis in granulation tissue and cholesteatomain middle ear reoperations. Folia Morphol (Warsz). 2004;63(1):119–21.
Martins O, Victor J, Selesnick S. The relationship between individual ossicular status and conductive hearing loss in cholesteatoma. OtolNeurotol. 2012;33(3):387–92. https://doi.org/10.1097/MAO.0b013e3182487fb0.
Maresh A, MartinsOF, Victor JD, Selesnick SH. Using surgical observations of ossicular erosion patterns to characterize cholesteatoma growth. OtolNeurotol. 2011;32(8):1239–42. https://doi.org/10.1097/MAO.0b013e31822e5b5d.
Abramson M. Collagenase in the mechanisms of action of cholesteatoma. Ann OtholRhinolLaryngol. 1971;80(3):414.
Abramson M. Collagenolytic activity in middle ear cholesteatoma. AnnOtol. 1969;78:112–25.
Abramson M, Huang CC. Localization of collagenase in human middle ear cholesteatoma. Laryngoscope. 1976;86:771–91.
Thompsen J. Bone resorption in chronic otitis media. In: McCabe BF, Sade J, Abramson M, editors. Cholesteatoma: first international conference. Birmingham, Alabama: Aescupulus Publishing Co; 1977. p. 136.
Hansen T, Unger RE, Gaumann A, Hundorf I, Maurer J, Kirkpatrick J, Kriegsmann J. Expression of matrix-degrading cysteine proteinase cathepsin K in cholesteatoma. United States Canadian AcadPathol. 2001;14(12):1226–31.
Kurihara A, Toshima M, Yuasa R, Takasaka T. Bone destruction mechanisms in chronic otitis media with cholesteatoma: specific production by cholesteatoma tissue in culture of bone-resorbing activity attributable to interleukin-1 alpha. Ann OtolRhinolLaryngol. 1991;100(12):989–98.
Imai R, Sato T, Iwamoto Y, Hanada Y, Terao M, Ohta Y, Osaki Y, Imai T, Morihana T, Okazaki S, Oshima K, Okuzaki D, Katayama I, Inohara H. Osteoclasts modulate bone erosion in cholesteatoma via RANKL signaling. J Assoc Res Otolaryngol. 2019;20(5):449–59. https://doi.org/10.1007/s10162-019-00727-1.
Sangal N, Yan J, Pryor J, Elias ML, Joseph B, Ulloa L, Jyung RW. Association of CARD8 activating polymorphism with bone erosion in cholesteatoma patients. Laryngoscope. 2021;131(2):E605–11. https://doi.org/10.1002/lary.28741.
Brook I. Aerobic and anaerobic bacteriology of cholesteatoma. Laryngoscope. 1981;91(2):250–3. https://doi.org/10.1288/00005537-198102000-00012.
Kenna MA, Bluestone CD, Reilly JS, Lusk RP. Medical management of chronic suppurative otitis media with cholesteatoma in children. Laryngoscope. 1986;96:146–51.
Fujikawa T, Tanimoto K, Kawashima Y, Ito T, Honda K, Takeda T, Sonobe A, Aoki N, Bai J, Tsutsumi T. Cholesteatoma has an altered microbiota with a higher abundance of staphylococcus species. Laryngoscope Investig Otolaryngol. 2022;7(6):2011–9. https://doi.org/10.1002/lio2.934. eCollection 2022.
Galli J, Calò L, Giuliani M, Sergi B, Lucidi D, Meucci D, Bassotti E, Sanguinetti M, Paludetti G. Biofilm's role in chronic cholesteatomatous otitis media: a pilot study. Otolaryngol Head Neck Surg. 2016;154(5):914–6. https://doi.org/10.1177/0194599816630548.
Paparella MM, Oda M, Hiraide F, Brady D. Pathology of sensorineural hearing loss in otitis media. Ann OtolRhinolLaryngol. 1972;81(5):632–47. https://doi.org/10.1177/000348947208100503.
Paparella MM, Schachern PA, Choo YB. The round window membrane: otological observations. Ann OtolRhinolLaryngol. 1983;92(6 Pt 1):629–34. https://doi.org/10.1177/000348948309200619.
English GM, Northern JL, Fria TJ. Chronic otitis media as a cause of sensorineural hearing loss. Arch Otolaryngol. 1973;98(1):18–22. https://doi.org/10.1001/archotol.1973.00780020022006.
Dumich PS, Harner SG. Cochlear function in chronic otitis media. Laryngoscope. 1983;93(5):583–6. https://doi.org/10.1002/lary.1983.93.5.583.
Walby AP, Barrera A, Schuknecht HF. Cochlear pathology in chronic suppurative otitis media. Ann OtolRhinolLaryngol Suppl. 1983;103(Suppl):1–19.
Rosito LPS, Sperling N, Teixeira AR, Selaimen FA, da Costa SS. The role of Tympanic membrane retractions in cholesteatoma pathogenesis. Biomed Res Int. 2018;2018:9817123. https://doi.org/10.1155/2018/9817123.
Rosito LPS, Canali I, Teixeira A, Silva MN, Selaimen F, Costa SSD. Cholesteatoma labyrinthine fistula: prevalence and impact. Braz J Otorhinolaryngol. 2019;85(2):222–7. https://doi.org/10.1016/j.bjorl.2018.01.005.
Dornhoffer JL, Milewski C. Management of the open labyrinth. Otolaryngol Head Neck Surg. 1995;112(3):410–4. https://doi.org/10.1016/S0194-59989570275-X.
Gomaa MA, Abdel Karim AR, Abdel Ghany HS, Elhiny AA, Sadek AA. Evaluation of temporal bone cholesteatoma and the correlation between high resolution computed tomography and surgical finding. Clin Med Insights Ear Nose Throat. 2013;6:21–8. https://doi.org/10.4137/CMENT.S10681.
Sethom A, Akkari K, Dridi I, Tmimi S, Mardassi A, Benzarti S, Miled I, Chebbi MK. Apport de la tomodensitométrie dans le bilanpréopératoire de l'otitemoyennechroniquecholestéatomateuse. A propos de 60 cas [Preoperative CT Scan in middle ear cholesteatoma]. Tunis Med. 2011;89(3):248–53.
Stephenson MF, Saliba I. Prognostic indicators of hearing after complete resection of cholesteatoma causing a labyrinthine fistula. Eur Arch Otorhinolaryngol. 2011;268(12):1705–11. https://doi.org/10.1007/s00405-011-1545-7.
Ikeda M, Nakazato H, Onoda K, Hirai R, Kida A. Facial nerve paralysis caused by middle ear cholesteatoma and effects of surgical intervention. Acta Otolaryngol. 2006;126(1):95–100. https://doi.org/10.1080/00016480500265950.
Quaranta N, Cassano M, Quaranta A. Facial paralysis associated with cholesteatoma: a review of 13 cases. OtolNeurotol. 2007;28(3):405–7. https://doi.org/10.1097/01.mao.0000265189.29969.c4.
Siddiq MA, Hanu-Cernat LM, Irving RM. Facial palsy secondary to cholesteatoma: analysis of outcome following surgery. J LaryngolOtol. 2007;121(2):114–7. https://doi.org/10.1017/S0022215106003227.
Psillas G, Constantinidis J. Facial palsy secondary to Cholesteatoma: a case-series of 14 patients. Audiol Res. 2023;13(1):86–93. https://doi.org/10.3390/audiolres13010008.
Ozkul Y, Songu M, Onal K, Imre A, Arslanoglu S, Horoz E, Bayrak F, Pinar E. Effect of surgical intervention on middle-ear cholesteatoma with associated facial paralysis. J LaryngolOtol. 2017;131(2):113–6. https://doi.org/10.1017/S0022215116009804.
Alice Lang Silva, Fábio André Selaimen , Isabel Saorin Conte, Marcela Lehmkuhl Damiani, Luciana Lima Martins Costa, Letícia Petersen Schmidt Rosito, Sady Selaimen da Costa. Aural polyps: what’s behind them? Scripta Scientifica Medica, 2021;53(2):62–7 Medical University of Varna.
Costa SS, Silva MN, Rosito LP, Selaimen FA. Um caso, duas lições: artériacarótida interna aberrantecausandocolesteatomaadquirido [One case, two lessons: an aberrant internal carotid artery causing acquired cholesteatoma]. Braz J Otorhinolaryngol. 2014;80(5):453–4. https://doi.org/10.1016/j.bjorl.2014.05.021.
Costa SS, de Souza LCA, Andrade MI. Procedures on the storm—reviewing the nomenclature. Rev Bras Otor. 1991a;57(4):170–9.
Costa, SSda, Paparella MM, Shachern P, Yoon TH. Chronic silent otitis media: a clinical-pathological study; a preliminar report. Rev bras otorrinolaringol. 1997: 57(2);81–4, abr.-jun.
Aberg B, Edstrom S, Bagger-Sjoback D, Kindblom LG. Morphologic development of experimental cholesteatoma. Arch Otolaryngol Head Neck Surg. 1993;119:272–5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
da Costa, S.S., Rosito, L.P.S., da Silva, M.N.L., Selaimen, F.A. (2023). Temporal Bone Cholesteatoma: The Full Picture. In: Goycoolea, M.V., Selaimen da Costa, S., de Souza, C., Paparella, M.M. (eds) Textbook of Otitis Media. Springer, Cham. https://doi.org/10.1007/978-3-031-40949-3_42
Download citation
DOI: https://doi.org/10.1007/978-3-031-40949-3_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40948-6
Online ISBN: 978-3-031-40949-3
eBook Packages: MedicineMedicine (R0)