Abstract
Background
Postoperative cerebral hyperperfusion (HP) is a notable complication that occurs more frequently in moyamoya disease (MMD) than in atherosclerosis. This study aimed to clarify the characteristics of intraoperative indocyanine green (ICG) videoangiography in MMD and atherosclerotic disease in terms of postoperative HP.
Methods
This prospective study included 47 patients with 60 sides that underwent superior temporal artery (STA)-middle cerebral artery (MCA) single bypass. ICG videoangiography was performed after revascularization. The ICG time intensity curve was recorded in the STA, proximal MCA, distal MCA, and superficial Sylvian vein, and the angiographic differences among adult MMD, pediatric MMD, and atherosclerosis were analyzed.
Results
Twenty-two patients (27 sides) had adult MMD, 14 patients (22 sides) had pediatric MMD, and 11 patients (11 sides) had atherosclerosis. Postoperative HP was significantly higher in adult MMD (40.7 %) than in pediatric MMD (18.2 %) and atherosclerosis (0 %). Adult MMD with HP was associated with a longer ICG peak time (P < 0.001). There was no correlation between the ICG peak time and preoperative cerebral blood flow or vascular reserve. The ratio of the vessel caliber was also higher in adult MMD with HP (P < 0.001).
Conclusions
ICG videoangiography provides different characteristics of bypass flow among adult MMD, pediatric MMD, and atherosclerosis. Poor run-off and stagnation of blood flow from the STA might contribute to postoperative HP in MMD. The occurrence of postoperative HP in MMD could depend on two factors: donor STA size and poor run-off and integrity of the blood brain barrier in the recipient MCA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease (MMD) is an uncommon cerebrovascular disease characterized by progressive stenosis of the terminal portion of the bilateral internal carotid arteries. This stenosis leads to the compensatory formation of an abnormal network of perforating blood vessels, termed moyamoya vessels, that provide collateral circulation [29, 30]. The clinical features of MMD substantially differ between children and adults. Most children with MMD develop transient ischemic attack (TIA) or cerebral infarction, whereas about half of adult patients develop intracranial bleeding, TIA/cerebral infarction, or both [19]. The clinical presentation and outcome of MMD remains varied and is based on angiographic findings and other factors including age, systemic factors, and the quality of the cerebral circulation and collateral networks [21]. Revascularization surgery is established for symptomatic MMD and asymptomatic MMD with impaired hemodynamics. Direct/indirect combined bypass surgery (superior temporal artery [STA]-middle cerebral artery [MCA] anastomosis with encephalomyosynangiosis) is the main treatment for patients with MMD, and indirect bypass surgery (encephalomyosynangiosis or encephaloduroarteriosynangiosis) is selected in patients in whom the STA is not developed [19].
Recently, hyperperfusion (HP) after STA-MCA bypass has been a focus in MMD. HP occurs more frequently in adult MMD than in pediatric MMD or atherosclerosis [8, 11, 14, 18, 33]. However, the mechanism for HP after STA-MCA bypass remains undetermined, and no modalities have been established to predict postoperative HP in patients with MMD. This study aimed to test (1) whether ICG videoangiography findings differ among adult MMD, pediatric MMD, and atherosclerosis and (2) whether perioperative ICG videoangiography has a potential to predict postoperative HP.
Materials and methods
Study population
From January 2010, consecutive patients with MMD and atherosclerosis who underwent STA-MCA anastomosis were prospectively analyzed. The indication for surgical revascularization for MMD was symptomatic or hemodynamic compromise on single-photon emission computed tomography (SPECT) [19]. The indication for surgical revascularization for atherosclerosis is still controversial even after the result of Carotid Occlusion Surgery Study (COSS) [2, 24, 25]. In this study, the patients with atherosclerosis were selected for surgical revascularization when they were symptomatic, intractable to medical treatment and have severe hemodynamic compromise on SPECT according to Japanese EC-IC bypass trial [22]. Patients were diagnosed as MMD according angiographic findings [10]: (1) stenosis or occlusion at the terminal portion of the internal carotid artery or proximal areas of the anterior cerebral artery and MCA and (2) abnormal vascular networks in the arterial territories near the occlusive or stenotic lesions as detected by magnetic resonance (MR) angiography and intra-arterial angiography. The patients were divided into adult MMD (>20 years of age) and pediatric MMD groups according to the previous report [33] since they have different properties of presentation [19].
Treatment strategy and ICG videoangiography
STA-MCA single anastomosis with encephalomyosynangiosis was performed by the same operator (I.N.) under general anesthesia. The method of intraoperative ICG videoangiography was approved by the ethics committee of Nagasaki University Hospital, and all patients provided written informed consent. ICG videoangiography was performed using the OPMI Pentero with integrated ICG technology (Carl Zeiss Co., Oberkochen, Germany). Intravenous injection of 12.5 mg of ICG (Daiichi Sankyo, Co., Tokyo, Japan) in 2.5 mL of normal saline was performed, and an ICG time intensity curve was developed. Normal cardiac function was confirmed preoperatively in all patients, and blood pressure, PaO2, and PaCO2 were maintained within the normal range during the surgery. After the surgery, the patients were monitored under strict control of blood pressure. MR imaging and SPECT evaluation were performed within 2 weeks before and 1 to 2 days after the surgery. MR imaging included diffusion-weighted imaging, fluid-attenuated inversion recovery (FLAIR) imaging, T1-weighted imaging, and T2-weighted imaging, and MR angiography. Cerebral blood flow (CBF) was evaluated with 123I-iodoamphetamine SPECT with or without acetazolamide administration for assessment of cerebrovascular reserve (CVR). CVR was calculated using the following formula: (acetazolamide challenging SPECT count – resting SPECT count) ⁄ resting SPECT count (%) [12].
The diagnostic criteria for postoperative symptomatic HP were (1) the presence of neurologic signs, including focal neurologic deficits and/or severe headache; (2) confirmed patency of STA-MCA bypass by MR angiography and the absence of any ischemic changes by diffusion-weighted imaging; (3) an obvious postoperative increase in CBF in the ipsilateral focal perfused area exceeding that in the contralateral side [6–8, 11, 14]; and (4) the absence of other pathologies such as compression of the brain surface by swelling of the temporal muscle graft for encephalomyosynangiosis, ischemic attack, or seizure [11]. To establish the reproducibility of our assessment for the presence of postoperative HP on SPECT, intraobserver and interobserver agreement applying Cohen’s kappa was determined by two readers (N.H. and M.M.) and one reader (N.H.) at an interval of more than 7 days. Cohen’s kappa was evaluated with established grading of agreement: 0 (no agreement), 0 to 0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (substantial), and 0.81 to 1.0 (nearly perfect). When HP developed, the patients were treated with strict blood pressure control using calcium channel antagonists, free radical scavengers, and sedative agents.
ICG image and statistical analysis
ICG images were analyzed with Image J (version 1.45 s; National Institutes of Health, USA), and an ICG time intensity curve was developed in four different regions of interest (ROIs): the STA, proximal MCA, distal MCA, and superficial Sylvian vein (Fig. 1). Based on the ICG time intensity curve, the ICG peak time was defined as the duration of an ICG intensity of >200 arbitrary units. The ratio of the donor STA/recipient distal MCA vessel caliber was also calculated. All data were analyzed by two experienced readers (N.H. and M.M.) by consensus; both were blinded to the radiological and clinical characteristics.
Operative views after STA-MCA bypass: microscopic view (a) and ICG videoangiography (b). Regions of interest were selected at the STA, proximal MCA, distal MCA, and SSV, and the ICG time intensity curve was automatically developed (c). STA: superficial temporal artery, MCA: middle cerebral artery, SSV: superficial Sylvian vein
Data are presented as means±standard error of the mean. Statistical analysis was performed with GraphPad Instat (version 3.05; La Jolla, CA, USA) and SPSS (version 15.0; SPSS Japan Inc., Tokyo, Japan). The independent-samples t-test and Fisher’s exact test were used to compare continuous and categorical characteristics, respectively, between patients with and without HP. One-way ANOVA with the Tukey-Kramer multiple comparison test was performed to compare ICG peak times among presentations in MMD. Correlations between ICG peak time and CBF or CVR were analyzed by Pearson’s rank correlation test, which measures the linear relationship between two variables, because we expected a linear relationship. Very weak, weak, moderate, strong, and very strong correlations were defined as 0 to 0.19, 0.20 to 0.39, 0.40 to 0.59, 0.60 to 0.79, and 0.80 to 1.00, respectively. Differences were defined as significant at a probability level of <0.05.
Results
Patient characteristics
The characteristics of the study population are listed in Table 1. Twenty-two patients (27 sides) had adult MMD, 14 patients (22 sides) had pediatric MMD, and 11 patients (11 sides) had atherosclerosis. The MMD group had a lower percentage of male patients compared with the atherosclerosis group (P = 0.0012). The adult MMD group comprised patients with TIA (n = 12), infarction (n = 5), hemorrhage (intracerebral or subarachnoid) (n = 5), and no symptoms (n = 5). The pediatric MMD group comprised patients with TIA (n = 14), headache (n = 4), no symptoms (n = 3), and infarction (n = 1). Preoperative SPECT analysis showed significantly lower CBF in atherosclerosis than in MMD (P = 0.02). Postoperative SPECT detected 11 patients (40.7 %) with HP in the adult MMD group, and seven patients (25.9 %) showed transient symptoms including dysarthria, numbness, and headache, which completely resolved in 1 week. On the other hand, only four patients (18.2 %) with pediatric MMD developed postoperative HP, and all were symptomatic. Overall, interobserver and intraobserver reproducibility were substantial to nearly perfect for the presence of HP (kappa coefficient = 0.86 and 0.73, respectively).
Postoperative bypass flow on ICG videoangiography
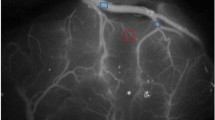
Bypass patency was confirmed in all patents with intraoperative ICG videoangiography and postoperative MR angiography. Interestingly, the ICG intensity in the recipient MCA adjacent to the anastomosis remained high until the late phase in some patients (Fig. 2a). The ICG peak time at the distal MCA was significantly longer in adult MMD with HP than without HP (P < 0.001) (Fig. 2b). The same trend was seen in pediatric MMD, but it did not reach statistical significance. There was no significant difference in ICG peak time among presentations in MMD. Moreover, there was a very weak correlation between the ICG peak time and preoperative CBF (P = 0.09, r2 = 0.08) or CVR (P = 0.65, r2 = 0.01) and a very weak correlation between HP and preoperative CBF or CVR (Fig. 2c).
ICG intensity in the distal MCA adjacent to the anastomosis (asterisk) remained high until the late phase (a). In adult MMD, the ICG peak time was significantly longer in patients with than without HP (***P < 0.001). The same trend was seen in pediatric MMD, but it did not reach statistical significance (b). There was no significant difference in ICG peak time among presentations in MMD (b). There was no correlation between the ICG peak time and preoperative CBF or CVR on SPECT in MMD (c, open circle indicates HP). CBF: cerebral blood flow, CVR: cerebral vascular reserve, SPECT: single-photon emission computed tomography, n.s.: not significant
Next, we analyzed the effect of the ratio of vessel caliber (donor STA/recipient MCA) on postoperative HP to determine whether vessel size mismatch affects HP. As expected, patients with adult MMD showing HP had significantly higher ratios than did patients with adult MMD without HP (P < 0.001), and the ratio was around 1.0 in pediatric MMD regardless of the presence of HP (Fig. 3). A representative case is shown in Fig. 4.
Representative images of a 43-year-old female patient with adult MMD showing postoperative HP. a left internal carotid angiogram suggested MMD, and STA-MCA bypass was performed. ICG videoangiography showed prolonged high ICG intensity in the distal MCA area (circle in b, ICG intensity curve in c). She complained of dysarthria and headache on the second postoperative day, and a high-signal intensity spot was detected on postoperative SPECT (d)
Discussion
Characteristics of ICG videoangiography and postoperative HP in MMD
Revascularization surgery has been established as a standard treatment for MMD to improve cerebral hemodynamics [19]. Increasing evidence has recently suggested that symptomatic HP may occur postoperatively in 15.0 % to 31.5 % of patients with MMD, which is quite different from that occurring after revascularization surgery for atherosclerotic disease despite performance of the same STA-MCA bypass surgery [8, 11, 14, 33]. This HP leads to transient neurological deterioration, seizures, or delayed intracerebral hemorrhage, and early prediction and careful management of HP is mandatory after the bypass for MMD [5]. However, the mechanism of postoperative HP in MMD remains undetermined, and no evidence has been established to explain the difference in the frequency of postoperative HP between MMD and atherosclerotic disease. In this study, we provide new evidence that intraoperative ICG dynamics differ among adult MMD, pediatric MMD, and atherosclerosis in terms of postoperative HP. Poor run-off and stagnation of the bypass flow might contribute to postoperative HP in adult MMD. Why patients with atherosclerosis did not show postoperative HP despite the same ICG flow pattern and even higher ICG intensity compared with patients with adult MMD in this study remains uncertain. Integrity of the host microvasculature in MMD could account for the difference in postoperative HP. Increased expression of serum matrix metalloproteinase-9, cytokines, angiogenic factors, and circulating endothelial progenitor cells are reportedly involved in the pathogenesis of MMD, and these factors could also contribute to HP via increasing the blood–brain barrier permeability after revascularization surgery [9, 16]. It is also reported that the specific pathology of a vascular structure with a poor network and distribution could affect the postoperative HP in MMD [18, 23]. Moreover, it is reported that autoregulatory vasodilation may quickly recover after STA-MCA bypass in pediatric MMD, and such vasodilation may require a longer time to recover in adult MMD [33] probably because of the longer course of microvessel degeneration in adult MMD. This might account for the difference of ICG dynamics between adult and pediatric MMDs. On the other hand, we found that the discrepancy between the STA and MCA calibers could affect postoperative HP, which did not always correlate with ICG peak time in this study (data not shown). A previous report showed that the STA flow and size correlated positively with postoperative MCA flow, and MCA size did not correlate with any of the postoperative MCA flow measurements, emphasizing the importance of STA size and flow rate as the main determinant of the postoperative MCA flow, which supports our result [20]. Awano et al. evaluated the bypass flow area after STA-MCA bypass using ICG and reported that bypass blood flow through the anastomosed STA was larger in patients with MMD than without MMD because of a larger gradient pressure. This finding may account, at least in part, for the occurrence of postoperative HP [1]. Although differences between the patients with and without HP were not assessed in their small study, their findings could contribute to the poor run-off and stagnation of ICG. Nevertheless, we believe that the STA/MCA ratio is not the only factor involved in the development of HP because it is generally larger in very young children. Multiple other factors could be involved in the occurrence of postoperative HP in patients with MMD, including STA/MCA mismatch, poor run-off, and integrity of the blood brain barrier in the MCA (Fig. 5).
Prediction of postoperative HP with ICG videoangiography in MMD
Two reports have shown that certain modalities, such as intraoperative laser Doppler or intraoperative thermography, have the potential to detect postoperative HP [17, 23]. Increases in regional CBF measured with laser Doppler or temperature increases around the anastomotic site during surgery may be indicators of postoperative HP [17, 23]. It is generally recognized that reduction of CBF and impaired CVR can anticipate the occurrence of HP after STA-MCA bypass for atherosclerosis as well as after carotid endarterectomy for carotid stenosis [36]. However, two recent studies assessed the hemodynamic/metabolic predictors of postoperative HP in patients with MMD, and both found that CBF and CVR are not predictors of symptomatic HP in patients with MMD [14, 33]. These results support our data. Further positron emission tomography (PET)-based parameters, including cerebral blood volume and the oxygen extraction fraction, are required to predict the development of postoperative HP in patients with MMD [14, 33]. Nevertheless, these modalities lack general versatility, and other modalities are required. Although quantification of cortical CBF with ICG videoangiography is still controversial and unestablished [15], we believe that routine ICG videoangiography could give additional information regarding poor run-off of the bypass flow by visual inspection, which might contribute to postoperative HP.
ICG is a near-infrared dye that was approved by the Food and Drug Administration to evaluate cardiocirculation and liver function and for use in ocular angiographies. Microscope-integrated intravenous ICG angiography has recently been a useful adjunct for intracranial aneurysm, extracranial-intracranial bypass, intracranial arteriovenous malformation (AVM), spinal AVM, spinal arteriovenous fistula (AVF), and spinal tumors [13, 26, 27, 31, 35]. Moreover, quantitative analysis of ICG transit curves opens new possibilities of assessing blood flow in cerebral vessels and brain tissue [3, 4, 15, 28, 32, 33], and recent papers reported quantification of ICG intensity to assess hypoperfusion after subarachnoid hemorrhage [28] or assess HP after revascularization surgery for atherosclerosis [15]. Some proposed parameters for cortical surface blood flow assessment include bolus peak time, time to peak, rise time, blood flow index (maximum intensity/rise time), and microvascular transit time. However, these parameters have not been established, and further validation of the evidence is mandatory [4, 15, 28, 34]. Particularly in MMD, surface cortical ICG intensity depends on the microvascular density in the ROI, which might give different information due to selection bias of the ROI in the operative field [4]. To avoid bias associated with microvessels on the cortical surface, we evaluated ICG time intensity curves focusing on the host STA, recipient MCA, and adjacent vessels. Interestingly, a previous report suggested that a postoperative increase in the ICG blood flow index could be an indicator of postoperative HP in atherosclerosis, although the patient number is very small [34]. This supports the view that ICG intensity assessment can predict postoperative HP in MMD as well.
Several limitations in this study should be addressed. First, ICG videoangiography for CBF assessment is not fully established, because ICG videoangiography has parameters that differ from those of SPECT or PET. Therefore, ICG parameters could not replace the standard CBF or CVR. Further accumulation of the evidence obtained from ICG videoangiography is mandatory, and new-generation software to standardize ICG parameters could address this limitation. Nevertheless, we believe that ICG videoangiography has the potential to predict HP in MMD before assessing hemodynamic parameters including CBF or CVR. Second, postoperative quantification of CBF on SPECT is lacking in some patients, especially those with pediatric MMD, because of low availability. Finally, we did not perform ICG videoangiography before STA-MCA bypass. Assessment of changes in the ICG intensity curves before and after STA-MCA bypass might give further information to support this study.
Conclusions
This study is the first to show that ICG videoangiography findings differ among adult MMD, pediatric MMD, and atherosclerosis. The occurrence of postoperative HP in MMD may depend on two factors: donor STA size and poor run-off and integrity of the blood brain barrier in the recipient MCA. ICG videoangiography may have the potential to predict postoperative HP in MMD.
References
Awano T, Sakatani K, Yokose N, Kondo Y, Igarashi T, Hoshino T, Nakamura S, Fujiwara N, Murata Y, Katayama Y, Shikayama T, Miwa M (2010) Intraoperative EC-IC bypass blood flow assessment with indocyanine green angiography in moyamoya and non-moyamoya ischemic stroke. World Neurosurg 73:668–674
Carlson AP, Yonas H, Chang YF, Nemoto EM (2011) Failure of cerebral hemodynamic selection in general or of specific positron emission tomography methodology?: Carotid Occlusion Surgery Study (COSS). Stroke 42:3637–3639
Czabanka M, Pena-Tapia P, Schubert GA, Woitzik J, Horn P, Schmiedek P, Vajkoczy P (2009) Clinical implications of cortical microvasculature in adult Moyamoya disease. J Cereb Blood Flow Metab 29:1383–1387
Czabanka M, Pena-Tapia P, Schubert GA, Woitzik J, Vajkoczy P, Schmiedek P (2008) Characterization of cortical microvascularization in adult moyamoya disease. Stroke 39:1703–1709
Fujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T (2012) Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis 33:436–445
Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T (2007) Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 67:273–282
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447
Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T (2011) Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery 68:957–964, discussion 964–955
Fujimura M, Watanabe M, Narisawa A, Shimizu H, Tominaga T (2009) Increased expression of serum Matrix Metalloproteinase-9 in patients with moyamoya disease. Surg Neurol 72:476–480, discussion 480
Fukui M (1997) Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare. Jpn Clin Neurol Neurosurg 99(2):S238–S240
Hayashi K, Horie N, Suyama K, Nagata I (2012) Incidence and clinical features of symptomatic cerebral hyperperfusion syndrome after vascular reconstruction. World Neurosurg 78:447–454
Horie N, Morikawa M, Nozaki A, Hayashi K, Suyama K, Nagata I (2011) "Brush Sign" on susceptibility-weighted MR imaging indicates the severity of moyamoya disease. AJNR Am J Neuroradiol 32:1697–1702
Horie N, So G, Debata A, Hayashi K, Morikawa M, Suyama K, Nagata I (2012) Intra-arterial indocyanine green angiography in the management of spinal arteriovenous fistulae: technical case reports. Spine 37:E264–E267
Kaku Y, Iihara K, Nakajima N, Kataoka H, Fukuda K, Masuoka J, Fukushima K, Iida H, Hashimoto N (2012) Cerebral blood flow and metabolism of hyperperfusion after cerebral revascularization in patients with moyamoya disease. J Cereb Blood Flow Metab 32:2066–2075
Kamp MA, Slotty P, Turowski B, Etminan N, Steiger HJ, Hanggi D, Stummer W (2012) Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: first experience in 30 patients. Neurosurgery 70:65–73, discussion 73–64
Kang HS, Kim JH, Phi JH, Kim YY, Kim JE, Wang KC, Cho BK, Kim SK (2010) Plasma matrix metalloproteinases, cytokines and angiogenic factors in moyamoya disease. J Neurol Neurosurg Psychiatry 81:673–678
Kawamata T, Kawashima A, Yamaguchi K, Hori T, Okada Y (2011) Usefulness of intraoperative laser Doppler flowmetry and thermography to predict a risk of postoperative hyperperfusion after superficial temporal artery-middle cerebral artery bypass for moyamoya disease. Neurosurg Rev 34:355–362, discussion 362
Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK (2008) Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis 25:580–586
Kuroda S, Houkin K (2008) Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7:1056–1066
Lee M, Guzman R, Bell-Stephens T, Steinberg GK (2011) Intraoperative blood flow analysis of direct revascularization procedures in patients with moyamoya disease. J Cereb Blood Flow Metab 31:262–274
Lee M, Zaharchuk G, Guzman R, Achrol A, Bell-Stephens T, Steinberg GK (2009) Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus 26:E5
Mizumura S, Nakagawara J, Takahashi M, Kumita S, Cho K, Nakajo H, Toba M, Kumazaki T (2004) Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med 18:13–21
Nakagawa A, Fujimura M, Arafune T, Sakuma I, Tominaga T (2009) Clinical implications of intraoperative infrared brain surface monitoring during superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. J Neurosurg 111:1158–1164
Powers WJ (2012) Letter by Powers Regarding Article, "Failure of cerebral hemodynamic selection in general or of specific positron emission tomography methodology? Carotid occlusion surgery study (COSS)". Stroke 43:e43, author reply e44
Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, Investigators C (2011) Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 306:1983–1992
Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52:132–139, discussion 139
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103:982–989
Schubert GA, Seiz-Rosenhagen M, Ortler M, Czabanka M, Scheufler KM, Thome C (2012) Cortical indocyanine green videography for quantification of acute hypoperfusion after subarachnoid hemorrhage: a feasibility study. Neurosurgery 71:260–267, discussion ons267-268
Suzuki J, Kodama N (1983) Moyamoya disease–a review. Stroke 14:104–109
Suzuki J, Takaku A (1969) Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20:288–299
Takagi Y, Sawamura K, Hashimoto N, Miyamoto S (2012) Evaluation of serial intraoperative surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography in patients with cerebral arteriovenous malformations. Neurosurgery 70:34–42, discussion 42–33
Terborg C, Groschel K, Petrovitch A, Ringer T, Schnaudigel S, Witte OW, Kastrup A (2009) Noninvasive assessment of cerebral perfusion and oxygenation in acute ischemic stroke by near-infrared spectroscopy. Eur Neurol 62:338–343
Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N (2012) Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 43:2610–2616
Uchino H, Nakamura T, Houkin K, Murata J, Saito H, Kuroda S (2013) Semiquantitative analysis of indocyanine green videoangiography for cortical perfusion assessment in superficial temporal artery to middle cerebral artery anastomosis. Acta Neurochir 155:599–605
Washington CW, Zipfel GJ, Chicoine MR, Derdeyn CP, Rich KM, Moran CJ, Cross DT, Dacey RG Jr (2013) Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg 118:420–427
Yoshimoto T, Houkin K, Kuroda S, Abe H, Kashiwaba T (1997) Low cerebral blood flow and perfusion reserve induce hyperperfusion after surgical revascularization: case reports and analysis of cerebral hemodynamics. Surg Neurol 48:132–138, discussion 138–139
Conflicts of interest
This work was supported in part by a Grant-in-Aid for Scientific Research to N.H. (23791611) and I.N. (24592134). The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horie, N., Fukuda, Y., Izumo, T. et al. Indocyanine green videoangiography for assessment of postoperative hyperperfusion in moyamoya disease. Acta Neurochir 156, 919–926 (2014). https://doi.org/10.1007/s00701-014-2054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2054-4