Abstract
The aim of the present study was to provide evidence for the establishment of sinus cholesteatoma, defined as postero-superior pars tensa retraction extending into the posterior tympanum and tympanic sinuses. Background: There is clinical evidence for formation of a retraction, but there is a lack of explanation for the transition from a retraction pocket to an active and expanding sinus cholesteatoma. Epidemiological studies on incidence of postero-superior retractions of pars tensa and follow-up studies on patients with similar pars tensa retractions were performed. Additionally, expression of proliferation marker and analysis of basement membrane were studied in samples of sinus cholesteatoma. The prevalence of pars tensa pathology was between 9.2 and 24% of investigated ears. In children with manifest secretory otitis there were some sinus cholesteatomas and 5–6% severe retractions, some of those became pre-cholesteatomas, requiring treatment and controls. Immunohistochemistry of sinus cholesteatomas showed that proliferating keratinocytes were very often found within epithelial cones growing towards the underlying stroma. These growth cones exhibit focal discontinuities of the basement membrane especially in areas of intense subepithelial inflammation. As a possible explanation based on clinical and immunohistochemical findings, we propose a four-step concept for pathogenesis of sinus cholesteatoma combining the retraction and proliferation theory: (1) The retraction pocket stage. (2) The proliferation stage of the retraction pocket, subdivided in (a) Cone formation, (b) Cone fusion. (3) Expansion stage of attic cholesteatoma. (4) Bone resorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
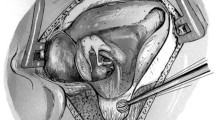
Acquired cholesteatomas of the middle ear are divided into: (1) Attic cholesteatoma, originating from the Shrapnell`s membrane and extending primary into the attic. (2) Sinus cholesteatoma, originating from the postero-superior retraction of pars tensa and extending primary into the tympanic sinuses. From here it may extend along the prominence of the facial nerve, medial to the incus body, into the posterior attic and antrum, while the anterior part of the tympanic cavity and the anterior attic are not involved (Fig. 1). (3) Tensa-retraction cholesteatoma, originating from an entirely retracted pars tensa, draping over the posterior and anterior walls of the tympanic cavity, extending from here into the hypotympanic cells and tubal orifice. Furthermore it may extend, medially to the malleus folds, towards the posterior and anterior attic.
Schematic illustration of extension of a sinus cholesteatoma, from the posterior tympanum into the posterior attic and antrum. The anterior and inferior parts of the tympanic cavity are not involved [30]
This classification, proposed by Tos for better understanding of the pathogenesis, has become widely accepted. It subdivides the pars tensa cholesteatomas into sinus cholesteatomas, with primarily posterior retraction and posterior pathology (Fig. 1) and into tensa retraction cholesteatoma with primary retraction of the entire pars tensa and primary pathology both in the anterior, inferior and posterior parts of the tympanic cavity [1] (Fig. 2). Such classification is also important for evaluation of the natural history, prognosis, surgical methods and results.
Several theories on pathogenesis of cholesteatoma have been described:
-
1.
Retraction theory [2], based on a retraction of pars tensa or the Shrapnell’s membrane as a result of chronic dysfunction of the Eustachian tube.
-
2.
Papillary proliferation theory [3, 4], based on infection leading to the proliferation of the epithelial cones in the basal layers of the keratinizing epithelium of the pars tensa or the Shrapnell’s membrane.
-
3.
Immigration theory [5], based on an ingrowth of the squamous epithelium through a preexisting peripheral perforation.
-
4.
Metaplasia theory [6–8], based on the metaplasia of the inflamed middle ear epithelium into keratinizing squamous epithelium.
Clinically, it is difficult to find any support for the immigration theory; we have never observed an acute perforation of the pars tensa or the Shrapnell’s membrane, allowing immigration of the keratinized epithelium through it. We have not observed any cholesteatoma starting somewhere in the antrum due to metaplasia and expanding outwards through the pars tensa or the Shrapnell’s membrane.
Indeed there is some evidence for retraction- and proliferation theories for a certain stage of cholesteatoma formation. There is clinical evidence for formation of a retraction, but there is no exact explanation for the transition from a retraction pocket to an active and expanding attic cholesteatoma. Previously Tos and Tos and Sudhoff [1, 9, 10] have indicated that a combination of the retraction theory and the proliferation theory could explain the pathogenesis of the acquired middle ear cholesteatomas.
In fact, in a study of pathogenesis of attic cholesteatoma we have documented high incidence of attic retractions in children and adults as a consequence of previous secretory otitis and tubal dysfunction [10]. Furthermore, we have in attic cholesteatoma by immunohistochemistry often demonstrated proliferating keratinocytes within the epithelial cones, growing towards the underlying perimatrix. We proposed a four-step concept for pathogenesis of attic cholesteatoma combining the retraction and proliferation theory: (1) The retraction pocket stage; (2) The proliferation stage of the retraction pocket, subdivided into (a) Cone formation, (b) Cone fusion; (3) Expansion stage of attic cholesteatoma; (4) Bone resorption [10].
The aim of the present study is to give evidence for a combination of retraction theory and proliferation theory in sinus cholesteatoma, by documenting high incidence of postero-superior pars tensa retractions in children and adults and by demonstrating with immunohistochemistry evidence for the formation of cholesteatoma by proliferation of the keratinizing epithelium of the postero-superior pars tensa retraction. We would thus like to perform a similar study on pathogenesis of sinus cholesteatoma as we performed on attic cholesteatoma [10].
Materials and methods
Clinical materials and methods
(a) Epidemiological study: In an epidemiological study of randomized, otherwise healthy, 4-year-old children, 5 repeated tympanometries every 3 months and then every year from age 5 to age 16 have been performed. From age 5 to age 16, nearly every year changes of the eardrum have been studied by repeated otomicroscopies [9, 11, 12]. The prevalence pars tensa pathology and in particular of postero-superior retractions have been investigated in each screening (Table 1A).
(b) Clinical studies: In a clinical study initially consisting of 527 ears from 4–8 years old children with secretory otitis treated with grommet insertion and adenoidectomy during the late sixties and early seventies at the ENT Department Gentofte Hospital, the prevalence of pars tensa pathology, in particular of postero-superior retractions have been studied several times by repeated otomicroscopies, first 3–8 years after surgery [9, 13], then 10–16 years after and again 17–22 years after surgery [9, 14, 15] (Table 1B).
(c) Clinical studies on surgical material: In daily otological and surgical practice, patients with postero-superior retractions of pars tensa have been followed through several years, observing eventual progression of retractions, becoming deeper and infected with crust formation [1, 9, 15] or formation of a specific polyp (herodium) within the postero-superior retraction [16], a sinus pre-cholesteatoma, most usually ending in sinus cholesteatoma. Studies on surgical incidence of 740 cholesteatomas, (137 children and 603 adults) operated on during 16 years from 1965 through 1980, in an area of the Gentofte Hospital comprising 300,000 inhabitants have been performed [17].
Immunohistochemical materials and methods
(a) Specimens were obtained from 17 patients requiring middle ear surgery for sinus cholesteatoma removing and investigating the entire cholesteatoma sac (average age, 29.5; 8 male, 9 female patients).
(b) Eleven biopsies of skin of the normal auditory canal skin or tympanic membrane from the same patients were investigated.
Immediately after surgery, the tissue was fixed in formalin, embedded in paraffin and examined histopathologically with routine HE staining. Immunohistochemical staining was performed by using the Alkaline-Phosphatase-anti-Alkaline-Phosphatase (APAAP) and Avidin-Biotin-Complex (ABC) labeling method as described elsewhere [14]. Paraffin sections (4 μm) were placed on poly-l-lysine-coated slides (Sigma, Munich, Germany). As monoclonal antibodies that are applied to formalin-fixed and paraffin-embedded tissue we used MIB 1 (Molecular Immunology Borstel 1) (Dianova, Hamburg, Germany) and collagen type IV (DAKO, Hamburg, Germany) [15, 16]. MIB 1 has been reported to recognize cell proliferation-associated human nuclear antigens present in the late G1, S, S2 and M phases of the cell cycle alike, but to be absent in the G0 and the early G1 phases. It reacts with recombinant parts of the Ki-67 antigen (1002 bp Ki-67 c-DNA fragment) and is a widely used proliferation marker. Anti-Ki-67 has been applied to cholesteatoma- and ear canal skin materials to assess the cell kinetics. On excised benign and malignant tumors the Ki-67/MIB 1 antigen proliferation marker and has a very high acceptance as a prognostic marker [17].
Collagen type IV is a major component of the basement membrane in the dermal–epidermal junction. It is a non-fibrillar collagen and constitutes the structural scaffolding of specialized sheets of extracellular matrix. Increased attention has been focused on the role of collagen type IV as a stabilizing protein of extracellular matrix in the process of tumor invasion and metastasis [18].
Results
Incidence of retractions
In a cohort of randomized, otherwise healthy children aged 5–16 years the prevalence of ears with some pathology of pars tensa was 9.2% at 5 years of age, 20.8% at 7 years of age and 24% at 16 years of age (Table 1A). The prevalence of atrophy with postero-superior retraction and fixation to incudostapedial joint or to the stapes has increased from 0.3% at the age of 5 years to 3.4% at the age of 16 years, which is relatively common if we consider that the retractions were found in the healthy teenage population (Table 1A).
In the material of manifest secretory otitis (13–15), treated actively with adenoidectomy and grommet insertions the incidence of ears with some pathology of pars tensa was high at all three postoperative evaluations, between 55 and 74% (Table 1B). These children were at least at two evaluations adults. There were between 3.2 and 5.1% postero-superior retractions, some sinus cholesteatomas and 5–6% severe retractions, some of those became pre-cholesteatomas, requiring treatment and controls (Table 1B)
Normal auditory meatal skin
In normal auditory meatal skin, MIB 1 positive proliferating cells were mainly found in the stratum basalis. An average MIB 1 score of 7.1% ± 3.9 was counted by the image analyzer system (Table 2 and Fig. 3). Positive cells were distributed regularly within the basal cell layer. MIB 1-positive cells could be observed only infrequently within the normal underlying connective tissue of the auditory meatal skin.
Avidin-biotin-complex peroxidase stain for collagen type IV showed a continuous linear immunoreacivity within the basement membrane of normal auditory meatal skin and middle ear mucosa (Fig. 4)
Sinus cholesteatoma
HE staining of the active sinus cholesteatomas showed in majority of the investigated cases epithelial cones. These cones are extensions of the basal cell layer down into the subepithelial connective tissue, the so-called perimatrix. With increasing length of the growth cone, we observed an increasing occurrence of intra-epithelial cornifications.
Positive immunoreactivity for the proliferation marker could be demonstrated in basal cell layer keratinocytes of epithelium of all 17 cases of cholesteatoma. The MIB 1 proliferation indices ranged from 8.1 to 39.6% with a mean value of 17.4 % ± 9.2 (Table 2 and Fig. 5). Proliferating keratinocytes were very often found within epithelial cones growing towards the underlying stroma.
The 14 out of 17 cases of positive immunoreactivity for collagen type IV revealed a distinct linear staining of the intrinsic basement membrane exhibiting focal discontinuities especially in areas of intense subepithelial inflammation and especially adjacent to the advancing front of the growth cones (Fig. 6).
Discussion
We have brought evidence of high incidences of attic retractions in otherwise healthy children who have had secretory otitis and long-term tubal dysfunction [1, 10]. The retractions were highly correlated to episodes of secretory otitis and chronic tubal dysfunction. However, only a minority of these children will have a progression of the retraction and only few will develop a cholesteatoma [1]. There seems to be several prerequisites for the establishment and growth of cholesteatoma, such as attic retraction of any degree, local infection within the retraction due to factors in the external ear canal or infection in the middle ear [10].
As a possible explanation based on clinical and immunohistochemical findings, we propose a pathogenesis of sinus cholesteatoma divided into several stages, combining the retraction and proliferation theory: (1) The retraction pocket stage. (2) The proliferation stage of the retraction pocket, subdivided into (a) Cone formation, (b) Cone fusion. (3) Expansion stage of attic cholesteatoma. (4) Bone resorption.
The sinus retraction pocket stage
The prevalence and incidence of sinus retraction of various severity is less compared to that of attic cholesteatoma (Table 1). Most retractions will never progress to cholesteatoma as migration and self-cleansing of the keratin is well functioning, due to normal migration of the squamous epithelium from the basal cell layers to the surface. In some retraction pockets the keratinized epithelium will start to proliferate and the self-cleansing mechanism will be disturbed.
The factors, which induce and promote proliferation of the epithelium within the retraction, can be the external factors located in the external acoustic meatus, e.g. external otitis with high epithelial turnover or blockage of debris transport due to cerumen at the entrance of the retraction pocket. This may lead to disturbed self-cleansing leading to accumulation of debris within the retraction, crust formation and local infection behind the crust or inclusion cysts of the squamous epithelium (Fig. 1). This local infection can induce cone proliferation. Clinically this condition could be regarded as pre-cholesteatoma. The internal (middle ear) factors are acute middle ear infection causing higher turnover of the epithelium, higher desquamation and accumulation of debris within the retraction leading to the same local infection and crust formation.
Other internal factors are deterioration of the tubal dysfunction and ventilation and of the attic and antrum, causing negative middle ear pressure, pulling parts of the retraction wall into the postero-superior portion of the pars tensa, making it deeper and causing impairment of its self-cleansing. Further internal factors are formation of inflammatory tissue, localized structural damage of the pars tensa with a loss of its regular assembly of the extracellular matrix and adhesion of the attic behind the retraction membrane, pulling it inside, making it deeper and irreversible.
The proliferation stage of the retraction pocket
Inflammatory stimuli of the subepithelial connective tissue or of the bottom of the retraction will alter the keratinocyte proliferation and lead to cone formation (Fig. 7) [19]. The local release of collagenases, which are able to degrade the basement membrane and cause its focal disruptions (Fig. 6), is involved in growth of the cones [20–22]. The basal keratinocyte proliferate due to inflammatory factors are known to be released from the perimatrix [14, 23–27]. This results in an enhanced proliferation of basal cells, results in an active down-growth and elongation of the epithelial cone. Once the cone is established, the intraepithelial maturation of the keratinocytes will differ from that of normal skin. In skin, the basal keratinocyte, which is detached from the basement membrane, will be moved vertically to the surface layer and desquamated as keratin shells (Fig. 7a). Within in the cone the process of keratinization is altered and will result in an accumulation of keratin in the center of the cone. The process of keratinocyte desquamation and differentiation is only altered in its orientation; that means that the basal cell cannot grow to the periphery of the retraction membrane, but to the center of the cone, far underneath of the surface of the retraction pocket (Fig. 7b). Under the pressure of the keratin, the micro-cholesteatoma will increase in size. Several cones will presumably accumulate keratin in the center as small lakes filled with keratin, which will increase in size and gradually fuse with adjacent ones and lead to the expansion the cholesteatoma (Fig. 7c).
Schematic illustration of the proliferation stage. a Normal intraepithelial keratinocytes differentiation and maturation in an epithelial cone orientated vertically towards the surface. Due to initial increase of keratinocyte proliferation the cones have started to grow (Thick arrow). b The keratinocyte differentiation is orientated towards the center of a long cone (arrows) forming small lakes of keratin—the micro-cholesteatoma. c The micro-cholesteatoma lakes are expanding, opening to the surface of the retraction and to the neighboring cones, making the cholesteatoma to expand for the length of a cone
The expansion stage
Fusion and expansion of several micro-cholesteatomas and further expansion to the periphery will cause disappearance of the overlying keratinized epithelium and the cholesteatoma has opened to the bottom of the retraction which hereby has grown in depth by the length of the one cone (Fig. 8a–d). This results in a new surface of the retraction pocket. As the process of proliferation at the bottom of the cone continues, a new accumulation of keratin in the cone will be soon formed in the same manner as described above. It can be imagined that this process continues repeatedly allowing further expansion of cholesteatoma and retraction. Now a vicious circle of the retraction is established in the following way: proliferation at the bottom of the cone, keratin formation within the cones, fusions of micro-cholesteatomas and further accumulation of keratin leading deterioration of cell cleaning.
Schematic illustration of the expansion stage. a Expansion of sinus cholesteatoma, and the lakes of keratin are opened to the surface of the retraction wall in the depth the cones are proliferating and growing. Lateral migration and cell cleaning is superficially still possible (arrow). b The cholesteatoma has expanded by the length of the cone in the depth new micro-cholesteatoma are formed within the new cones. c The keratin lakes are fused, moving the border of the matrix further towards the attic. d Further expansion of attic cholesteatoma. Establishment of a vicious circle in following way: proliferation at the bottom of the cone, keratin formation within the cones, fusions of micro-cholesteatomas and further accumulation of keratin leading to further deterioration of cell cleaning
Bone resorption and further expansion
Once the expansion stage of sinus cholesteatoma is established, further well-known complication of cholesteatoma will appear. Active ingrowth continues and bone resorption of the adjacent ossicles and scutum will be initiated [28, 29].
References
Tos M (1981) Relationship between secretory otitis in childhood and chronic otitis and its sequelae in adults. J Laryng Otol 95:1011–1022
Bezold F (1890) Cholesteatom, Perforation der Membrana flaccida und Tubenverschluß. Z Hals Nasen Ohrenheilk 20:5–29
Lange W (1925) Über die Entstehung der Mittelohrcholesteatoma. Z Hals Nas Ohrenheilk 11:250–271
Ruedi L (1978) Pathogenesis and surgical treatment of the middle ear cholesteatoma. Acta Otolaryngol (Stockh) Suppl 361:1–45
Habermann J (1888) Zur Entstehung des Cholesteatoms des Mittelohres. Arch Ohrenheilkd 27:43–51
Wendt H (1873) Desquamative Entzündung des Mittelohres (“Cholesteatom des Felsenbeins”). Arch Ohr Nasen Kehlkopfheilk 14:428–446
Ulrich K (1917) Beiträge zur Genese des Mittelohrcholesteatoms. Zschr Ohr hk 75:159–166
Sadé J (1971) Cellular differentiation in the middle ear lining. Ann Otol Rhinol Laryngol 80:376–384
Tos M (1990) Chronic otitis media and cholesteatoma. In: English GH (ed) Otolaryngology, vol. I. Lippincott, Philadelphia 23:1–30
Sudhoff H, Tos M (2000) Pathogenesis of attic cholesteatoma. Clinical and Immunohistochemical support for combination of retraction theory and proliferation theory. Am J Otol 21:786–792
Tos M. Stangerup SE, Holm-Jensen S, Sørensen CH (1984) Spontaneous course of secretory otitis and changes of the eardrum. Arch Otolaryngol 110:281–289
Stangerup SE, Tos M, Arnesen R, Larsen P (1994) A cohort study of point prevalence of eardrum pathology in children and teenagers from age 5 to age. Eur Arch Otol Rhinol Laryngol 251:399–403
Tos M, Poulsen G (1979) Changes of pars tensa in secretory otitis. ORL 41:313–328
Tos M, Stangerup SE, Larsen P, Hvid G, Andreassen UK (1989) The relationship between secretory otitis and cholesteatoma. In: Tos M, Peitersen E, Thomsen J (eds) Cholesteatoma and mastoid surgery. Kugler, Amsterdam pp 325–330
Tos M (1997) Pathogenesis of sinus and tensa retraction cholesteatoma. In: Sanna M (ed) Cholesteatoma and mastoid surgery. CIC Edizioni Internazionali, Rome, pp 3–8
Larsen P, Tos M (1992) Ear polyps in posterior superior retraction pockets, Herodion. ORL 54:328–333
Tos M (1988) Incidence,ethiology and Pathogenesis of cholesteatoma in children. Ann Oto Rhinol Laryngol 40:110–117
Sudhoff H., Bujia J, Holly A, Kim C, Fisseler-Eckhoff A (1994) Functional characterization of middle ear mucosa residues in cholesteatoma samples. Am J Otol 15:217–221
Cattoretti G, Becker MHG, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J (1992) Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168:357–363
Weber L, Kirsch E, Müller P, Krieg T (1984) Collagen type distribution and macromolecular organization of connective tissue in different layers of human skin. J Invest Dermatol 82:156–160
Kamel OW, Franklin WA, Ringus JC, Meyers JS (1989) Thymidine labeling index and Ki67 growth fraction in lesions of the breast. Am J Pathol 134:107–113
Liotta LA (1986) Tumor invasion and metastases - role of the extracellular matrix: Rhoads memorial award lecture. Cancer Res 46:1–7
Sudhoff H, Bujia J, Fisseler-Eckhoff A, Schulz-Flake C, Holly A, Hildmann H (1995) Expression of the cell-cycle-related antigen (MIB 1) in cholesteatoma and auditory meatal skin. Laryngoscope 105:1227–1231
Schönermark M, Mester B, Kempf HG, Blaser J, Tschesche H, Lenarz T (1996) Expression of matrix-metalloproteinases and their inhibitors in human cholesteatomas. Acta Otolaryngol 16:451–456
Stark T, Sudhoff H, Fisseler-Eckhoff A, Borkowski G, Luckhaupt H, Hildmann H (1977) Alterations of basement membrane in middle ear choles-teatoma. In: Sanna M (ed) Proceedings of the 5th international conference on choles-teatoma and mastoid surgery, CIC Edizioni Internationali, Roma, pp 238–247
Sudhoff H, Bujia J, Fisseler-Eckhoff A, Borkowski G, Holly A, Koc C, Hildmann H (1996) Basement membrane in middle ear cholesteatoma. Immuno-histochemical and ultra-struc-tural observations. Ann Otol Rhinol Laryngol 105:804–810
Sudhoff H, Dazert S, Gonzales AM, Borkowski G, Park SY, Baird A, Hildmann H, Ryan AF (2000) Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am J Otol 21:793–798
Hamzei M, Ventriglia G, Hagnia M, Antonopolous A, Bernal-Sprekelsen M, Dazert S, Hildmann H, Sudhoff H (2003) Osteoclast stimulating and differenttiating factors in human cholesteatoma. Laryngoscope 113:436–442
Jung JY, Chole RA (2002) Bone resorption in chronic otitis media: the role of the osteoclast. ORL J Otorhinolaryngol Relat Spec 64:95–107
Tos M (1995) Manual of middle ear surgery, vol 2. Thieme, Stuttgart New York
Acknowledgments
Supported by grant 158/99 from FORUM, University of Bochum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudhoff, H., Tos, M. Pathogenesis of sinus cholesteatoma. Eur Arch Otorhinolaryngol 264, 1137–1143 (2007). https://doi.org/10.1007/s00405-007-0340-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-007-0340-y