Abstract
Indocyanine green (ICG) use has become more prominent in multiple surgical fields. Within the field of neurosurgery, ICG has been used in neurovascular, endoscopic endonasal approaches, ventricular surgery, and neurooncological procedures. Within neurovascular surgery, ICG has been used for aneurysm repair, arteriovenous malformations, and extra-anatomic bypass. In aneurysm repair ICG use has been compared with Doppler ultrasonography and the gold standard of digital subtraction angiography (DSA). Compared to these other methods, ICG can reduce operative time and cost and is easier to interpret. In aneurysm repair ICG can quickly allow the surgeon to recognize stenosis, incomplete exclusion, and compromised flow through small perforating vessels that may be under the detection level of DSA. Despite these apparent advantages, ICG is limited by the fact that it can only assess visible vessels. This limitation can partly be improved with the use of endoscopic ICG. While most operative microscopes have added the ability to view ICG, endoscopic use of ICG has only more recently been reported. ICG has also found use in the treatment of arteriovenous malformations, particularly in the operative planning stage, and in extra-anatomic bypass and the detection of potential early anastomotic complications. Further studies between select imaging modalities will help define the specific situations where ICG, DSA, or Doppler ultrasonography will be the most appropriate imaging.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Indocyanine green (ICG) has become well-established as an adjunct in multiple surgical fields as it augments reality to allow for accurate identification of anatomic structures and assessment of blood flow [1]. Due to the binding of indocyanine to plasma proteins, it is maintained in the vasculature and has become recognized as versatile tool in vascular neurosurgery. Assuring the integrity of tissue perfusion is the most important principle in neurovascular surgery [2]. The first described use of fluorescent visualization of cerebral blood vessels in neurovascular surgery was in 1967 by Feindel et al. [3], and in 1994, Wrobel et al. [4] described a case of ICG angiography in aneurysm surgery. However, it wasn’t until 2003 when Raabe et al. [5] used microscope-integrated indocyanine green (M-ICG) angiography to assess vessel patency that neurosurgery as a field began to widely investigate and adopt the technique [6]. Prior to this time, intraoperative digital subtraction arteriography (DSA) was required for assessment of cerebral blow flow [5]. Raabe et al. [5] demonstrated that ICG angiography was a simple method to provide real-time information on multiple phases of blood flow down to vessels less than 0.5 mm in diameter, thus opening the door to a multitude of neurosurgical applications (Fig. 13.1). The use of indocyanine has since broadened to other areas within neurosurgery with the four main uses being neurovascular, endoscopic endonasal approaches, ventricular surgery, and neurooncological procedures; this chapter will focus on the neurovascular uses of indocyanine green. Within neurovascular procedures ICG is commonly used for assessing aneurysm morphology and perforator vessel preservation. The ability to identify the obliteration or patency of vessels is also pertinent in arteriovenous malformations and dural fistula surgery.
For the exposed operative field (left), ICG videoangiography (right) provides a real-time assessment of the flow through both large and small (recurrent artery of Heubner) vessels. LA1 left A1 segment of the ACA, LA2 left A2 segment of the ACA, RA1 right A1 segment of the ACA, RA2 right A2 segment of the ACA [7]
The use of ICG angiography has been fairly easy to integrate into the field of neurosurgery, largely due to the prevalence of microscopic surgery. Multiple companies have equipped their microscopes with near infrared sensitive digital cameras capable of viewing the ICG diffusion within the cerebral vessels [6]. With integration of ICG angiography into the operative microscope, simplicity and speed are improved.
ICG Use in Aneurysm Repair
Aneurysm treatment depends on complete exclusion of the aneurysm sac and patency of the supplying arteries. While alternative treatments such as coiling and stenting have made advances, microsurgical clipping should still be considered the durable and definitive treatment for intracranial aneurysms [8]. Sindou et al. [9] found that incomplete clipping accounts for up to 4–8% of all clipping cases [6]. If imaging is only completed postoperatively, the findings may result in repeat surgery, and studies suggest a 2–8% rate of residual aneurysm filling and a 4–12% rate of parent or branching artery occlusion on postoperative angiography [2]. Prior to ICG, neurosurgeons had several intraoperative methods to assess aneurysm blood flow such as contact Doppler ultrasonography, blood flowmetry, and the gold standard of contrast-enhanced (digital subtraction) angiography (DSA). Starting with the case series by Raabe et al. in 2003, ICG angiography has been studied in aneurysm clipping. ICG offers an easy, rapid, and minimally invasive method of allowing evaluation of complete aneurysm exclusion, neck remnant, and blood flow in parent and perforating arteries (Fig. 13.2). Many of the studies since Raabe et al. [5] have compared ICG to Doppler ultrasonography or DSA to assess anatomy pre- and post-clipping, clip positioning, presence of aneurysm residuals, and patency of normal vessels. It is recommended that the surgeon use an objective intraoperative technique rather than relying on visual assessment alone [6].
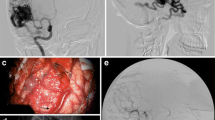
Middle cerebral artery aneurysm with the neck dissected and clear view of the parent vessel and its two branches (a), after retraction showing the neck of the clipped MCA aneurysm (b), and intraoperative ICG videoangiography showing the patency of the branches of the MCA and complete exclusion of the aneurysm from the circulation (c) [10]
ICG, while not without its limitations, does offer benefit over some of the existing methods of flow detection. DSA, which has been shown to have a significant impact on 7–34% of cases when used intraoperatively, is expensive, requires additional specially trained staff, and has a complication rate of 0.4–3.5% [7]. A minimum of 20 min is typically needed to set up and perform intraoperative DSA, a length of time that may exceed the ischemic limit of cerebral tissue in certain cases. Clip adjustment after DSA is associated with a 33% rate of stroke. Doppler ultrasonography exhibits good correlation with postoperative angiography and is inexpensive and noninvasive and does not add significant time to the case, but interpretation of signals is subjective, and such factors as the angle of insonation, thickness of the vessel wall, and investigation point may affect the outcome of the assessment [2, 6].
Aneurysm clipping is often completed with two doses of ICG; one dose being administered prior to clipping and the second to confirm the position of the clip and exclusion of the aneurysm. With an up to 42-min visualization window, no additional doses are needed in the pre-clipping step, and the patency of parent and surrounding perforating arteries can be easily assessed. However, a second dose is typically required for post-clipping evaluation due to “trapping” of the ICG in the aneurysm which could simulate incomplete clipping [11]. Eliava et al. [6] demonstrated in their series that it takes approximately 10 min to complete aneurysm clipping after ICG administration. The use of ICG also allowed the surgeon to quickly recognize stenosis or incomplete exclusion and reposition the clip accordingly. Vessels less than 1 mm in diameter are well assessed with the operating microscope and ICG allowing for the accurate assessment of perforating arteries [2]. These small perforating arteries may be below the level of detection for DSA, and Oliveira et al. [12] showed a 1.6% rate of clip adjustments based on ICG detection of compromised flow through perforating arteries.
Despite a greater than 90% correlation with intra/postoperative DSA, ICG angiography cannot completely remove all risk of improperly placed clips [2]. Eliava et al. [6] described a case where postoperative selective angiography revealed a remnant aneurysmal neck that was unable to be evaluated intraoperatively due to inability to directly view the area because of the location. Several other studies have demonstrated a small rate of unexpected neck residuals and close vessel occlusion at a rate of 4–6% [7, 8, 13]. In the series of 15 patients, one patient also had mild stenosis of the internal carotid artery that was only noted on control angiography and not on ICG angiography. Studies have demonstrated clip repositioning rates of 2–38% with the use of ICG; part of this variation is due to the lack of standardization of ICG technique, potential limited experience, and varied patient groups. ICG angiography was noted to be crucial to case success in three cases where blood flow, not visible by Doppler ultrasonography, was visible by ICG angiography. A comparison of intraoperative DSA to ICG, with 100 patients in each group, by Hardesty et al. [14] demonstrated no reliable difference in the risk of postoperative ischemia (4% vs 3%), unexpected aneurysm filling (4% vs 2%), or parent vessel compromise (2% vs 2%).
ICG is not without its drawbacks. Mainly, the fluorescence can only be traced within the limits of visibility; important structures may be easily obscured by vessel branches, a clip, or the aneurysm itself. A comparison of contrast-enhanced angiography and ICG by Washington et al. [7] did not favor ICG. In this study, the conclusion of 14.3% of cases was discordant, largely due to the fact that only visible vessels were assessed by ICG. Within the seven cases of discordance from this study, five of the cases underwent clip adjustment leading to complete aneurysm occlusion or restoration of patency in branch vessels. No specific aneurysm characteristics were more likely to contribute to discordance on multivariate analysis, but there was a trend toward anterior communicating artery location leading to discordance on univariate analysis. This finding aligns with the findings of Gruber et al. [15] and Dashti et al. [13] that deep-seated aneurysms are poorly imaged with ICG. Similarly, Roessler et al. [16] demonstrated a 9.1% identification rate with selective angiography of small (<2 mm) neck aneurysm remnants and a residual 6 mm aneurysm following ICG. These findings suggest that DSA may still serve a vital role in complex aneurysms where hidden parts of the parent, branching, and perforating vessels and undissected portions of the aneurysm dome are difficult to directly visualize with ICG (Fig. 13.3). ICG can also be affected by calcified or atherosclerotic vessels and thrombosis within the aneurysm. However, intraoperative DSA also has its limitations with a reported 7.9% residual aneurysm and 4.8% rate of distal arterial branch occlusion detected on postoperative DSA. Despite the aforementioned finding, the study of 295 patients by Roessler et al. [16] is one of the largest to date and also demonstrated a 9% repositioning rate and a 4.5% rate of incomplete clipping after ICG administration, findings that may not have been detected with Doppler or flowmetry.
(a) Preoperative anteroposterior angiography with right internal carotid injection in a patient with an anterior communicating artery aneurysm. (b) Indocyanine green videoangiography after clipping. There is no evidence of residual aneurysm. The direction of the arrow denotes the direction of the clip. Postoperative anteroposterior angiography (c, d) showed residual aneurysm. The patient underwent reclipping, and angiography (e) after reclipping showed complete obliteration of the aneurysm [17]
The use of ICG has advanced beyond microscope integrated use, and in 2013 the first described use of endoscopic-ICG (E-ICG) use for neurovascular use was reported by Bruneau et al. [18]. Aneurysm clipping was performed on an unruptured anterior communicating artery aneurysm using a similar two bolus protocol and a 0° NIR-optimized endoscope. The E-ICG provided high-resolution, panoramic views with additional information on the aneurysm exclusion and parent and perforating artery patency that would be potentially obscured without an angled camera. In 2014, Mielke et al. [19] published a series of 26 patients with ruptured and non-ruptured aneurysms comparing M-ICG and E-ICG. The series showed that in 11 of 26 patients, E-ICG added information by increasing fluorescence time, offering different viewing angles and enlarged views of the vessels and aneurysms. An additional series by Yoshioka and Kinouchi [20] and Cho et al. [21] demonstrated successful use of E-ICG in aneurysm clipping. Cho et al. [21] specifically selected ICA-PCoA and ICA-AChA aneurysms due to the higher incidence of incomplete clipping and branch compromise (Fig. 13.4). With the use of endoscopic ICG, all necessary anatomic information and assessment of the clipping was possible despite limited microscopic views. The main advantage of endoscopy is enhanced visualization, better magnification, and dynamic vision which improves the ability to view less accessible regions, particularly posterior to the clip and beyond the direct view of the microscope. Critical to the success of E-ICG procedures is the maintenance of a clear surgical field. Without a clear field, visual perception may be distorted and “false” vascular structures visualized.
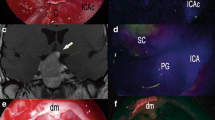
A 59-year-old man with an unruptured left internal carotid artery (ICA) anterior choroidal artery (AChA) aneurysm measuring approximately 7 mm (case 8). (a) The AChA (thick arrow) and PCoA (thin arrow) were observed on medioposterior view of three-dimensional reconstructed left ICA angiography. (b, c) The orifice of the AChA (arrows) at the aneurysm neck was identified with the microscope (b) (with a magnifying view at the right lower side) and endoscope (c), with a hard atherosclerotic anterior aneurysm wall (asterisks). (d, e) After clipping, the orifice of the AChA was not visualized with the microscope (d); however, it was shown to be compromised by the standard clip (arrow) with the endoscope (e). (f, g) A miniclip was instead applied, and the orifice of the AChA (arrows) was shown on microscopic indocyanine green angiography with no indocyanine green filling within the sac. (h, i) Dual-channel endoscopic indocyanine green angiography of fluorescent (left panels) and visible color light (right panels) imaging also visualized the intact orifice (arrows in h) and trunk proper (circles in i) of the AChA [21]
Compared with other forms of vascular assessment, ICG angiography is approximately ten times more expensive than Doppler ultrasonography and four times more expensive than flowmetry. ICG is considered a cost-effective replacement to intraoperative DSA. Studies comparing the cost of ICG and other standard flow assessments are lacking with Nishiyama et al. [22] being one of the few to look at per-patient cost of intraoperative imaging.
Washington et al. [7] note that a combination of ICG and DSA may be the most effective for maximizing the safety and efficacy of aneurysm surgery. Care should be taken when relying on ICG for aneurysms where the field of view is limited or there are complex flow dynamics.
ICG Use in Arteriovenous Malformation
The goal of surgical intervention of arteriovenous malformations (AVM) is the removal of the AVM nidus and complete excision. Microsurgical resection is the only treatment option that immediately eliminates the risk of hemorrhage from the AVM and any remaining residual nidus with a high chance of re-rupture. DSA can be used intraoperatively for assessment of AVMs, but it comes with increased operating time and expense. The DSA images are not integrated into the microscopic view like ICG and must be interpreted separately, diverting the surgeon’s attention away from the operative field.
ICG can help distinguish vessels in physiological and pathological states; with arterialized veins emitting fluorescence in the late arterial phase. Using ICG can help the surgeon identify AVM arteries and veins early and plan operative steps. Deep-seated AVMs are somewhat troublesome for ICG as the location is not amenable to direct visualization; in these cases DSA is the gold standard, but ICG can be a helpful adjunct.
ICG Use in Extracranial-Intracranial Bypass
ICG has always had a leading role in bypass surgery, and use with neurovascular bypass was incorporated after the benefit was demonstrated in coronary artery bypass. Extracranial-intracranial bypass plays an important role in management of complex intracranial aneurysms, moyamoya disease, and cerebral ischemia. Early bypass graft occlusion and bypass failure are the main obstacles of EC-IC bypass surgery, which leads to cerebral ischemia and resulting morbidity. Intraoperative assessment of bypass graft function can reduce complications, but techniques such as Doppler ultrasonography and thermal artery imaging are limited in respect to image quality and spatial resolution, thus limiting the ability to detect flow in perforating arteries. DSA is the gold standard but is associated with limitations as mentioned previously. Historically, the immediate postoperative bypass patency rate has been cited between 89% and 96%. Multiple factors contribute to the patency of the graft including graft and donor vessel diameter, atherosclerotic changes in the donor vessel, cerebral blood flow demand, and flow direction within the bypass [23]. ICG has been reliably shown to detect stenosis and nonfunctioning bypasses and can help judge flow and anatomic relationships at the anastomotic site. Woitzik et al. [24] demonstrated a patency rate of EC-IC bypass of 100% after introducing the usage of ICG; prior to ICG the patency rate was less than 90%. The use of ICG localized the site of failure to the anastomosis and allowed for surgical revision and restored flow in six cases reported by Woitzik et al. [24].
While ICG can rapidly allow identification of parent and recipient arteries, further advancement in the quantitative and qualitative evaluation of blood flow is underway. Awano et al. [25] measured ICG perfusion area to assess hemodynamic changes following superficial temporal artery (STA) and middle cerebral artery (MCA) bypass surgery in moyamoya disease and non-moyamoya ischemic stroke. The group showed a significant increase in cortical oxygen saturation in the moyamoya disease cohort thought to be attributable to the increase of blood flow demand and pressure gradient between the STA and recipient vessels [26]. Januszewski et al. [23] attempted to classify the type of flow through bypass grafts to predict early graft failure and need for intraoperative revision. The group characterized flow through the graft into three types: type I being robust and anterograde (early arterial phase), type II being delayed anterograde flow (usually corresponding to capillary or venous phase), and type III being delayed anterograde flow without continuity to the bypass site or no flow at all. This classification makes use of the three phases of ICG distribution: arterial, capillary, and combined arteriovenous due to the recirculation of dye. On postoperative imaging, four occluded grafts (11%) were found in the series, all of which were type II or III. Based on these findings, Januszewski recommended revision of most type II flow grafts as failure is not likely to be related to competitive flow. ICG was found to be accurate and comparable to DSA in this series.
Discussion of Hyperperfusion After STA-MCA
ICG can also be of use in detecting patients who may be at risk for cerebral hyperperfusion. Two studies performed by Horie et al. [27] and Uchino et al. [28] examined ICG intensity-time curves and semiquantitative analysis of ICG-video angiography (ICG-VA), respectively. This can be applied in both patients with moyamoya disease and atherosclerotic disease. A blood flow index, calculated on the basis of the ICG intensity-time curve, with an increase of greater than threefold, may be predictive of the occurrence of hyperperfusion. Additionally, ICG-VA and analysis with specialized software may allow for recognition of flow parameters and hemodynamic changes that may be indicative of transient neurologic event complications, but to date no evidence has shown a correlation with improved patient outcomes.
Intraoperative Vessel Identification
Intraoperative vessel identification can also be performed with ICG-VA in aneurysm bypass and indirect bypass. While preoperatively there are multiple modalities to assist in selection of an appropriate donor vessel for EC-IC bypass, ICG can be used intraoperatively for mapping and preparation of the donor vessel. ICG-VA has also been used for selective targeting of the most suitable recipient artery, and this has been well-described in the treatment of complex MCA aneurysm. As a terminal branch of the MCA (M4) is preferred as a recipient vessel in this case, ICG-VA can detect the direction of flow and delayed or reversed filling time of M4 branches during arterial phase and other vascular structures during the capillary and venous phases. A primary and secondary identification protocol has been described by Esposito et al. [29, 30] in which delayed filling of superficial M4 branches during either phase demarcates the supplied cortical area and any artery within this area can be selected as the recipient. The secondary identification consists of a provocative temporary occlusion. ICG-VA can then be repeated after anastomosis to rule out residual filling of the aneurysm. A “flash fluorescence” technique has also been described by Lawton (Fig. 13.5). This technique helps identify an adequate recipient artery through occlusion of the afferent artery and subsequent flash fluorescence with ICG of efferent arteries after removal of the clip. This method can reduce unnecessary sylvian fissure dissection and allows for anastomosis to be performed on the cortical surface without brain retraction. Transdural ICG-VA use in STA-MCA bypass has helped in delineation of superficial vessel anatomy and allowed for tailoring of the dural incision and prevention of vessel damage during dural opening and microsurgical dissection.
Use of flash fluorescence technique to identify the efferent arteries of the aneurysm using ICG-VA. (a) Identification of candidate bypass recipient arteries among the surface M4 branches is difficult but could be improved using the following steps. (b) Temporary clip occlusion of the aneurysm inflow (afferent arteries) proximal to the aneurysm. (c) ICG-VA demonstrating initial fluorescence in uninvolved arterial branches. (d) Removal of temporary clip for aneurysm reperfusion. (e) Fluorescence seen in the efferent arteries to identify the most suitable recipient on the cortical surface for the bypass [26]
There is also a report by Yokoyama et al. [31] of E-ICG use in the treatment of recurrent skull base tumors. ICA angiography was performed after identification of a possible branch feeding the tumor; this information was used as the basis for deciding whether to deliver intra-arterial chemotherapy [11].
Standard ICG Dosing
The standard dose of ICG is 0.2–0.5 mg/kg [26]. Januszewski et al. [23] described a standard injection, through a peripheral vein, of 25 mg of ICG dissolved in 10 mL of water. Cavallo et al. [26] reported dissolving 25 mg in 5 mL of water and flushing immediately with 10 mL of normal saline. Redosing is suggested at 20 min to ensure adequate clearance of the tracer with the total cumulative dose not to exceed 5 mg/kg.
Conclusion
Over the past decade, the use of ICG angiography in neurosurgery has expanded. This technique has found use in multiple aspects of neurosurgery and offers additional tool in the surgeon’s kit. Series in neurovascular bypass, aneurysm exclusion, and AVM have demonstrated the utility of ICG-VA. This technology may even help in the early recognition of postsurgical complications with the application of software that can analyze flow using ICG intensity. ICA-VA can be time-saving in some cases and simpler to interpret but is limited in its ability to view deeper vessels. This limitation has been somewhat overcome by the introduction of E-ICG. Additional studies are still needed to further determine when ICG is superior to other imaging methods.
References
Alius C, Tudor C, Badiu CD, Dascalu AM, Smarandache CG, Sabau AD, Tanasescu C, Balasescu SA, Serban D. Indocyanine green-enhanced colorectal surgery—between being superfluous and being a game-changer. Diagnostics. 2020;10:742.
Balamurugan S, Agrawal A, Kato Y, Sano H. Intra operative indocyanine green video-angiography in cerebrovascular surgery: an overview with review of literature. Asian J Neurosurg. 2011;6(2):88–93.
Feindel W, Yamamoto YL, Hodge CP. Intracarotid fluorescein angiography: a new method for examination of the epicerebral circulation in man. Can Med Assoc J. 1967;96:1–7.
Wrobel CJ, Meltzer H, Lamond R, Alksne JF. Intraoperative assessment of aneurysm clip placement by intravenous fluorescein angiography. Neurosurgery. 1994;35:970–3.
Raabe A, Beck J, Gerlach R, Zimmerman M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52(1):132–9.
Eliava SS, Shekhtman OD, Pilipenko YV, Okishev DN, Kheyreddin AS, Kisar’ev SA, Kaftanov AN. Intraoperative indocyanine green fluorescence angiography in brain aneurysm surgery. The first experience of the technique use and a literature review. Problems Neurosurg Named After N.N. Burdenko. 2015;1:28–36.
Washington CW, Zipfel GJ, Chicoine MR, Derdeyn CP, Rich KM, Moran CJ, Cross DT, Dacey RG Jr. Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg. 2013;118:420–7.
Ozgiray E, Akture E, Patel N, Baggott C, Bozkurt M, Niemann D, Baskaya MK. How reliable and accurate is indocyanine green video angiography in the evaluation of aneurysm obliteration? Clin Neurol Neurosurg. 2013;115(7):870–8.
Sindou M, Acevedo JC, Turjman F. Aneurysmal remnants after microsurgical clipping: classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir (Wien). 1998;140:1153–9.
Norat P, Soldozy S, Elsarrag M, Sokolowski J, Yagmurlu K, Park MS, Tvrdik P, Yashar M, Kalani S. Application of indocyanine green videoangiography in aneurysm surgery: evidence, techniques, practical tips. Front Surg. 2019;6:1–7.
Catapano G, Sgulo F, Laleva L, Columbano L, Dallan I, de Notaris M. Multimodal use of indocyanine green endoscopy in neurosurgery: a single-center experience and review of the literature. Neurosurg Rev. 2018;41:985–98.
de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green video videoangiography. Neurosurgery. 2007;61(3 Suppl):63–72.
Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J. Microscope integrated indocyanine green video-angiography in cerebrovascular surgery. Acta Neurochir Suppl. 2011;109:247–50.
Hardesty DA, Thind H, Zabramski JM, Spetzler RF, Nakaji P. Safety, efficacy, and cost of intraoperative indocyanine green angiography compared to intraoperative catheter angiography in cerebral aneurysm surgery. J Clin Neurosci. 2014;21(8):1377–82.
Gruber A, Dorfer C, Standhardt H, Bavinzski G, Knosp E. Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery. 2011;68:657–73.
Roessler K, Krawagna M, Dorfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014;36:1–7.
Sharma M, Ambekar S, Ahmed O, Nixon M, Sharma A, Nanda A, Guthikonda B. The utility and limitations of intraoperative near-infrared indocyanine green videoangiography in aneurysm surgery. World Neurosurg. 2014;82(5):607–13.
Bruneau M, Appelboom G, Rynkowski M, Van Cutsem N, Mine B, De Witte O. Endoscope-integrated ICG technology: first application during intracranial aneurysm surgery. Neurosurg Rev. 2013;36:77–84; discussion 84–5.
Mielke D, Malinova V, Rohde V. Comparison of intraoperative microscopic and endoscopic ICG angiography in aneurysm surgery. Neurosurgery. 2014;10(Suppl 3):418–25; discussion 425. https://doi.org/10.1227/NEU.0000000000000345.
Yoshioka H, Kinouchi H. The roles of endoscope in aneurysmal surgery. Neurol Med Chir (Tokyo). 2015;55:469–78. https://doi.org/10.2176/nmc.ra.2014-0428.
Cho W, Kim JE, Kang H, Ha EJ, Jung M, Lee C, Shin I, Kang U. Dual-channel endoscopic indocyanine green fluorescence angiography for clipping of cerebral aneurysms. World Neurosurg. 2017;100:316–24.
Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, et al. Endoscopic indocyanine green video angiography in aneurysm surgery: an innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg. 2012;117:302–8.
Januszewski J, Beecher JS, Chalif DJ, Dehdasjti AR. Flow-based evaluation of cerebral revascularization using near-infrared indocyanine green videoangiography. Neurosurg Focus. 2014;36:1–8.
Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by nearinfrared indocyanine green videoangiography. J Neurosurg. 2005;102:692–8.
Awano T, Sakatani K, Yokose N, Kondo Y, Igarashi T, Hoshino T, et al. Intraoperative EC-IC bypass blood flow assessment with indocyanine green angiography in moyamoya and non-moyamoya ischemic stroke. World Neurosurg. 2010;73:668–74.
Cavallo C, Gandhi S, Zhao X, Belykh E, Valli D, Nakaji P, Preul MC, Lawton MT. Applications of microscope-integrated indocyanine green videoangiography in cerebral revascularization procedures. Front Surg. 2019;6:1–10.
Horie N, Fukuda Y, Izumo T, Hayashi K, Suyama K, Nagata I. Indocyanine green videoangiography for assessment of postoperative hyperperfusion in moyamoya disease. Acta Neurochir. 2014;156:919–26. https://doi.org/10.1007/s00701-014-2054-4.
Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 2012;43:2610–6. https://doi.org/10.1161/strokeaha.112.654723.
Esposito G, Dias S, Burkhardt JK, Bozinov O, Regli L. Role of indocyanine green videoangiography in identification of donor and recipient arteries in cerebral bypass surgery. Acta Neurochir Suppl. 2018;129:85–9. https://doi.org/10.1007/978-3-319-73739-3_12.
Esposito G, Regli L. Selective Targeted cerebral revascularization via microscope integrated indocyanine green videoangiography technology. Acta Neurochir Suppl. 2014;119:59–64. https://doi.org/10.1007/978-3-319-02411-0_10.
Yokoyama J, Ishibashi K, Shiramizu H, Ohba S. Impact of endoscopic indocyanine green fluorescence imaging on superselective intra-arterial chemotherapy for recurrent cancer of the skull base. Anticancer Res. 2016;36:3419–24.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jones, B., Almenoff, M. (2023). Use of Fluorescence Guidance in Neurosurgery. In: Szoka, N., Renton, D., Horgan, S. (eds) The SAGES Manual of Fluorescence-Guided Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-40685-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-40685-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40684-3
Online ISBN: 978-3-031-40685-0
eBook Packages: MedicineMedicine (R0)