Abstract
Cholesteatoma is a destructive lesion of the temporal bone that gradually expands and causes complications by erosion of the adjacent bony structures. Bone resorption can result in destruction of the ossicular chain and otic capsule with consecutive hearing loss, vestibular dysfunction, facial paralysis and intracranial complications. Surgery is the only treatment of choice. The etiopathogenesis of cholesteatoma, however, is still controversial. This review was designed to understand the reasons for these disparities and to reduce or eliminate them. Future studies focused on developmental, epidemiological, hormonal and genetic factors as well as on treatment are likely to contribute to further understanding of cholesteatoma pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholesteatoma, once established in the middle ear, mastoid or petrous bone, is a destructive lesion that gradually expands and leads to complications by destruction of the adjacent structures. Erosion caused by bone resorption of the ossicular chain and otic capsule may result in hearing loss, vestibular dysfunction, facial paralysis and intracranial complications. Du Verney probably gave the first description of a cholesteatoma-like mass, which he called "steatoma," in 1683 [37]. The French pathologist Cruveilhier defined cholesteatoma as "tumeur perleé," or pearly tumor of the temporal bone, in 1829 [32]. The term "cholesteatoma" was coined by the German physiologist Johannes Müller in 1838, identifying a layered pearly tumor of fat [85]. The term "cholesteatoma" must be considered a misnomer as this benign tumorous lesion ("-oma") contains neither cholesterine ("chol-") nor fat ("-stea-") [43, 124]. Though many otologists expressed dissatisfaction with this term, others such as "pearly tumor," "margaritoma" or "keratoma" were not destined to last [134]. Three conditions seem to be necessary for the development of cholesteatoma: (1) the unique anatomical situation of the tympanic membrane, (2) chronic or recurrent infections and inflammation of the submucosal tissue in the middle ear and (3) mechanisms known from tissue repair.
Cholesteatoma is characterized by an intrusion of keratinizing stratified squamous epithelium into the middle ear. As soon as the epithelium begins to hyperkeratinize, the destructive behavior of cholesteatoma is triggered [141]. The discussion about how epidermic structures get into the middle ear is still controversial. The present report summarizes and evaluates theories and hypotheses on the etiopathogenesis of cholesteatoma.
Epidemiology
The annual incidence of cholesteatoma was found to be about 3 per 100,000 in children and 9.2 per 100,000 in adult Caucasian inhabitants in Finland and Denmark. There is male predominance [57, 104, 105, 148]. The incidence is about 1.4 times higher in males than in females (age groups: <50 years). The mean +/- SD age of children with congenital cholesteatoma is 5.6+/-2.8 years, while that of children with acquired cholesteatoma is 9.7+/-3.3 years [88]. Epidemiology shows a high prevalence of cholesteatoma among Caucasians followed by people of African descent, while it is scarcely seen in (Non-Indian) Asians [104]. According to Kemppainen et al., cholesteatomas show the same prevalence in all social groups [57]. Interestingly, it was confirmed that the prevalence of cholesteatoma in the Inuit Eskimos is significantly lower than average. The most likely explanation for this observation is that the larger nasopharynx in this ethic group facilitates middle ear aeration and prevents the sequelae of chronic ear disease [107].
Classification
Since the initial stages of research in cholesteatoma there have been numerous discussions about its classification. Classically, three forms have been distinguished: (1) One "type" occurs behind an intact tympanic membrane and is often called "congenital," "primary" or "genuine" cholesteatoma. (2) "Primary acquired" cholesteatoma appears as a limited diverticulum of the pars flaccida, and there is little or no history of otorrhea. (3) "Secondary acquired" cholesteatoma appears with posterosuperior eardrum perforations and extension of the disease process into the antrum, mastoid, attic and middle ear. Granulation tissue, polyps and foul-smelling otorrhea are common [79, 158]. A subsequent classification, according to Tos, is based on the site of origination of cholesteatoma, which is an important factor for the surgical procedure and prognosis: (1) attic cholesteatoma, defined as a retraction of the pars flaccida or Shrapnell's membrane (Fig. 1) extending into the attic or aditus, and eventually into the antrum, mastoid or tympanic cavity, (2) sinus cholesteatoma, as a posterosuperior retraction or perforation of the pars tensa extending to the tympanic sinus, posterior tympanum and beyond and (3) tensa cholesteatoma, as a retraction and adhesion of the entire pars tensa involving the tympanic orifice of the Eustachian tube (it may also extend further into the attic) [145]. In 1964, the Committee of Conservation of Hearing of the American Academy of Ophthalmology and Otolaryngology published a detailed classification for surgery of chronic ear infections [31]. In 1986, Meyerhoff and Truelson attempted to classify cholesteatoma according to its pathophysiology, location, eustachian tube, ossicular defects and presence or absence of complications. They divided the lesions into [77]: (1) primary acquired, (2) secondary acquired, (3) tertiary acquired and (4) congenital. In 1988, Tos proposed an otoscopic classification, which divided cholesteatoma into [144]: (1) attic, (2) pars tensa I (marginal disease) and (3) pars tensa II (central disease). Mills et al. added the fourth category to the Tos classification to include cholesteatomas occurring behind an intact tympanic membrane [82].
Lesions occurring within the temporal bone were classified by Sanna in 1993 into [119]: (1) supralabyrinthine, (2) infralabyrinthine, (3) massive labyrinthine, (4) infralabyrhintine-apical and (5) apical types. According to the sites affected with cholesteatoma, it is useful to classify: S1 for the site where cholesteatoma has started, S2 for when the disease spreads to another site, S3 for when the disease spreads to the third site, S4 for when the disease spreads to the fourth site and S5 for cases in which the primary site and four or more sites are involved. This classification distinguishes between seven sites: the attic and antrum, middle ear, mastoid, eustachian tube, labyrinth and middle fossa [118]. This staging is a practical solution for describing the extent of disease and is clinically relevant. It can be applied to all lesions except for those of the petrous apex, which cannot be diagnosed otoscopically [118].
With the advancement of reconstructive ossicular surgery, a classification of the condition of the ossicular chain is essential to compare different surgical procedures in relation to the primary disease process. The classification is a modification of that of Wullstein and Austin [11, 118, 159]: O0 if the ossicular chain is intact, O1 if the incus is eroded with discontinuity of the ossicular chain, O2 if the the incus and stapes arch are eroded and O3 if the malleus handle and incus are absent and the stapedial arch is eroded.
Another classification considers preoperative complications: It is usually related to the stage of disease and is important during management. Five complications are observed: fistula of the lateral semi-circular canal, facial palsy, total sensorineural hearing loss, sinus thrombosis and intracranial invasion. Three stages are distinguished [118]: C0 for no complications, C1 for occurrence of one complication and C2 for two or more complications.
Tos also suggested a classification of acquired cholesteatoma based on the site of appearance of cholesteatoma: attic cholesteatoma, sinus cholesteatoma and tensa cholesteatoma [147]. Combining otoscopic evaluation of the extent of the lesion, assessment of ossicular condition and the preoperative complications is useful to determine the surgical procedure and compare the results of surgery.
A profound understanding of the pathogenesis of middle ear cholesteatoma is particularly important, since it is the destructive nature of this lesion that is responsible for most of its complications (Fig. 2). The tendency of cholesteatoma to erode bone and the lack of effective, nonsurgical treatment underline the importance of investigating the basic pathomechanisms of this disease. It is generally accepted that cholesteatomas may be classified according to their theoretical mechanism of pathogenesis into two general categories: congenital or acquired.
Congenital cholesteatoma: definition and theories on pathogenesis
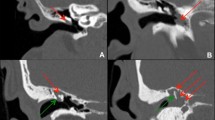
Congenital cholesteatomas have been defined by Derlacki and Clemis as embryonic remnants of epithelial tissue behind an intact tympanic membrane, the patients having no prior history of ear infection or surgery [35] (Fig. 3). Although this definition is widely accepted, it is associated with several uncertainties, as it is essentially a diagnosis of exclusion. Intratympanic cholesteatomas are more common in children than in adults, as is generally expected in congenital diseases [35]. Until the publication of a number of relatively large case series during the last decade [69, 97, 103], congenital cholesteatoma was generally considered to be extremely rare. Congenital cholesteatoma generally grows in the anterior superior quadrant, initially into the posterior superior quadrant and the attic and finally into the mastoid [63]. Spreading within three distinct anatomical sites suggests a natural classification into three types [88]: type 1 if lesions are limited to the middle ear but do not involve the ossicles, except for the malleus manubrium; type 2 if lesions involve the ossicular mass in the posterior superior quadrant and the attic; type 3 if lesions involve the mastoid. Type 1 is controlled by extended exploratory tympanotomy and does not require a second-look reexploration. Type 2 lesions are approached by an extended tympanotomy, but may necessitate atticotomy and canal wall-up tympanomastoidectomy (with or without opening of the facial recess). They require a second-look reexploration. Ossicular reconstruction may be necessary. Type 3 lesions are approached in the same manner as type 2 lesions, but occasionally a canal wall-down tympanomastoidectomy could be necessary. As the type progresses from 1 to 3, the frequency of recurrence increases [88]. Two histologic types of congenital cholesteatoma, an open and closed form, have been described by Karmody et al. [54].
Theories on etiopathogenesis of congenital cholesteatoma
Lucae observed cholesteatoma that appeared to originate from the mucosa of the tympanic cavity in a patient with a normal middle ear and tympanic membrane [72]. This seems to be the first description of what is now considered to be a congenital cholesteatoma. Many theories have been published about the origin of congenital cholesteatoma. In 1854, von Remak postulated that the origin of dermoids and other related tumors is a follicle of skin that has been dislocated during early embryonic life [108]. In 1855, Virchow suggested that congenital cholesteatoma originates from connective tissue [152]. Ruedi postulated that inflammatory injury to the tympanic membrane of the fetus produces microscopic perforations through which the proliferating basal epidermal cell layer invaginates into the middle ear to form a congenital cholesteatoma [113]. Paparella and Rybak suggested that ectodermal implants result from fusion planes of the first and second branchial arches [96]. Aimi proposed an epithelial migration theory assuming that ectodermal cells of the developing external auditory canal accidentally migrate through the tympanic isthmus into the middle ear where they can form congenital cholesteatoma [4]. Another theory was presented by Northrop et al., who hypothesized that viable epithelial cells found in the amniotic fluid could be the source of congenital cholesteatoma [90]. Teed in 1936 and Michaels in 1986 identified an epidermoid remnant in the anterosuperior quadrant of the middle ear of temporal bones of human fetuses, which Michaels called the epidermoid formation (EF) [78, 143] (Fig. 4). It could not be found after the 33rd week of gestation, and it was postulated that its persistance results in the development of congenital cholesteatoma. A recently published paper by Karmody et al. gives histological evidence of the squamous epithelial remnant in two postpartum patients [54]. McGill et al. and Wang et al. confirmed the presence of the epithelial remnant in fetal temporal bones, but could not find evidence of keratinization [75, 153]. Keratinization was never observed in any EF by Kayhan et al. [56]. Contrary to previous reports, they found EFs not only in ears of fetuses, but also in ears of infants and children. Although EFs may persist in some ears, possibly developing into congenital cholesteatomas, our findings do not provide direct support for this concept.
A new acquired inclusion theory presented by Tos is based on the fact that the place of origin of the anterosuperior mesotympanic cholesteatoma is the area of the malleus handle and malleus neck, and of the posterosuperior cholesteatoma, the long process of the incus. Under common pathological conditions, the risk of retraction and adhesion of the eardrum to these ossicles is high. Some of the keratinized squamous epithelial cells may be left behind, trapped in the tympanic cavity forming an inclusion cholesteatoma [146]. Levine et al. recently published another case of postnatal persistence of epidermoid remnants in the human middle ear [70]. However, a clear transition from epidermoid formation to unequivocal cholesteatoma has not yet been demonstrated.
Lee and co-workers found evidence that epidermoid formations are often located close to a developmental epithelial fold, which could close off to produce a cyst [68]. These features support the concept that the epidermoid formation is a precursor of the small anterosuperior congenital cholesteatoma [80].
The reason for persistence of the epidermoid formation in some cases is, of course, subject to speculation. A certain event in cellular regulation that normally results in the regression of the epidermoid formation, or its transformation into middle ear epithelium, may fail to occur. Thus, the unambiguous demonstration of the transition of an epidermoid formation into cholesteatoma must remain subject to further studies.
Primary epidermoid cysts are congenital cholesteatomas [106]. These epithelial remnants of the embryonal period may occur throughout the body with the highest prevalence in the meninges, the brain, the skull base and the temporal bone. This lesion resembles acquired varieties morphologically in that it shows concentric layers of keratin in a cavity that is lined by squamous epithelium and encapsulated by pseudoconnective stroma. Primary epidermoid cyst occurrning in the temporal bone, in other bones of the skull base, within the meninges, the brain and in other parts of the body is known as a congenital cholesteatoma [106]. The pathology is similar to that of the acquired varieties: a squamous epithelial-lined cavity filled with concentric layers of keratin. A pseudoconnective stroma encapsulates it. Progressive exfoliation of keratinous material into the interior of the cyst produces a waxy white material that leads to a slow expansion of the cyst [106]. Viable squamous epithelial cells originating from the amniotic fluid have been found present in the middle ears of neonates. However, the authors found no evidence that these cells could implant into the middle ear to form a congenital cholesteatoma [90].
The epithelial migration theory suggests that ectoderm from the embryonic external auditory canal surmounts the hypothetical restrictive mechanism of the tympanic ring and migrates into the middle ear [4]. This theory could be a possible explanation for the occurrence of congenital cholesteatoma in the posterior and inferior parts of the middle ear, close to the site of the embryonic tympanic ring [63]. Kountakis et al. found that epidermoid cells from the cerebellopontine angle in vitro exhibit migratory properties similar to those of cholesteatoma cells from the middle ear and mastoid cavity. This includes mass migration, a variable migratory rate, random direction of migration and a similar migratory profile. These migratory properties are unique to epithelium derived from the first brachial groove system such as epithelium of the tympanic membrane and cholesteatoma of the middle ear and mastoid [64]. Congenital cholesteatoma could derive from nests of squamous epithelial cells, which occasionally keratinize in the lateral wall of the embryonic tympanic cavity below the level of the pars flaccida. Such epidermoid formation is usually present between the 10th and the 33rd gestational week [78]. The cause of a congenital cholesteatoma could also be remnants of occasionally keratinizing squamous epithelial cells in the lateral wall of the embryonic tympanic cavity below the level of the pars flaccida. These remnants of cells, called epidermoid formations, were observed as early as the 10th week of gestation and consistently disappeared until the 33rd week of gestation [78]. However, discussion about whether the majority of cholesteatomas in the anterior mesotympanum are congenital or acquired remains controversial [63, 146].
The origin of congenital cholesteatoma remains uncertain, but a substantial body of evidence indicates that most congenital cholestaetomas originate as embryonic epidermoid formations in the anterior mesotympanum that fail to involute [63]. Tos presented several clinical and histological reasons for doubts about the epithelial formation theory, rather supporting the acquired inclusion theory as an explanation of the mesotympanic cholesteatoma [146].
Recently, attention has been focused on the telomere length to establish a molecular biological approach for differentiating between congenital and acquired cholesteatoma. Telomeres in congenital cholesteatomas are shorter than those in the acquired variant, which shares telomere length with inconspicuous skin cells of the external ear canal. The level of telomerase activity in congenital cholesteatoma is low. The telomere length in congenital cholesteatoma is shorter than in normal external ear canal skin, whereas in acquired cholesteatoma tissue, the telomere length is almost the same as in normal external ear canal skin. Congenital cholesteatoma may originate from fetal-stage tissue remnants or aberrant tissues [62].
Clinical features of congenital cholesteatoma
Although acquired and congenital cholesteatoma are likely to derive from different pathomechanisms, there is evidence that some cases of congenital cholesteatoma, if untreated, grow and become indistinguishable from acquired cholesteatoma. Congenital cholesteatomas may expand and become super-infected, resulting in bone destruction and chronic ear infection similar to that caused by acquired cholesteatoma [45].
Congenital cholesteatoma in industrialized countries is associated with a lower incidence of intracranial complications, a relatively higher incidence of facial nerve impairment and ossicular destruction and wide-spread malformations, but better postoperative hearing results than the acquired form [34].
Acquired cholesteatoma: definition and theories on pathogenesis
Pathogenesis of "acquired" or "secondary" cholesteatoma has been subject to extensive debates for more than a century. There is evidence for at least four different mechanisms for the development of this disease: (1) squamous metaplasia of middle ear epithelium due to infection, (2) epithelial invasion/immigration through a perforation of the tympanic membrane, (3) invasive hyperplasia of the basal cell layers, (4) retraction pockets and invaginations of the tympanic membrane due to eustachian tube dysfunction and (v) a conceivable combination of the mentioned theories [67, 145] (Fig. 5).
Squamous metaplasia theory
In 1864, von Tröltsch regarded the stratified keratinizing squamous epithelium of cholesteatoma as a metaplastic product of the middle ear mucosa under the pressure of dried, caseous pus [149]. Wendt (1873) suggested that the non-keratinizing epithelium of the middle ear and the mastoid could undergo a metaplastic transformation into keratinizing epithelium. This could result in cholesteatoma as a result of inflammation [154]. Sadé supported this theory and pointed out that the pluripotent middle ear epithelium could be stimulated by inflammation into a metaplastic stratified squamous epithelium with keratinization [115]. The metaplastic epithelium grows becasue of the accumulation of keratin, and recurrent infection and inflammation leads to lysis and perforation of the tympanic membrane resulting in the typical appearance of attic cholesteatoma. The metaplasia theory is supported by the observation that middle ear mucosa biopsies obtained from pediatric otitis media patients with effusion occasionally display islands of keratinizing epithelium [114]. Chole and Frush described metaplasia of the normal middle ear mucosa into a lightly keratinizing squamous epithelium in rats under extreme vitamin A deprivation [25]. However, there is no direct histological or experimental evidence that these mechanisms lead to cholesteatoma formation [42].
Immigration theory
Habermann in 1888 and Bezold in 1890 proposed the epithelial immigration or invasion theory, suggesting that squamous epithelium migrates from the margin of a tympanic membrane perforation into the middle ear spaces [15, 46]. In 1901, Politzer observed that the epidermis of the external auditory canal can grow over a perforation of the tympanic membrane into the middle ear cavity. He assumed that after perforation of the tympanic membrane, a second infection would be the cause of epithelial migration [46], (Fig. 6). Immigration of epithelium from the tympanic membrane into the middle ear of infected guinea pig bulla resulting in cholesteatoma was shown by Friedmann [42]. Similarly, Steinbach, Wright et al. and Masaki et al. demonstrated that a mixture of propylene glycol and antibiotics caused perforations and immigration of epidermis into the middle ear of chinchillas and rabbits [73, 130, 156].
Basal hyperplasia theory
Lange observed in 1925 that epithelial cells from the keratinizing epithelium of the pars flaccida could invade into the subepithelial space and form an attic cholesteatoma [67]. Ruedi provided experimental and clinical evidence for this theory of cholesteatoma pathogenesis [113]. Further studies with human specimens by Lim et al. and also animal experiments have confirmed that the basement membrane focally dissolves and gives access for the keratinocytes to invade the subepithelial space [26, 42, 71, 157]. Pseudopodia from the basal cell layer seem to break the basal lamina, allowing epidermal keratinocytes to invade the lamina propria [137]. The entrapped cells keratinize and form inclusion cysts that may expand and form cholesteatoma.
Retraction pocket or invagination theory
The retraction pocket or invagination theory was presented by Wittmaack in 1933 and is generally accepted as the most common pathogenetic mechanism in acquired cholesteatoma [155]. According to this theory, the pars flaccida, or occasionally the pars tensa, of the tympanic membrane retracts into the middle ear. The underlying pathomechanism of retraction pocket formation is likely to be related to negative pressure, inflammation or both. The retention of accumulating keratin within the deep retraction pocket establishes cholesteatoma. The loss of drainage of the retraction pocket is thought to induce the expansion into the middle ear and mastoid spaces. An alterated epithelial migration pattern in these deep retraction pockets was observed by Sadé [115, 116].
Clinically, each of these pathogenetic mechanisms appears to account for acquired cholesteatoma. Although there is experimental and observational evidence for each of the four mentioned theories, none of them is unequivocally proven.
The pathogenesis of two further types of acquired cholesteatoma is quite apparent: (1) the iatrogenic cholesteatoma, manifesting after previous surgery for non-cholesteatomous middle ear disease, like tymanoplasty or grommet insertion and (2) posttraumatic cholesteatoma, which originates from trauma scattering squamous epithelum into the middle ear [76, 160].
Topology and subclassification of acquired cholesteatoma
Attic cholesteatoma
Attic cholesteatoma is defined as a retraction of the pars flaccida or Shrapnell's membrane extending into the attic and eventually into the antrum, mastoid or tympanic cavity [147]. Several theories on the pathogenesis of attic cholesteatoma have been framed: the retraction theory, the papillary proliferation theory, the immigration theory and the metaplasia theory. A combination of the retraction theory and the proliferation theory has been suggested as an explanation for the pathogenesis of attic cholesteatoma [132]. The authors divided the pathogenetic process into several stages. The first stage is a retraction pocket. Most retractions will never progress to cholesteatoma because of migration of the squamous epithelium from the basal cell layer to the surface; thus, self-cleansing of the retraction pocket functions well. In a few retraction pockets, the keratinized epithelium starts to proliferate, and the self-cleansing mechanism is disturbed. This may lead to accumulation of debris within the retraction, crust formation and local inflammation behind the crust. Clinically, this condition could be regarded as precholesteatoma. At this point, the proliferation stage of the retraction pocket begins. It leads to cone formation: The basal keratinocytes proliferate resulting in elongation of epithelial cones. Once a cone is established, the intraepithelial maturation of the keratinocytes is different from that of normal skin. Keratin accumulates in the center of the cone. Under the pressure of the keratin, the microcholesteatoma increases in size and fuses, and the cholesteatoma expands. Expansion of several microcholesteatomas is the next stage in the pathogenesis of attic cholesteatoma. The bottom of the retraction pocket is open and the cholesteatoma grows deeper into the middle ear spaces in this stage. Once the expansion stage of attic cholesteatoma is established, bone resorption of the adjacent ossicles and scutum is initiated [132].
Sinus cholesteatoma
Sinus cholesteatoma is defined as a postero-superior retraction or perforation of the pars tensa extending to the tympanic sinus, posterior tympanum and beyond [147].
The sinus tympani has been the focus of clinical interest because cholesteatoma has the tendency to invade it. It was found that the sinus tympani is bounded laterally by a constant ledge of bone anterior to the facial nerve. The microscope enables the surgeon to examine this region because the orifice of the retraction pocket is more or less perpendicular to the axis of the external auditory canal. Authors emphasize the importance of removal of the lateral lip of bone to expose the orifice of the sinus tympani [12].
Tensa cholesteatoma
Tensa cholesteatoma is defined as retraction and adhesion of the entire pars tensa involving the tympanic orifice of the eustachian tube. It may also extend further into the attic [147]. Lesions involving the pars tensa are not uncommon in clinical practice [128]. Mechanisms such as metaplasia, ectopic epidermis remnants or ingrowth of meatal epidermis have been proposed to explain the pathogenesis of cholesteatoma behind an intact tympanic membrane. Histopathologic findings in temporal bones emphasize the role of an acquired epidermal remnant. Cholesteatoma behind an intact tympanic membrane can originate from a resolved retraction of the pars tensa [133]. Retraction of the pars tensa minimizes middle ear volume and thus according to Boyle's law (volume x pressure is constant) acts as a pressure buffer. A direct correlation was found between the degree of atelectasis and the middle ear volume replaced by the atelectasis. Retraction of the pars tensa may counteract negative middle ear pressure, depending on the degree of retraction and the extent of mastoid pneumatization [117]. The management includes surgical procedures ranging from grommet insertion to extensive tympanoplasty [128].
Methodological concepts in cholesteatoma research
Regardless of their underlying pathogenesis, all cholesteatomas share similar properties. Their common clinical hallmarks are invasion, migration, hyperproliferation, altered differentiation, aggressivness and recidivism. Animal experiments, clinical trials, cell and tissue cultures as well as the application of modern technologies (e.g., immunohistochemistry, in-situ hybridization, polymerase chain reaction and microarrays) might improve understanding of the biological properties and the pathophysiology of this lesion.
Epithelial proliferation and differentiation
Pathogenesis of acquired and congenital cholesteatoma brings up two fundamental questions: (1) what is the origin of the keratinizing squamous epithelium and (2) what influences invasive and hyperproliferative behavior of the epidermis?
Cholesteatoma can be regarded as a disorder of growth control. This allows the formulation of two different hypotheses. It is generally accepted by now that the stratified squamous epithelium is derived from the epidermis of the tympanic membrane and external canal skin. With regard to the second question, the behavior of cholesteatoma tissue may be induced by a loss of growth-inhibiting signals, by an increase in growth-promoting factors or both of these changes. Before conclusions like these can be made, it is necessary to objectively confirm the well-known clinical observation that cholesteatoma shows high growth activity.
Cell cycle analysis and proliferation markers
Several comparative investigations have been performed to assess the epithelial cell kinetics of cholesteatoma using normal meatal skin as a control (Fig. 7). In one of these studys, MIB 1 (Ki-67) immunostaining, which reacts with a nuclear antigen expressed by dividing cells, was applied [136]. Specimens of normal auditory meatal skin expressed an average MIB 1 score of 7%. Cholesteatoma samples expressed an average MIB 1 score of 17% and heterogeneity of proliferating epithelial areas [136] (Fig. 7). Epithelial cones growing toward the underlying stroma exhibited high mitotic activity. The results of this study confirm a highly significant increase in the proliferation rate of cholesteatoma keratinocytes with an MIB 1 score that was 2.3 times higher than the score for keratinocytes of normal external auditory meatal skin.
As a result of the various approaches to evaluating proliferative activity, cytokeratin 13/16, proliferating cell nuclear antigen (PCNA) and thrombomodulin (TM) have recently been introduced as markers of cellular proliferation [100]. PCNA is a 36 kD nuclear antigen that appears at S and G1 phases of the cell cycle [16]. PCNA positive cells have been detected in the suprabasal and parabasal layer as well as in the basal layer of cholesteatoma epithelium. Compared to normal skin, cholesteatoma epithelium exhibits a significantly higher percentage of PCNA positive cells. Staining intensity was different in the various areas of the epithelium and did not depend on the thickness of the epithelium [91]. Park et al. conducted studies on induced cholesteatoma in Mongolian Gerbils demonstrating that induced cholesteatoma keratinocytes have higher mitotic capacity than normal keratinocytes also seen in the suprabasal layer. These studies suggest that extensive accumulation of keratinous mass in the external auditory meatus may induce changes in cellular differentiation and proliferation. The authors explain the increased mitotic activity by the high number of newly formed keratinocytes in the suprabasal layer as well as in the basal layer and by the increased turnover rate of the basal cells [99]. As the experiments conducted by Soyer et al. in specimens of psoriasis and melanocytic skin tumors demonstrated, antigen Ki-67 is a marker of cell proliferation of the epidermis [127]. The presence of Ki-67 positive cells has been observed in the suprabasal layer and stroma of cholesteatoma in a significantly higher percentage than in normal skin [20, 91]. Thrombomodulin (TM) is a cell surface glycoprotein that forms a high affinity non-covalent complex with thrombin and is a differentiation marker for spinous layer keratinocytes [84]. Therefore, strongly positive staining for TM in the suprabasal layer is very meaningful [100]. Quantification of the proliferating activity of the epithelium allows the estimation of cholesteatoma aggressiveness, the intensity of bone destruction and the likelihood of recurrence [22]. Further studies using proliferation markers such as PCNA, AgNOR (argyrophilic nuclear organizer regions) and thymidine labeling showed comparable results and proved the high proliferative activity of cholesteatoma epithelium [22, 93, 136, 139]. For instance, Sudhoff et al. observed a similar proliferative activity in both the congenital and the acquired variant of cholesteatoma in AgNOR staining [139].
Having in mind the observation that cholesteatoma does have such a high proliferative activity, it is necessary to inquire if it can be a form of low-grade neoplasia. Analysis of the DNA content of cholesteatoma could provide evidence for or against this hypothesis.
DNA content
In a study performed by Desloge et al., the DNA content of ten cholesteatoma and six postauricular skin specimens was compared using flow cytometry; the DNA content of some cholesteatoma specimens was also analyzed using image analysis [36]. In one cholesteatoma specimen, an abnormal aneuploid DNA content was found, whereas the remaining nine cholesteatomas and the six postauricular skin specimens had a normal euploid DNA content. The authors conclude that considering the lack of overt genetic instability as evidenced by the presence of a normal euploid DNA content, cholesteatomas cannot be regarded as low-grade neoplasms [36].
Lipids
Cholesteatomas do not contain fat, although biochemical examinations of the matrix have revealed high concentrations of cholesterol and cholesterol ester [47, 112]. Cholesterol imprints may be seen histologically. The intracellular lipids are present in the keratinocytes of the stratum granulosum. They are synthesized and stored in the Odland bodies. Extracellular lipids in cholesteatoma are metabolized from the contents of Odland bodies and consist of fatty acids, ceramides and cholesterol. The synthesis of barrier lipid is normally controlled by the composition of the barrier. Immediately after barrier breakthrough, signal molecules are liberated that start the cytokine cascade. The balance between lipid secretion and lipid loss is disturbed in cholesteatomas [38, 89]. Defective growth signal transduction and increased cytokine expression has been demonstrated in cholesteatoma [86, 142].
Cytokeratins and differentiation markers
Several studies have shown the invasive and hyperproliferative behavior of epidermis in cholesteatoma. It could be demonstrated to be surely benign. As a next step, it is necessary to ask for the origin of the keratinizing squamous epithelium in cholesteatoma. To investigate this question, numerous immunohistologic studies have been performed to compare the localization of markers of epidermal differentiation in cholesteatoma and normal external ear canal skin [19, 65, 151]. Cytokeratins (CK) are intermediate filament proteins that are exclusively present in epithelial cells. The expression of different types of CKs (numbered 1–20) varies with the type of epithelium and the stage of differentiation. Because of these properties, CKs seem suitable for a better understanding of the mechanisms of squamous cell metaplasia of the middle ear.
As the pathogenesis of cholesteatoma (migration versus metaplasia) has been subject to vigorous debates, similar cytokeratin (CK) patterns could be demonstrated in keratinocytes of cholesteatoma, normal auditory canal skin and tympanic membrane skin [65]. Kakoi and co-workers studied the differentiation markers involucrin and filaggrin as well as glycoproteins and found an abnormal distribution of all three of them in both basal cells and suprabasal cell layers of cholesteatoma [53].
However, not all of the cholesteatomas showed hyperproliferative properties: The expression of CKs 6, 16 and 19 in cholesteatoma epithelium showed hyperproliferative patterns. Patterns of the terminal differentiation of CKs 1, 5, 10 and 14 in cholesteatoma were basically the same as those in skin. In cholesteatoma, each CK gradually decreased in molecular weight in the granulated layer and debris [53].
Using gel electrophoresis and immunohistochemistry, Bujia and co-workers demonstrated the presence of cytokeratin 16 in cholesteatoma samples [19]. Their results provided additional support for the hyperproliferative character of cholesteatoma epithelium.
Analyzing the cytokeratin pattern, Kujipers et al. concluded that the matrix of cholesteatoma is not the result of a metaplastic change. They found cholesteatoma epithelium similar to that of tympanic membrane and ear canal skin, but in various states of proliferation, dependent on the degree of inflammation present [65]. The cytokeratin patterns are known to be affected during the formation of aural cholesteatoma. In a recent study in an animal model of aural cholesteatoma, Kim and Chen employed immunohistochemical methods to investigate the distribution of cytokeratin and the binding patterns of lectin [58]. They conclude that the origin of aural cholesteatoma may be external auditory canal epidermal cells and that the characteristics of these cells do not change during cholesteatoma development. According to Kim et al., the patterns of cytokeratin expression correspond well with the state of keratinocyte proliferation, migration and differentiation. The authors used an animal model with experimental induction of cholesteatomas in gerbils by external auditory canal ligation (ECL). This animal model allowed the investigation of CK expression in various clinical stages of cholesteatoma. The results of this study support the concept that the normal keratinizing epithelium of the tympanic membrane undergoes certain changes as it forms a cholesteatoma in the external canal and middle ear. An increased expression of CK5/6 and CK13/16 is found in the suprabasal epithelial layer of the external auditory canal in stages I through III cholesteatoma. This gives further evidence that cholesteatoma epithelium shows increased proliferation of keratinocytes, especially in the pars tensa of the tympanic membrane near the malleus. Additionally, the increased expression of CK4 in the external meatal skin close to the tympanic membrane and the transition of the CK13/16 expression from the basal to the suprabasal epithelial layer suggest that the epithelium migration might be increased from the outer to the inner portion of the auditory canal [60].
Investigations by Kim and co-workers based on an animal model of induced retraction pocket cholesteatomas also revealed that aural cholesteatoma is a disease with a spectrum of pathological conditions. In this study, the authors found that CK expression is altered only in focal parts of retraction pocket cholesteatoma and that the significant CK change is located at sites adjacent to cholesteatoma, not it the cholesteatoma itself. This might explain that transmigration and hyperproliferation of squamous epithelium occurs in areas adjacent to the cholesteatoma [59].
Arriaga et al. used monoclonal antibodies against cytokeratin subtypes in cholesteatoma to try to inhibit growth of keratinocytes in case of residual disease [10]. They investigated the keratinocyte cytotoxicity of monoclonal antibodies directed against a cytokeratin subtype relatively unique to cholesteatoma. Their experiments showed that monoclonal antibodies do not have an in vitro activity against keratinocytes. However, additional investigation of a possible role of cytokeratin monoclonal antibodies is required with the goal of developing a clinically useful biologic adjunct for cholesteatoma management.
Tumor-suppressor genes, transcription factors and apoptosis
Cases have been described where congenital cholesteatoma underwent spontaneous elimination [62]. Not every ectodermal tissue invaded or remaining as a remnant in the mesenchymal tissue grows and forms cholesteatoma. Under normal conditions, such ectodermal tissue is naturally eliminated by means of apoptosis [62]. It has been proposed that the development of human cholesteatoma is due to the altered control of cellular proliferation, which tilts the balance toward the aggressive, invasive growth of squamous epithelium into middle ear. However, whether the cause of this altered control is a defect in the mechanisms and underlying genes that control proliferation, or cytokines released from infiltrating inflammatory cells or another mechanism, is unknown [8] (Figs. 8 and 9.
The nuclear phosphoprotein p53 tumor suppressor gene plays a pivotal regulatory role in cell cycle control and apoptosis. Congenital, primary, secondary acquired and recurrent cholesteatomas have been analyzed by Albino and co-workers for the altered expression of p53 [6]. Albino's experiments show that the expression of p53 in cholesteatoma is 9–20 times higher than in normal postauricular skin or tympanic membrane. A possible explanation for these findings could be a defect in the mechanisms that p53 engages (i.e., cell cycle control or apoptosis or both) in cholesteatoma. Furthermore, Albino's results suggest that there is no intrinsic difference between any clinical group of cholesteatomas, at least with respect to p53 expression and, presumably, p53 function [6].
Recent publications have shown that the c-jun protein promotes keratinocyte proliferation and p53 induces apoptosis of various cells. C-jun functions as a transcription factor for many genes and the p53 protein functions as a negative regulator of cellular proliferation and is involved in the apoptosis pathway that induces DNA damage. In a study by Shinoda and co-workers, the presence of c-jun and p53 in cholesteatoma was demonstrated by immunoblotting assays [126]. Cholesteatoma tissue incubated with anti-c-jun antibody showed a staining of keratinocytes of the basal and spinous layers of the epithelium. The c-jun protein was located in the basal layer of normal skin, and the p53 protein was present in the nucleus of keratinocytes in the granular layer of cholesteatoma epithelium. Keratinocytes of normal external ear canal skin and normal human skin were only slightly stained in the granular layer of the epidermis. These results suggest that c-jun and p53 proteins may play a role in keratinocyte differentiation, proliferation and apoptosis in cholesteatoma [129].
An increased rate of keratinocyte cell death compared to normal skin causes an accumulation of keratin debris in the middle ear. The death of keratinocytes (terminal differentiation) in the epidermis may constitute a form of apoptosis [102]. In recent studies, apoptotic cells were found in the suprabasal layers of cholesteatoma epithelium in a higher percentage than in normal skin [91, 98]. The preliminary data of a study conducted by Choufani and co-workers indicate that cholesteatomas with a high apoptotic index are the most prone to recurrence. The distribution of apoptotic cells in the cholesteatomas' epidermal compartment was the second most reliable variable in the prediction of recurrence of the disease selected by discriminant analysis. The distribution of apoptotic cells in nonrecurrent cholesteatomas was more heterogeneous than in recurrent ones. This also means that recurrent cholesteatomas exhibit a higher fraction of apoptotic cells [28].
According to Miyazaki et al., the process of terminal differentiation in the cholesteatoma epidermis proceeds in the same manner as in normal skin. Moreover, according to this study, the following scenario of events appears conceivable: First, while the cholesteatoma epidermis is in a state of hyperproliferation due to the elevation of the levels of various cytokines, regulation is achieved through normal progression of terminal differentiation and apoptosis. This leads to an abnormal accumulation of keratinous debris, which is the characteristic feature of middle ear cholesteatoma [83].
An antiapoptotic compound known as galectin3 is a protein that may influence the events in cholesteatoma epithelium. Sheikholeslam-Zadeh et al. discovered that this protein promotes antiapoptotic effects and may thus be a protective mechanism against the intensified apoptosis present particularly in recurrent cholesteatoma [125].
Apoptosis in cholesteatoma is markedly intensified compared to skin. Hyperproliferation in cholesteatoma epithelium on the other hand counteracts apoptosis. The balance between hyperproliferation and apoptosis creates a higher cell turnover, which may be an important factor limiting the preneoplastic defect [39].
In a variety of cells, c-myc oncogene has been found to be highly linked to the control of growth and differentiation. The expression of c-myc was studied in cholesteatoma epithelium using a monoclonal antibody against the 67 kD c-myc gene product. Holly et al. found that the incidence of c-myc is significantly increased in nuclear staining of cholesteatoma epithelium [48]. Simultaneously, in cytoplasmatic staining, c-myc was observed in both skin and cholesteatoma, with a stronger reactivity in the latter. These results also suggest a participation of the c-myc oncogene in the reported dysregulation of cholesteatoma epithelium. Kojiama et al. observed that the proliferation of epidermal cells in cholesteatoma is not uncontrolled as it is in malignant tumors. They observed an increase in the proliferation rate and apoptotic cell death in cholesteatoma epidermis [61].
Annexin II has previously been discovered to be involved in DNA replication and metabolism, bone resorption and osteoclast formation. Immunoalkaline-phosphatase staining by Huang et al. selectively localized annexin II in the keratinocytes in the basal and spinous layers of cholesteatoma tissue [49]. In normal human skin, annexin II was expressed mainly in the cytoplasmatic membrane of keratinocytes in the basal layer without significant staining of the nuclei. However, annexin II was expressed in both the cytoplasmatic membrane and the nuclei of the keratinocytes in basal and spinous layers of human cholesteatomas. This observation could be an indication for a physiologic role of annexin II in keratinocyte cell hyperproliferation during the development of human cholesteatoma [61].
Growth factors and cytokines
A characteristic sign of acquired cholesteatoma is the infiltration of the stroma with immune cells [5]. Many markers are responsible for the special behavior of acquired cholesteatoma, such as interleukin-1α, tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), lymphocyte functional antigen-1 (LFA-1), etc. Cholesetatoma matrix is characterized by a keratinocyte dysregulation with an aggressive growth that leads to the destruction of normal middle ear mucosa. The abnormal behavior of cholesteatoma epithelium seems to be induced by the presence of a heavy immune cell infiltrate releasing high amounts of different cytokines and growth factors. In normal epidermis, the restriction of keratinocyte proliferation to the basal cell layer is controlled by the local expression of transforming growth factor-alpha (TGF-alpha) and epidermal growth factor (EGF), and by the down regulation of epidermal growth factor-receptor (EGF-R) in the suprabasal cell layers. Several studies have reported defects in the regulation of the EGF-R system in cholesteatoma [18, 17, 135]. In contrast to normal external meatal skin, the high expression of the EGF-R in cholesteatoma is not confined to basal kertinocytes, but is also found in suprabasal keratinocytes in the stratum spinosum and granulosum. As many as 75% of the keratinocytes in cholesteatoma express EGF-R, whereas only 10% of the keratinocytes of the external meatal skin express EGF-R [14].
Psoriatic keratinocytes express the receptor for IFNγ (interferon-gamma) [150]. IFNγ increases the in vitro expression of EGFR. It may thus be a mediator for the overexpression of EGFR in psoriatic keratinocytes [41]. Both, IFNγ and IL-1 induce the expression of the intercellular adhesion molecule-1 (ICAM1), which is a member of the immunoglobin superfamily and is the ligand of the lymphocyte function-associate antigen-1 (LFA1) [101]. In a study conducted by Ottaviani and co-workers, a cytokine and adhesion molecule pattern indicating an activation state has been observed in all cholesteatoma specimens. In the perimatrix, the endothelial cells express the inducible molecules ICAM1 and ELAM1 (endothelial-derived leukocyte adhesion molecule-1), which play a role in recruiting inflammatory cells and modulating immune response. The basal layer of cholesteatoma matrix is positive for ICAM1. The overexpression of IFNγR in the matrix reinforces the hypothesis that the hyperproliferation of the epithelial cells of secondary cholesteatoma is mediated through lymphokines [94].
Further studies investigate the presence of interleukin-1 (IL-1), transforming growth factor-alpha (TGF-alpha) and epidermal growth factor (EGF) [120, 135]. The findings were compared to surrounding stroma with an enhanced immune cell infiltrate. Cholesteatoma epithelium showed a high staining intensity of IL-1, TGF-alpha and EGF-R. In contrast, middle ear mucosa did not show any positive reactions for the mentioned factors. The authors found a high concentration of lymphocytes and macrophages in the stroma surrounding cholesteatoma (Fig. 10). Most of these cells expressed TGF-alpha and IL-1, showing activation. Keratinocytes of the middle ear mucosa do not appear to react to the inflammatory stimuli released by cholesteatoma. This observation suggests important differences in the cell biological features of the keratinocytes in middle ear mucosa and cholesteatoma. Wright and co-workers investigated external and middle ear pathology in TGF-alpha-deficient animals [158]. The experiments were performed in the waved-1 mutant mouse, which is severely deficient in TGF-alpha: Morphologic changes of the external and middle ear were studied histologically at 2 weeks to 6.5 months of age. Abnormalities found in the mutants included epidermal hyperplasia of the external ear canal (EAC) and tympanic membrane (TM) and enlargement of specialized sebaceous glands adjacent to the cartilaginous EAC. Sebum and desquamated keratin progressively accumulated within the EAC, pushing the TM into the middle ear. However, the authors could not support the idea of TGF-alpha being an important player in the pathogenesis of cholesteatoma [158].
Besides, platelet-derived growth factor (PDGF), granulocyte-macrophage colony stimulating factor (GM-CSF) and cytokines such as tumor necrosis factor-alpha (TNF-alpha) seem to play a significant role in cholesteatoma pathogenesis [44, 49, 50, 161, 162] (Fig. 11). Investigations on growth factors in cholesteatoma by Ishibashi et al. revealed a significantly higher expression of keratinocyte growth factor (KGF) mRNA in cholesteatoma compared to normal skin, while no significant difference in KGFR mRNA expression was noted between cholesteatoma tissue and skin [51]. These findings suggest that excessive KGF synthesis may contribute to the hyperproliferative state in cholesteatoma.
Kato and co-workers recently reported that cholesteatoma cells produce IL-6 and TNF-alpha [55]. IL-6 and TNF-alpha produced in middle ear cholesteatoma might aggravate the disease by the wide range of their biological activities on the mucociliary system, cell proliferation and bone metabolism [55, 161, 162]. IL-1alpha and IL-8 from the cholesteatoma epithelium may be responsible for bone destruction and osteoclast activation. Certain substances relieved from the subepithelial granulation tissue seem to stimulate IL-1 alpha and IL-8 production in cholesteatoma [29].
Results published by Bujia and co-workers point out an over-expression of IL-1 accompanied by a decreased secretion of IL-1-receptor antagonist in middle ear cholesteatoma [21]. Furthermore, IL-1- receptor antagonist production was on a significantly lower level than total IL-1 production, resulting in a predominance of active IL-1. This may lead to an increased activation of osteoclasts and cause bone resorption [21].
Inflammatory reaction
Inflammation is always observed in cholesteatoma specimens. It represents a sequence of complex, interrelated events that occur as a response to tissue injury. Inflammation is induced by an immune response to different stimuli. An inflammatory response is essential for the repair and restoration of structural and functional integrity of damaged tissue. Irrespective of the nature of the occurring events, the wound healing process follows a predictable pattern, partly comparable to cholesteatoma development. The occurring events can be divided into early or acute inflammation, a proliferative phase characterized by fibroblast and endothelial cell proliferation and finally matrix synthesis and scar formation. In case of persistent inflammatory stimuli, such as in cholesteatoma, the response may be chronic, resulting in pathological alteration of tissue behavior. Cholesteatoma pathogenesis can be interpreted as the reaction of migrating epithelium to an ongoing inflammation and bacterial infection, because cholesteatomas are usually associated with inflammatory reactions in the middle ear cavity, and inflammatory granulation tissue often appears along the invading epithelium in active cholesteatomas [71, 113].
An intense infiltration of the stroma of cholesteatoma by immunologically activated T-cells and macrophages indicates a chronic inflammatory response. Inflammation of the subepithelial connective tissue may contribute to the aberrant behavior of cholesteatoma epithelium [95]. The density of the macrophages in normal ear canal skin was found to be much higher in the upper dermis than in the lower dermis [95] (Fig. 10). A great number of phagocytic cells closely resembling dermal macrophages was found in the stroma of the cholesteatomas. These cells probably contribute to an active immune process [87]. Mast cell concentration in cholesteatomas is approximately three to seven times higher than in other tissues. Besides, 19–34% of the mast cells in cholesteatoma are found in the suprabasal layers of the squamous epithelium. This observation can only be made in grossly inflamed tympanic membrane specimens, but not in other control tissues, including minimally inflamed tympanic membranes. Albino and co-workers conclude from these results that mast cells may play a previously overlooked role in cholesteatoma pathology [7].
Extracellular matrix
The physiological functions of extracellular matrix (ECM) macromolecules such as collagen, fibronectin, intergrins and glycosaminoglycans are linked with cell adhesion, cell migration, cell growth and differentiation. Quantitative and qualitative alterations of ECM may be induced by cytokines that modify connective tissue cell metabolism. Penetration of the submucosal space by middle ear infection and cell necrosis starts the wound healing cascade. In wound healing, connective tissue fibroblasts and macrophages play a pivotal role. Cytokines of wound healing promoting the re-epithelization of mucosal defects and scaring become effective on the intact squamous cell layer of the outer surface of the ear drum at the same time. Thus, a proliferation of the undamaged epithelial layer is induced. Persistence of the inflammation may cause permanent wound healing, proliferation of fibroblasts and epithelium in the perimatrix.
Dallari et al. investigated the expression of integrin adhesion molecules in primary acquired and recurrent cholesteatomas and compared it to common epidermal cysts and normal human skin [33]. In these experiments, cholesteatoma epithelium exhibited a markedly augmented expression of alpha v integrin subunit and a corresponding accumulation of vitronectin in the surrounding stroma. In contrast, the expression pattern of alpha 2 beta 1, alpha 3 beta 1 and alpha 6 beta 4 integrins as well as the distribution of laminin, collagen IV and fibronectin were similar in cholesteatomas, epidermal cysts and normal human skin [33].
Consistent with other authors, we found immunohistochemical and ultrastructural alterations in the distribution of the basement membrane zone (BMZ) components collagen type IV, collagen type VII and fibronectin in human middle-ear cholesteatoma compared to auditory meatal skin [129, 137]. Collagen type IV immunoreactivity of skin and middle ear mucosa displays a continuous line in the BMZ, whereas in cholesteatoma focal discontinuities are frequently observed. In cholesteatoma, on the other hand, fibronectin immunoreactivity is markedly increased in the extrinsic BMZ and subepithelial connective tissue. The ultrastructural arrangement of the BMZ of cholesteatoma and skin is grossly the same. However, it exhibits distinct alterations of the lamina fibroreticularis and lamina densa (Fig. 12). Our results outline cholesteatoma as a disease with disturbed cell matrix interactions analogous to those of wound re-epithelization. Lang and co-workers also found a marked expression of tenascin. Fibronectin reactivity was observed as a continuous band along the epidermal-stromal junction, extending to the deeper stroma [66]. Additionally, in cases of intensified TGF-beta expression, beginning collagen fibril formation was observed in adjacent deeper stroma layers, indicating beginning stromal fibrosis. These observations suggest that TGF-beta may be involved in the stimulation of the synthesis of tenascin, fibronectin and collagen. Furthermore, the enhanced expression of tenascin and fibronectin provides evidence for a dysregulated cell-matrix interaction in the enhanced proliferative process of cholesteatoma formation.
Degradation of extracellular matrix (ECM) and proteolysis
As the ECM displays obvious alterations in cholesteatoma, it is reasonable to investigate degrading enzymes capable of disturbing the ECM. Among other enzymes, Abramson investigated collagenase in the subepithelial connective tissue of cholesteatoma, granulation tissue from the middle ear and the dermis of canal skin [1, 2]. He could not prove the presence of collagenase in the keratin layer, epithelium or the epidermal appendages. Expression of collagenase enhanced by chronic inflammation attacks the intact collagen molecule, making it susceptible to further digestion by other proteases that are also products of inflammation. This process brings about resorption of connective tissue and bone. The proteolytic erosion of the temporal bone may play an important role in the process of cholesteatoma progression. Recently, a new family of proteolytic enzymes, the matrix-metalloproteinases (MMP), has been identified. MMPs seem to play a pivotal role in matrix and bone homeostasis as well as inflammatory osteolytic diseases, e.g., osteoarthritis and periodontitis. They are controlled in a sophisticated manner by specific inhibitors and activation cascades. Schönermark et al., Stark et al. and Banerjee and co-workers investigated the expression of MMPs and MMP-inhibitors in human cholesteatoma tissue [13, 14, 123, 129]. By immunocytochemistry and zymography, the expression of MMP-1, MMP-2, MMP-9 and MMP-3 was demonstrated to be strictly confined to the basal and suprabasal cell layer of cholesteatoma epithelium. Neutrophil collagenase showed a more disseminated expression in the epithelium and granulation tissue. The tissue inhibitor of MMPs, TIMP-1, could be detected only in very limited areas of the granulation tissue in a quite disseminated manner [129]. Interpreting these findings, a derailment of the normally tightly controlled MMP system in favor of proteolysis was postulated and found to play an active role in the molecular mechanism of cholesteatoma invasion into the temporal bone [123, 129].
Bone resorption
Bone resorption is stimulated by a variety of factors, including inflammation, local pressure, keratin and specific cytokines, such as interleukins (IL-1 α, IL-1 β and IL-6), interferon-beta (INF β) and parathyroid-hormone-related protein (PTHrP), which are all known to be released by cholesteatoma [3, 120] (Fig. 13). Inflammatory mediators such as cytokines, prostaglandins, NO (nitric oxide), growth factors and neurotransmitters are released in the chronic inflammatory process in cholesteatoma and may initiate osteoclast recruitment and bone resorption [52]. TNF-α (tumor necrosis factor alpha) is known to play a pivotal role in bone resorption [5]. It has a primary effect on osteoblasts, which then stimulate osteoclastic bone resorption [40]. Bone resorption comprises a sequence of events, beginning with the recruitment of mononuclear cells from bone marrow and multinucleation of these cells that then become osteoclasts (Fig. 14). These specialized cells are capable of resorbing the organic and anorganic matrix of bone. Activation of osteoclasts requires products of arachidonic acid metabolism, the previously mentioned cytokines as well as unkown factors. Bone resorption is also controlled by an obligatory relationship between local osteoblasts and osteoclasts [9, 24, 131]. Cinamon and al. observed that the cholesteatoma-afflicted bone samples have an irregular border with a non-continuous periosteum. A loss of order and continuity of the cement lines between the bone lamellae was also observed. Most of the affected bones lack osteoblasts and osteocytes at sites of contact with cholesteatoma. Small, irregular eosophilic vesicles at the interface have also been observed [30]. Non-collagenous proteins such as osteopontin, bone sialoprotein, osteonectin and osteocalcin are mainly produced by osteoblasts and appear at different stages of the bone remodeling process. These proteins support cellular adhesion to the matrix, osteoclastic differentiation and regulation of the mineralization process, and besides have an affinity for bacterial adhesion [110, 111, 163].
The observation of sparse osteoblasts and a distinct difference in the content of non-collagenous proteins in the bone matrix adjacent to cholesteatoma may suggest an additional destructive impact through "down regulation" of osteoblasts [30]. Proteases such as plasminogen activators and matrix metalloproteinases (MMPs) have also been accused of playing a role in the degradation of bone matrix [23]. MMP-2 and MMP-9 both have been reported to contribute to bone remodeling by degrading basement membrane type IV collagens [74]. Presence of MMP-9 and MMP-2 has been observed in basal and suprabasal epithelial cell layers of cholesteatoma [121, 123]. The expression of MMP-9 (but not MMP-2) was increased in cholesteatoma compared to external ear canal skin samples. A possible explanation for the up-regulation of MMP-9 in cholesteatoma epithelial cells could be an immunological response to bacterial products stimulating macrophages to invade tissue and to produce cytokines. These cytokines may stimulate epithelial cells to produce proteases and cytokines [121]. However, further studies by the same authors showed none of the analyzed growth factors (EGF, TNF-α and TGF-β) increased significantly in correlation with MMP-9 activity values. This may be due to the fact that MMP up-regulation underlies a complex regulation that is not controlled by a single cytokine [122].
In 1980, Chole et al. discovered that Mongolian gerbils develop cholesteatoma with age [27]. They used this animal model to demonstrate that osteoclastic bone resorption is triggered by pressure [92]. Richardson et al. administered the bisphosphonate risedronate subcutaneously to neonatal mice, which resulted in a significant decrease of PTH-activated (parathyroid-hormone) protein calcium release in explanted calvaria in vitro [109]. Bisphosphonates resemble a promising new class of drugs in the treatment of bone resorption disorders, but require further investigations.
Angiogenesis
Angiogenesis is particularly important in a large number of normal and pathological processes, including wound healing and inflammation. The localization and distribution of angiogenic growth factors, basic fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF) in middle ear cholesteatoma has therefore been studied by many authors [81, 138]. Basic fibroblast growth factor plays a pivotal role in wound healing. It is chemotactic and mitogenic for fibroblasts, endothelial cells and keratinocytes. It can also stimulate the production of collagenase and plasminogen activators to enhance fibroblast proliferation and angiogenesis. FGF-2 could be demonstrated in cholesteatoma perimatrix. However, cholesteatoma matrix tissue without inflammation or any sign of wound healing has not expressed FGF-2 [81].
An enhanced expression of VEGF and FGF-2 in cholesteatoma in relation to middle ear mucosa and auditory meatal skin points out rapidly growing activated keratinocytes and endothelial cells. Vascularization within the perimatrix of cholesteatoma showed a five-fold increase compared to middle ear mucosa and a two-fold increase compared to skin (Fig. 15). We found a close relationship between the density of capillaries, degree of inflammation and expression of the investigated angiogenic factors [138]. Further studies confirm that with increasing degrees of inflammation, an enhanced vascularization within the perimatrix occurs. Because monocytes, macrophages and infiltrating leukocytes are known to produce cytokines such as FGF-2, VEGF, EGF, TGF-α, TGF-β, IL-1 and IL-6, angiogenesis might be induced by these inflammatory cells. Moreover, it could also be demonstrated that keratinocytes of cholesteatoma are able to release angiogenic factors. Proliferation of endothelial cells could be induced by the release of angiogenic factors from cholesteatoma matrix or by inflammatory cells of the subepithelial connective tissue. Additionally, the dual growth factor phenomenon of autocrine stimulation of cell proliferation and paracrine stimulation of the surrounding cells may play an essential role.
Angiogenesis enables and supports the migration of keratinocytes into the middle ear cavity [140]. As proliferating tissues such as middle ear cholesteatoma require an enhanced blood supply, angiogenesis appears to be a prerequisite for the expansion of cholesteatoma matrix within the middle ear.
Conclusions
The studies published to date support several suppositions concerning the pathogenesis of cholesteatomas. Cholesteatoma is a wound-healing process rather than a neoplastic lesion. Research to date does not indicate any genetic instability in cholesteatomas, which is a critical feature of malignant lesions. The activation of hyperproliferative cells in all epidermal layers of cholesteatoma potentially constitutes a response to both internal events as well as external stimuli represented by cytokines released by infiltrating inflammatory cells of the underlying subepithelial connective tissue.
The pathogenesis of cholesteatoma is certainly diverse, with different pathways leading to the same destructive lesion. There is no evidence for any obvious molecular or cellular differences among the various types of cholesteatomas (primary and secondary acquired or congenital). Approximately 30 years after his first publication on cholesteatoma in 1855, Virchow pointed out that he could not precisely explain the exact pathogenesis of this disease. Although there are several new ideas and results on its pathogenesis, Virchow's statement still remains valid today. Continued research should pay attention to the precise molecular and cellular dysfunction involved in the pathogenesis of cholesteatoma. This could ameliorate the understanding of this destructive middle ear lesion and be helpful in the development of new therapeutic strategies. To date, the only reasonable treatment for cholesteatoma is still surgery.
References
Abramson M (1964) Collagenolytic activity in middle ear cholesteatoma. Ann Otol Rhinol Laryngol 78: 112–124
Abramson M, Huang CC (1977) Localization of collagenase in human middle ear cholesteatoma. Laryngoscope 87: 771–791
Ahn JM, Huang CC, Abramson M (1990) Interleukin-1 causing bone destruction in middle ear cholesteatoma. Otolaryngol Head Neck Surg 103: 527–536
Aimi K (1983) Role of the tympanic ring in the pathogenesis of congenital cholesteatoma. Laryn-goscope 93: 1140–1146
Akimoto R, Pawankar R, Yagi T, Baba S (2000) Acquired and congenital choleteatoma: determination of tumor nectosis factor-alpha, intercellular adhesion molecule-1, interleukin-1-alpha and lymphocyte functional antigen-1 in the inflammatory process. ORL 62: 257–265
Albino AP, Reed JA, Bogdany JK, Sassoon J, Desloge RB, Parisier SC (1998) Expression of p53 protein in human middle ear cholesteatomas: pathogenetic implications. Am J Otol 19: 30–36
Albino AP, Reed JA, Bogdany JK, Sassoon J, Parisier SC (1998) Increased numbers of mast cells in human middle ear. cholesteatomas: implications for treatment. Am J Otol 9: 266–272
Albino PA, Kimmelmann CP, Parisier SC (1998) Cholesteatoma: a molecular and cellular puzzle. Am J Otol 19: 7–19
Amling M, Delling G (1996) Zellbiologie des Osteoklasten und molekulare Mechanismen der Knochenresorption. Pathologe 17: 358–367
Arriaga MA, Dixon P (1998) Cytotoxicity of cytokeratin monoclonal antibody against kera-tinocytes: a possible therapeutic adjunct for cholesteatoma? Am J Otol 19: 26–29
Austin DF (1969) Types and indications of staging. Arch Otolaryngol 89: 235–242
Baki FA, El Dine MB, El Saiid I, Bakry M (2002) Sinus tympani endoscopic anatomy. Otolaryngol Head Neck Surg 127: 158–162
Banerjee AR, James R, Narula AA (1998) Matrix metalloproteinase-2 and matrix metalloproteinase-9 in cholesteatoma and deep meatal skin. Clin Otolaryngol 23: 345–347
Banerjee AR, James R, Narula AA, Lee RJ (1998) Matrixmetalloproteinase-1 in cholesteatoma, middle ear granulations and deep meatal skin: a comparative analysis. Clin Otolaryngol 23: 515–519
Bezold F (1890) Cholesteatom, Perforation der Membrana flaccida und Tubenverschluß. Z Hals Nasen Ohrenheilk 20: 5–29
Bravo R, Frank R, Blundell P, McDonald-Bravo H (1987) Cyclin/PCNA is the auxillary protein of DANN polymerase-delta. Nature 326: 515–517
Bujia J, Holly A, Schilling V, Negri B, Pitzke P, Schulz P (1993) Aberrant expression of epidermal growth factor receptor in aural cholesteatoma. Laryngoscope 103: 326–329
Bujia J, Holly A, Kim C, Sudhoff H, Ostos P, Kastenbauer E (1996) Epidermal growth factor receptor (EGF-R) in human middle ear cholesteatoma: An analysis of protein production and gene expression. Am J Otol 17: 1–4
Bujia J, Schilling V, Holly A, Stammberger M, Kastenbauer E (1993) Hyperproliferation-associated keratin expression in human middle ear cholesteatoma. Acta Otolaryngol (Stockh) 113: 364–368
Bujía J, Holly A, Sudhoff H, Antolí- Candela F, Tapia MG, Kastenbauer E (1996) Identification of proliferating keratinocytes in middle ear cholesteatoma using the monoclonal antibody Ki-67. ORL Otorhinolaryngol Relat Spec 58: 23–26
Bujia J, Kim C, Ostos P, Sudhoff H, Kastenbauer E, Hültner I (1996) Interleukin-1 (Il-1) and Il-1-R antagonist (Il-1-RA) in middle ear cholesteatoma: an analysis of protein production and biological activity. Eur Arch Otorhinolaryngol 253: 252–255
Bujia J, Sudhoff H, Holly A, Hildmann H, Kastenbauer E (1996) Immunohistochemical detection of proliferation cell nuclear antigen in middle ear cholesteatoma. Eur Arch Otorhinolaryngol 253: 21–24
Chambers TJ, Darby JA, Fuller K (1985) Mammalian collagenase predisposes bone surfaces to osteoclastic resorption. Cell Tissue Res 241: 671–675
Chole RA (1993) Bone resorption in aural cholesteatoma. In: Fourth International Conference on Cholesteatoma and Mastoid Surgery. Kugler, Amsterdam, pp 79–83
Chole RA, Frush D (1982) Quantitative studies of eustachian tube epithelium during experimental vitamin A deprivation and reversal. Kugler, Amsterdam
Chole RA, Tinling SP (1985) Basal lamina breaks in the histogenesis of cholesteatoma. Laryngoscope 95: 270–275
Chole RA, Henry KR, McGinn MD (1981) Cholesteatoma: spontaneous occurence in the Mongolian gerbil Meriones unguiculatus. Am J Otol 2: 204–210
Choufani G, Mahillon V, Decaestecker C, Lequeux T, Danguy A, Salmon I, Gabius H-J, Hassid S, Kiss R (1999) Determination of the levels of expression of sarcolectin and calcyclin and of the percentages of apoptotic but not proliferating cells to enable distinction between recurrent and nonrecurrent cholesteatomas. Laryngoscope 109: 1825–1831
Chung JW, Yoon TH (1998) Different production of interleukin-1alpha, interleukin-1beta and interleukin-8 from cholesteatomatous and normal epithelium. Acta Otolaryngol (Stockh) 118: 386–391
Cinamon U, Kronenberg J, Benayahu D (2000) Structural changes and protein expression in the mastoid bone adjacent to cholesteatoma. Laryngoscope 110: 1198–1203
The Committee of Conservation of Hearing of the American Academy of Ophthalmology and Otolaryngology (1965) Standard classification for surgery of chronic ear disease. Arch Otolaryngol 81: 204–205
Cruveilhier J (1829) Anatomie pathologique du corps humain. Bailliere, Paris
Dallari S, Cavani A, Bergamini G, Girolomoni G (1994) Integrin expression in middle ear cholesteatoma. Acta Otolaryngol (Stockh) 114: 188–192
Darrouzet V, Duclos J-Y, Portmann D, Bebear J-P (2002) Congenital middle ear cholesteatomas in children: our experience in 34 cases. Otolaryngol Head Neck Surg 126: 34–41
Derlacki EL, Clemis JD (1965) Congenital cholesteatoma of the middle ear and mastoid. Ann Otol Rhinol Laryngol 74: 706–727
Desloge RB, Carew JF, Finstad CL, Steiner MG, Sassoon J, Levenson MJ, Staiano-Coico L, Parisier SC, Albino AP (1997) DNA analysis of human cholesteatomas. Am J Otol 18: 155–159
DuVerney JG (1683) Traite de l'organe de l'ouie, contenant Structure. Les ufages et leas maladies des toutes les parties de l'orelle. E. Michallet, Paris
Elias PM, Wood LC, Feingold KR (1999) Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat 10: 119–126
Ergun S, Carsloo B, Zheng X (1999) Apoptosis in meatal skin, cholesteatoma and squamous cell carcinoma of the ear. Clin Otolaryngol 24: 280–285
Felix R, Fleisch H, Elford PR (1989) Bone-resorbing cytokines enhance release of macrophage colony stimulating activity by the osteoblastic cell MC3T3-E1. Calcif Tissue Int 44: 356–360
Fransson J, Hammar H (1992) Epidermal growth in skin equivalent. Arch Dermatol Res 284: 343–348
Friedmann I (1955) The comparative pathology of otitis media—experimental and human. II. The histopathology of experimental otitis of the guinea pig, with particular reference to experimental cholesteatoma. J Laryngol Otol 69: 588–601
Friedmann I (1971) Epidermoid cholesteatoma and cholesterol granuloma. Ann Otol Rhinol Laryngol 68: 57–80
Fujioka C, Huang CC (1994) Platelet-derived growth factor in middle ear cholesteatoma. Eur Arch Otorhinolaryngol 251: 199–204
Grundfast KM, Ahuja GS, Parisier SC, Culver SM (1995) Delayed diagnosis and fate of congenital cholesteatoma (keratoma). Arch Otolaryngol Head Neck Surg 121: 903–907
Habermann J (1888) Zur Entstehung des Cholesteatoms des Mittelohres. Arch Ohrenheilkd 27: 43–51
Hayashida T, Iwamori M, Kitsuwa T, Nagai Y, Nomura Y (1984) Biochemical study of cholesteatoma and cholesterol granuloma occurrence of delta 7 cholesterol in the tissue of cholesteatoma. ORL J Otorhinolaryngol Relat Spec 46: 242–247
Holly A, Sittinger M, Bujia J (1995) Immunohistochemical demonstration of c-myc oncogene product in middle ear cholesteatoma. Eur Arch Otorhinolaryngol 252: 366–369
Huang CC, Yi ZX, Chao WY (1988) Effects of granulation tissue conditioned medium on the in-vitro differentiation of keratinocytes. Arch Otorhinolaryngol 245: 325–329
Huang T, Yan SD, Huang CC (1989) Colony-stimulating factor in middle ear cholesteatoma. Am J Otolaryngol 10: 393–398
Ishibashi T, Shinogami M, Kaga K, Fukaya T (1997) Keratinocyte growth factor and receptor mRNA expression in cholesteatoma of the middle ear. Acta Otolaryngol (Stockh) 117: 714–718
Jung JY, Chole RA (2002) Bone resorption in chronic otitis media: the role of the osteoclast. ORL 64: 95–107
Kakoi H, Tamagawa Y, Kitamura K, Anniko M, Hiraide F, Kitajima Y (1995) Cytokeratin expression patterns by one- and two-dimensional electrophoresis in pars flaccida cholesteatoma and pars tensa cholesteatoma. Acta Otolaryngol (Stockh) 115: 804–810
Karmody CS, Bybhatti SV, Vltonen H, Northrop C (1998) The origin of congenital cholesteatoma. Am J Otol 19: 292–297
Kato A, Ohashi Y, Masamoto T, Sakamoto H, Uekawa M, Nakai Y (1998) Interleukin-6 and tumour necrosis factor alpha synthesized by cholesteatoma cells affect mucociliary function in the eustachian tube. Acta Otolaryngol [Suppl] 538: 90–97
Kayhan FT, Mutlu C, Schachern PA, Le CT, Paparella MM (1997) Significance of epidermoid formations in the middle ear in fetuses and children. Arch Otolaryngol Head Neck Surg 123: 1293–1297
Kemppainen HO, Puhakka HJ, Laippala PJ, Sipila MM, Manninen MP, Karma PH (1999) Epidemiology and aetiology of middle ear cholesteatoma. Acta Otolaryngol 119: 568–572
Kim CS, Chung JW (1999) Morphologic and biologic changes of experimentally induced cholesteatoma in Mongolian gerbils with anticytokeratin and lectin study. Am J Otol 20: 13–18
Kim H-J, Tinling SP, Chole RA (2001) Expression patterns of cytokeratins in retraction pocket cholesteatomas. Laryngoscope 111: 1032–1036
Kim H-J, Tinling SP, Chole RA (2002) Expression patterns of cytokeratins in cholesteatomas: evidence of increased migration and proliferation. J Korean Med Sci 17: 381–388
Kojima H, Tanaka Y, Tanaka T, Miyazaki H, Shiwa M, Kamide Y, Moriyama H (1998) Cell proliferation and apoptosis in human middle ear cholesteatoma. Arch Otolaryngol Head Neck Surg 124: 261–264
Kojima H, Miyazaki H, Shiwa M, Tanaka Y, Moriyama H (2001) Molecular biological diagnosis of congenital and aquired cholesteatoma on the basis of differences in telomere length. Laryngoscope 111: 867–873
Koltai PJ, Nelson M, Castellon RJ, Garabedian E-N, Triglia J-M, Roman S, Roger G (2002) The natural history of congenital cholesteatoma. Arch Otolaryngol Head Neck Surg 128: 804–809
Kountakis SE, Chang CYJ, Gormley WB, Cabral FR (2000) Migration of indtradural epidermoid matrix: embryologic implications. Otolaryngol Head Neck Surg 123: 170–173
Kuijpers W, Vennix PP, Peters TA, Ramaekers FC (1996) Squamous metaplasia of the middle ear epithelium. Acta Otolaryngol (Stockh) 116: 293–298
Lang S, Schilling V, Wollenberg B, Mack B, Nerlich A (1997) Localization of transforming growth factor-beta-expressing cells and comparison with major extracellular components in aural cholesteatoma. Ann Otol Rhinol Laryngol 106: 669–673
Lange W (1925) Über die Entstehung der Mittelohrcholesteatoma. Z Hals Nas Ohrenheilk 11: 250–271
Lee TS, Liang JN, Michaels L, Wright A (1998) The epidermoid formation and its affinity to congenital cholesteatoma. Clin Otolaryngol 23: 449–454
Levenson MJ, Parisier SC, Chute P, S. W, Juarbe CA (1986) A review of 20 congenital cholesteatomas of the middle ear in children. Otolaryngol Head Neck Surg 94: 560–567
Levine JL, Wright CG, Pawlowski KS, Meyerhoff WL (1998) Postnatal persistence of epidermoid rests in the human middle ear. Laryngoscope 108: 70–73
Lim D, Birck H, Saunders W (1977) Aural cholesteatoma and epidermization: a fine morphological study. In: McCabe BF, Sade J, Abramson M (eds) Cholesteatoma. First Intenational Conference. Aesculapius, Briminham, pp 233–252
Lucae A (1873) Beiträge zur Kenntnis der Perlgeschwülste des Felsenbeins. Arch Ohr Nas Kehlk Hk 7: 255
Masaki M, Wright CG, Lee DH, Meyerhoff ML (1989) Epidermal ingrowth through tympanic membrane following middle ear application of propylene glycol. Acta Otolaryngol (Stockh) 108: 169–174
Mc Cawley LJ, O'Brien P, Hudson LG (1998) Epidermal growth factor (EGF)- and scatter factor factor/hepatocyte growth factor (SF/HGF)-madiated keratinocyte migration is coincident with induction of matrix-metalloproteinase (MMP)-9. J Cell Physiol 176: 255–265
McGill TJ, S. Merchant, G. B. Healy, Friedman EM (1991) Congenital cholesteatoma of the middle ear in children: a clinical and histopathological report. Laryngoscope 101: 606–613
McKennan KX, Chole RA (1989) Post-traumatic cholesteatoma. Laryngoscope 99: 779–782
Meyerhoff WL, Truelson J (1986) Cholesteatoma staging. Laryngoscope 96: 935–939
Michaels L (1986) An epidermoid formation in the developing middle ear; possible source of cholesteatoma. J Otolarnygol 15: 169–174
Michaels L (1987) Cholesteatoma. Ear, Nose Throat Histopathology. Springer, New York Heidelberg Berlin
Michaels L (1988) Origin of congenital cholesteatoma from a normally occurring epidermoid rest in the developing middle ear. Int J Pediatric Otolaryngol 15: 51–65
Milewski C, Fedorowski A, Stan AC, Walter GF (1998) Basic fibroblast growth factor (b-FGF) in the perimatrix of cholesteatoma. HNO 46: 804–808
Mills RP, Padgham ND (1991) Management of childhood cholesteatoma. J Laryngol Otol 105: 343–345
Miyazaki H, Kojima H, Tanaka Y, Shiwa M, Koga T, Moriyama H (1999) Terminal differentiation of epithelial cells in middle ear choleseatoma: investigation of patterns of expression of protein kinase C-? and protein kinase C-? Laryngoscope 109: 1785–1792
Mizutani H, Ohyanagi S, Hayashi T, Groves RW, Suzuki K, Shimizu M (1996) Functional thrombomodulin expression on epithelial skin tumors as a differentiation marker for suprabasal keratinocytes. Br J Dermatol 135: 187–193
Müller J (1838) Über den feineren Bau und die Formen der krankhaften Geschwülste. Reimer, Berlin
Myers EN, Park K, Chun Y, Lee D, Hwang S (1999) Signal transduction pathway in human middle ear cholesteatoma. Otolaryngol Head Neck Surg 120: 899–904
Negri B, Schilling V, Bujia J, Schulz P, Kastenbauer E (1992) Immunotype findings in macrophages in aural cholesteatomas. Eur Arch Otorhinolaryngol 249: 87–90
Nelson M, Roger G, Koltai PJ, Garabedian E-N, Triglia J-M., Roman S, Castellon RJ, Hammel JP (2002) Congenital cholesteatoma: classification, management, and outcome. Arch Otolaryngol Head Neck Surg 128: 810–814
Nemes Z, Steinert PM (1999) Bricks and mortar of the epidermal barrier. Exp Mol Med 31: 5–19
Northrop C, Piza J, Eavey RD (1986) Histological observations of amniotic fluid cellular content in the ear of neonates and infants. Int J Pediatr Otorhinolaryngol 11: 113–127
Olszewska E, Chodynicki S, Chyczewski L (2003) Some markers of proliferative activity in cholesteatoma epithelium in adults. Otolaryngol Pol 57: 85–89
Orisek BS, Chole RA (1987) Pressures exerted by experimental cholesteatomas. Arch Otolaryngol Head Neck Surg 113: 386–391
Ottaviani F, Sambataro G, Mantovani M, Lavezzi AM, Matturi L (1989) Middle ear cholesteatoma. A cytodiagnostic study of cellular proliferative activity. Kugler and Ghedini, Amsterdam
Ottaviani F, Neglia CB, Berti E (1999) Cytokines and adhesion molecules in middle ear cholestetoma. A role in epithelial growth? Acta Otolaryngol 119: 462–467
Palva T, Taskinen E (1990) Inflammatory cells in chronic middle ear disease. Acta Otolaryngol (Stockh) 109: 124–129
Paparella MM, Rybak L (1978) Congenital cholesteatoma. Otolaryngol Clin North Am 11: 113–120
Parisier SC, Levenson MJ, Edelstein DR, Bindra GS, Han JC, Dolitsky JN (1989) Management of congenital pediatric cholesteatomas. Am J Otol 10: 121–123
Park HJ, Park K (1999) Expression of Fas/APO-1 and apoptosis of keratinocytes in human cholesteatoma. Laryngoscope 109: 613–616
Park K, Chun Y-M, Park H-J, Lee Y-D (1999) Immunohistochemical study of cell proliferation using BrdU labeling on tympanic membrane, external auditory canal and induced cholesteatoma in Mongolian gerbils. Acta Otolaryngol (Stockh) 119: 874–879
Park K, Park H-J, Chun Y-M (2001) Immunohistochemical study on proliferative activity of expwrimental cholesteatoma. Eur Arch Otorhinolaryngol 258: 101–105
Paukkonen K, Naukkarinen A, Horsmanheimo M (1995) The development of manifest psoriatic lesions is linked with the appearance of ICAM-1 positivity on keratinocytes. Arch Dermatol Res 287: 165–170
Polakowska RP, Placentini M, Bartlett R, Goldsmith LA, Haake AR (1994) Apoptosis in human skin development: morphogenesis periderm and stemcells. Dev Dynamics 199: 176–188
Potsic WP, Wetmore RF, March RR (1997) Congenital cholesteatoma: 15 years experience at the childrens hospital of Philadelphia. In: Fifth International Conference on Cholesteatoma and Mastoid Surgery. CIC Edizioni Internationali, Rome, pp 422–431
Potsic WP, Korman SB, Samadi DS, Wetmore RF (2002) Congenital cholesteatoma: 20-year experience at the Children's Hospital of Philadelphia. Otolaryngol Head Neck Surg 126: 409–414
Quantin L, Fernández SC, Moretti J (2002) Congenital cholesteatoma of external auditory canal. Int J Pediatr Otorhinolaryngol 62: 175–179
Rashad U, Hawthorne M, Kumar U, Welsh A (1999) Unusual cases of congenital cholesteatoma of the ear. J Laryngol Otol 131: 52–54
Ratnesar P (1977) Aeration: a factor in the sequele of chronic ear disease along the Labrador and northern Newfoundland Coast. In: McCabe B, Sade J, Abramson M (eds) Cholesteatoma: First international conference. Aesculapius, Birmingham, pp 302–307
Remak R (1854) Ein Beitrag zur Entwicklungsgeschichte der Geschwulste. Deutsche Klin 6: 170
Richardson AC, Tinling SP, Chole RA (1993) Risedronate activity in the fetal and neonatal mouse. Otolaryngol Head Neck Surg 109: 623–633
Roach HI (1994) Why does bone matrix contain non collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin, and bone sialoprotein in bone mineralisation and resorption. Cell Biol Int 18: 617–628
Robey PG, Boskey AL (1996) The biochemistry of bone: review. Stanford University School of Medicine, Stanford, California
Robinson AC, Hawke M (1991) The motility of keratinocytes in cholesteatoma: an ultrastructural approach to epithelial migration. J Otolaryngol 20: 353–359
Ruedi L (1978) Pathogenesis and surgical treatment of the middle ear cholesteatoma. Acta Otolaryngol (Stockh) [Suppl] 361: 1–45
Sadé J (1979) The atelectatic ear. Churchill Livingstone, New York
Sadé J (1971) Cellular differentiation in the middle ear lining. Ann Otol Rhinol Laryngol 80: 376
Sadé J, Berco E, Buyanover D, Brown M (1981) Ossicular damage in chronic middle-ear inflammation. Acta Otolaryngol 92: 273–283
Sadé J (2000) The buffering effect of middle ear negative pressure by retraction of the pars tensa. Am J Otol 21: 20–23
Saleh HA, Mills RP (1999) Classification and staging of cholesteatoma. Clin Otolaryngol 24: 355–359
Sanna M, Mazzoni A, Landolfi M, Aristegni M (1994) Treatment of petrous bone cholesteatoma. Acta Otorrinolaringol Esp 45: 143–152
Schilling V, Negri B, Bujia J, Schulz P, Kastenbauer E (1992) Possible role of interleukin 1a and interleukin 1b in the pathogenesis of cholesteatoma of middle ear. Am J Otol 13: 350–355
Schimdt M, Grünsfelder, Hoppe F (2000) Induction of matrix metalloproteinases in keratinocytes by cholesteatoma debris and granulation issue extracts. Eur Arch Otorhinolaryngol 257: 425–429
Schimdt M, Grünsfelder, Hoppe F (2001) Up-regulation of matrix metalloprotease-9 in middle ear cholesteatoma: correlations with growth factor expression in vivo? Eur Arch Otorhinolaryngol 258: 472–476
Schönermark M, Mester B, Kempf HG, Blaser J, Tschesche H, Lenarz T (1996) Expression of matrix-metalloproteinases and their inhibitors in human cholesteatomas. Acta Otolaryngol 116: 451–456
Schuknecht HF (1993) Pathology of the ear, Series 2. Lea and Feabinger, Philadelphia
Sheikholeslam- Zadeh R, Decaestecker C, Delbrouck C, Danguy A, Salmon I, Zick Y, Kaltner H, Hassid S, Gabius H-J, Kiss R, Choufani G (2001) The levels of expression of galectin- 3, but not of galectin- 1 and galectin- 8, correlate with apoptosis in human cholesteatomas. Laryngoscope 111: 1042–1047
Shinoda H, Huang CC (1995) Expressions of c-jun and p53 proteins in human middle ear cholesteatoma: relationship to keratinocyte proliferation, differentiation, and programmed cell death. Laryngoscope 105: 1232–1237
Soyer HP, Smolle J, Smolle- Juettner FM, Kerl H (1989) Proliferation antigens in cutaneous melanocytic tumors: an immunohistochemical study comparing the transferrin receptor and the Ki-67 antigen. Dermatologica 173: 3–9
Srinivasan V, Banhegyi G, O'Sullivan G, Shermann IW (2000) Pars tensa retraction pockets in children: treatment by excision and ventilation tube insertion. Clin Otolaryngol 25: 253–256.
Stark T, Sudhoff H, Fisseler-Eckhoff A, Borkowski G, Luckhaupt H, Hildmann H (1997) Alterations of basement membrane in middle ear cholesteatoma. In: Sanna M (ed) Fifth international conference on cholesteatoma and mastoid surgery. CIC Edizioni Internationali, Rome, pp 238–247
Steinbach E (1982) Experimental studies on cholesteatoma formation. Kugler, Amsterdam
Steinbach E, Hildmann, H. (1972) Re-use of incus in cholesteatoma and in chronic mucosal disease. Z Laryngol Rhinol 51: 659–664
Sudhoff H, Tos M (2000) Pathogenesis of attic cholesteatoma: clinical and immunohistochemical support for combination of retration theory and proliferation theory. Am J Otol 21: 786–792
Sudhoff H, Linthicum FHJ (2001) Cholesteatoma behind an intact tympanic membrane: histopathologic evidence for a tympanic membrane origin. Otol Neurotol 22: 444–446
Sudhoff H, Hildmann H (2003) Gegenwärtige Theorien zur Cholesteatomentstehung. HNO 51: 71–83
Sudhoff H, Bujia J, Holly A, Kim C, Fisseler-Eckhoff A (1994) Functional characterization of middle ear mucosa residues in cholesteatoma samples. Am J Otol 15: 217–221
Sudhoff H, Bujia J, Fisseler-Eckhoff A, Schulz-Flake C, Holly A, Hildmann H (1995) Expression of the cell-cycle-related antigen (MIB 1) in cholesteatoma and auditory meatal skin. Laryngoscope 105: 1227–1231
Sudhoff H, Bujia J, Fisseler-Eckhoff A, Borkowski G, Holly A, Koc C, Hildmann H (1996) Basement membrane in middle ear cholesteatoma. Immunohistochemical and ultrastructural observations. Ann Otol Rhinol Laryngol 105: 804–810
Sudhoff H, Fisseler-Eckhoff A, Borkowski G, Luckhaupt H, Stark T, Hildmann H (1997) Cholesteatoma and angiogenesis. In: Sanna Me (ed) Fifth international conference on cholesteatoma and mastoid surgery. CIC Edizioni Internationali, Rome, pp 264–272
Sudhoff H, Fisseler-Eckhoff A, Stark T, Borkowski G, Luckhaupt H, Cooper J, Michaels L (1997) Agyrophilic nuclear organizer regions in auditory skin and cholesteatoma. Clin Otolaryngol 6: 545–548
Sudhoff H, Dazert S, Gonzales AM, Borkowski G, Park SY, Baird A, Hildmann H, Ryan AF (2000) Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am J Otol 21: 793–798
Svane-Knudsen V, Halkier-Sorensen L, Rasmussen G, Ottosen PD (2001) Cholesteatoma: a morphologic study of stratum corneum lipids. Acta Otolaryngol 121: 602–606
Tanaka Y, Kojima H, Miyazaki H, Koga T, Moriyama H (1999) Roles of cytokines and cell cycle regulating substances in proliferation of cholesteatoma epithelium. Laryngoscope 109: 1102–1107
Teed RW (1936) Cholesteatoma verum tympani: its relationship to the first epibranchial placode. Arch Otolaryngol 24: 455–474
Tos M (1988) Incidence, etiology and pathogenesis of cholesteatoma in children. Otol Rhinol Laryngol 40: 110–117
Tos M (1993) Manual of middle ear surgery. Thieme, Stuttgart, New York
Tos M (2000) A new pathogenesis of mesotympanic (congenital) cholesteatoma. Laryngoscope 110: 1890–1897
Tos M, Lau T (1989) Recurrence and the condition of the cavity after surgery for cholesteatoma using various techniques. Kugler and Ghedini, Amsterdam
Tos M, Stangerup S-E, Larsen P, Siim C, Andreassen U (1989) The relationship between secretory otitis and cholesteatoma. In: Tos M, Thomsan J, Peiteresen E (eds) Proceedings of the 3rd international conference on cholesteatoma and mastoid surgery. Kugler Ghedini, Amsterdam pp 325–330
von Tröltsch A (1864) The diseases of the ear, their diagnosis and treatment. William Wood, New York
Van Den Oord JJ, De Ley M, De Wolf-Peeters C (1993) Distribution of gamma interferon receptors in normal and active psoriatic skin. In: Schlossman SF BL, Gilks W, et al (eds) Fifth international workshop and conference. Oxford University Press, Boston, pp 1994–1995
Vennix PP, Kuijpers W, Peters TA, Tonnaer EL, Ramaekers FC (1996) Keratinocyte differentiation in acquired cholesteatoma and perforated tympanic membranes. Arch Otolaryngol Head Neck Surg 122: 825–832
Virchow R (1855) Über Perlgeschwülste (Cholesteatoma Joh. Müller). p 371
Wang RG, Hawke M, Kwok P (1987) The epidermoid formation (Michaels structure) in the developing middle ear. J Otolaryngol 16: 327–330
Wendt H (1873) Desquamative Entzündung des Mittelohres ("Cholesteatom des Felsenbeins"). Arch Ohr Nasen Kehlkopfheilk 14: 428–446
Wittmaack K (1933) Wie entsteht ein genuines Cholesteatom? Arch Otorhinolaryngol 137: 306
Wright CG, Meyerhoff WL, Burns DK (1985) Middle ear cholesteatoma: an animal model. Am J Otolaryngol 6: 327–341
Wright CG, Bird LL, Meyerhoff WL (1991) Tympanic membrane microstructure in experimental cholesteatoma. Acta Otolaryngol (Stockh) 111: 101–111
Wright CG, Robinson KS, Meyerhoff WL (1996) External and middle ear pathology in TGF-alpha-deficient animals. Am J Otol 17: 360–365
Wullstein H (1956) Theory and practice of tympanoplasty. Laryngoscope 66: 1076–1093
Wullstein HL, Wullstein SR (1980) Cholesteatoma. Etiology, nosology and tympanoplasty. ORL J Otorhinolaryngol Relat Spec 42: 313–335
Yan SD, Huang CC (1991) Lymphotoxin in human middle ear cholesteatoma. Laryngoscope 101: 411–415
Yan SD, Huang CC (1991) The role of tumor necrosis factor-alpha in bone resoption in cholesteatoma. Am J Otol 12: 82–89
Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG (1992) Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res 281: 275–294
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olszewska, E., Wagner, M., Bernal-Sprekelsen, M. et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol 261, 6–24 (2004). https://doi.org/10.1007/s00405-003-0623-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-003-0623-x