Abstract
Melatonin synthesized by plants is known as phytomelatonin. It is a ubiquitous indolamine, largely investigated for its key functioning in the regulation of many morpho-physiological activities in plants. The biosynthesis of melatonin in plants starts from tryptophan through many similar successive enzymatic steps. The role of phytomelatonin in many transcriptional processes through altering the genes confirms its significant involvement as a multi-regulatory molecule that leads the many aspects of plant development. The current chapter addresses the history and biosynthesis of phytomelatonin. The potential role of phytomelatonin in agriculture as a growth regulator, photosynthesis, antioxidant defense system, plant hormone interaction, and secondary metabolism are also described briefly.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
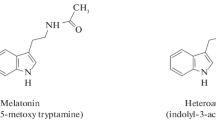
Melatonin (N-acetyl-5-methoxytryptamine), one of the most important biochemicals in living organisms, serves as a secondary metabolite associated with a variety of physiological, hormonal, and natural processes at the cell, tissue, and organ levels. The tryptophan metabolic pathway produces the hormone melatonin as its by-product. Studies have revealed that it is still a part of an evolutionary process and that its origins may be linked to prokaryotes (Manchester et al. 2015; Zhao et al. 2021a). In animals, as a chronobiological hormone that alerts the brain and other peripheral organs to darkness, melatonin is particularly significant. It regulates sleep-wake cycles and synchronizes life activity into seasonal periods and reproductive activities. It is an endogenous synchronizer for both endocrine via neurotransmitter release and other physiological rhythms (Lerner et al. 1959).
Melatonin of plant origin is known as phytomelatonin (N-acetyl-5-methoxytryptamine). Phytomelatonin has been found in almost all plant species examined since its discovery in 1995. However, the actual quantity of phytomelatonin significantly varies among plant species. Typically, the phytomelatonin content in aromatic medicinal plants is higher compared to other plants. The conditions for growth and development are the major factors, which exhibit the potent impact on endogenous phytomelatonin content. Plant cellular organelles such as chloroplasts and mitochondria are involved in the synthesis of phytomelatonin. The presence of phytomelatonin synthesizing enzymes in cytoplasm and endoplasmic reticulum indicates the involvement of these organelles in melatonin production. However, it is still unknown if the production of melatonin in various cellular compartments may be influenced by plant species, types of stress, and serotonin levels. Phytomelatonin is also present in the vascular tissue, but its role in transportation and assimilation of plant nutrients still needs to be explored. Till now, plant roots have not been explored for the presence of melatonin.
The presence of high amount of melatonin in plants triggered plant scientists to explore its regulatory functions in plant growth and development. Phytomelatonin is mentioned as a pleiotropic hormone that exhibit a wide range of advantageous effects that generally enhance physiological processes such as seed germination and growth, photosynthesis including pigment content, photorespiration, stomatal conductance, and water economy, seed and fruit yield, osmoregulation, and control of various metabolic pathways such as carbohydrates, lipids, nitrogen compounds, sulfur, and phosphorus cycles. Phytomelatonin stimulates the formation of simple phenols, flavonoids, anthocyanins, carotenoids, and numerous terpenoids in relation to secondary metabolism. Phytomelatonin delays leaf senescence and encourages rooting processes. Melatonin is also reported to control ethylene levels and lycopene content in fruits during post-harvest handling. Thus, phytomelatonin exhibit significant impact on overall metabolism of ripening of agri-produce. Melatonin is also reported to induce parthenocarpy during fruiting by regulating genes involved in gibberellin pathways. Phytomelatonin also aids in crop health by controlling damage caused due to pathogen infections by promoting systemic acquired resistance. Several physiological investigations also have mentioned phytomelatonin as a defensive biomolecule that can efficiently resist various kinds of physical, chemical, and biological stresses. Phytomelatonin is also a potent plant hormone regulator which exhibit a significant impact on auxin, gibberellins, cytokinins, abscisic acid, ethylene, jasmonic acid, salicylic acid, and brassinosteroids. Thus, melatonin is frequently known as a “plant master regulator.” All these above-mentioned effects of phytomelatonin are concentration-dependent, thus it is necessary to adopt effective approaches for melatonin detection in plant samples.
The role of phytomelatonin in controlling plant-ecosystem interactions has also been highlighted by the characteristics of melatonin in phytoremediation, plant innate immunity, and plant-rhizomicrobial population.
1.2 History of Phytomelatonin Discovery and Major Findings
1.2.1 Melatonin Discovery in Plants (Phytomelatonin)
As mentioned in Sect. 1.1, till 1995, melatonin was only recognized as an animal hormone. But in 1993, van Tassel and O’Neill found melatonin in Pharbitis nil L by employing radioimmunoassay (RIA) and gas chromatography (GC) equipped with mass spectrometry (MS). But this finding was not released until 1995 (Arnao 2014). Interestingly, in 1995 melatonin levels were measured in Nicotiana tabacum L. and five culinary plants was published by Dubbels and colleagues using RIA and HPLC-MS (Dubbels et al. 1995). Two months later, a new study measured melatonin in numerous plant food extracts using liquid chromatography (HPLC) and fluorescence detection was published (Hattori et al. 1995). The same year, another article was published on the presence of melatonin in Chenopodium rubrum L. by Czech researchers (Kolar et al. 1995). Followed by these findings, multiple investigations that have quantified melatonin in different plants which established the present of this chemical in both plants and mammals. After the discovery of plant melatonin, another study on metabolic processes similar to those associated with animal melatonin was published. The first goal of this study was to show how it might have a role as a chrono-regulatory protein in photomorphogenic processes like flowering. The Czech researchers Kolá and Macháková devoted many years to explore the impact of phytomelatonin on Chenopodium rubrum L. flowering and how circadian rhythms affects the phytomelatonin levels, but their attempts were unsuccessful (Kolar et al. 1997, 2003; Wolf et al. 2001; Kolar 2003). In 2000, another research by a Canadian team led by Dr. Saxena on St. John’s wort (Hypericum perforatum L.) cell culture discovered the stages in phytomelatonin biosynthesis pathway that were comparable to those of mammals. It was suggested that melatonin might function as an auxin in vitro cell culture as melatonin and indole-3-acetic acid (IAA) are chemically comparable (Murch et al. 2000, 2001; Murch and Saxena 2002). In 2004, a study was published to demonstrate growth-stimulating impact of melatonin on etiolated hypocotyls of lupin (Lupinus albus L.). The stimulatory potential of melatonin was reported to be 63% higher compared to the IAA (Hernández-Ruiz et al. 2004). This stimulatory potential of melatonin was later verified in several Poaceae species (Hernández-Ruiz et al. 2005; Arnao and Hernández-Ruiz 2006).
In 2004, another study revealed the protective effects of melatonin in carrot (Daucus carota L.) cells exposed to cold stress (Lei et al. 2004). Since 2008, several studies reported the protective effect of melatonin against different kinds of stress such as the impact of melatonin on the germination of Brassica oleracea L. seeds under copper toxicity (Posmyk et al. 2008, 2009). and protective effect of melatonin against abiotic (drought, salinity, waterlogging, cold, heat, metal toxins, herbicides, UV radiation) and biotic (bacteria, fungi, virus) stressors (Arnao and Hernández-Ruiz 2014, 2015, 2019; Debnath et al. 2019; Zhan et al. 2019; Kul et al. 2019; Wang et al. 2018; Fan et al. 2018; Sharif et al. 2018; Kanwar et al. 2018; Yu et al. 2018; Sharma and Zheng 2019; Sharma et al. 2020). All these stressors causes a spike in reactive oxygen and nitrogen species (ROS/RNS), which in turn triggers the biosynthesis of phytomelatonin in tissues. The enzymes involved in the biosynthesis of melatonin such as tryptophan 5-hydroxylase (T5H), tryptophan decarboxylase (TDC), serotonin N-acetyltransferase (SNAT), acetylserotonin methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT) exhibit increased gene expression in response to induced stress (Arnao and Hernández-Ruiz 2018, 2019; Hernández-Ruiz and Arnao 2018). In 2007, melatonin was reported to induce the formation of adventitious roots in lupin by stimulating the formation of root primordia in the pericycle (Arnao and Hernández-Ruiz 2007).
A study published in 2009 revealed the delay in chlorophylls loss due to darkness in barley plants followed by melatonin treatment (Arnao and Hernández-Ruiz 2009a, b). Researchers conducted in later years revealed the control of transcription factors connected to senescence and chlorophyll biosynthesis in leaf senescence by melatonin (Wang et al. 2012).
Overall, only 35 articles related to phytomelatonin were published between 1995 and 2005. Whereas, the number of publications (264) increased almost linearly between 2009 and 2017, and an exponential rise in publications (388) was observed from 2018 to 2020. Whereas, in year 2021, there were 287 articles published, that number was rose to 372 articles in 2022. Till March 2023, 98 publications related to phytomelatonin are already available online. The number of publication related to phytomelatonin is expected to be increased significantly in near future due to the significant role of melatonin in plant species.
1.2.1.1 Major Studies and Breakthroughs Related to Phytomelatonin
Phytomelatonin is a superior antioxidant with a much higher protective capacity compared to the traditional antioxidants such as ascorbic acid and vitamin E. Its ability to control gene expression quickly led to the development of novel theories. For a molecule to be classified as a plant hormone, it is important to know its production, degradation, potential conjugation, transportation, receptors, signaling pathways and physiological effects. In case of phytomelatonin, many of these parameters are well-documented except identification of its receptor which is the most challenging task to deal with (Arnao and Hernández-Ruiz 2018, 2019). In 2018, Dr. Chen and co-workers discovered phytomelatonin receptor known as PMTR1 in plasma membrane of Arabidopsis thaliana. Phytomelatonin can increase its expression in various tissues, as it works with the G-protein subunit (GPA1). The dissociation of G-protein subunits Gγb and Gα is caused by PMTR1-phytomelatonin binding initiates an NADPH oxidase-dependent H2O2 generation (RBOH) that increases Ca2+ inflow and encourages K+ efflux which in turn lead to stomatal closing (Wei et al. 2018). This was the most important finding which established phytomelatonin as a hormone. However, it is also important to describe the phytomelatonin receptor in other plant species. Phytomelatonin was known for its regulatory functions in the redox network, where it stimulates the activation of many genetic resources during the times of stress conditions to rebalance the redox homeostatsis. Phytomelatonin was also mentioned as a major plant regulator of the redox network (Arnao and Hernández-Ruiz 2019; Hernández-Ruiz and Arnao 2018; Garrido et al. 2010). Phytomelatonin has also been shown to act as a chronoregulator in plants (Arnao and Hernández-Ruiz 2020), but the experimental confirmation for the relationship between biological clock and redox network is still pending. If this hypothesis is true, phytomelatonin (a plant master regulator) proves to functions similar to the animal melatonin in a way it transfers biological clock oscillations to the redox network which provides an appropriate response to achieve redox homeostasis in stressful conditions. Since 2010, different parts of various wild and edible plants as well as plant-based cooked and fermented food products (juices, musts, wines, and infusions) have been explored for the presence of phytomelatonin (Arnao and Hernández-Ruiz 2020).
Phytomelatonin mentioned to exhibit several physiological functions (Arnao and Hernández-Ruiz 2020) such as regulation of lipids and carbohydrates metabolism, regulation of nitrogen, sulfur, and phosphorus cycles, stimulation of secondary metabolism to produce carotenoids, anthocyanins, and flavonoids under stress. Melatonin causes parthenocarpy in pears, which raises the amount of gibberellins (Liu et al. 2018). In Arabidopsis, melatonin levels are controlled by diurnal rhythms and are linked to Circadian Clock Associated 1, gene that regulates circadian rhythms. Additionally, exogenous melatonin and circadian variations co-regulated the expression of AtCBFs and C-repeat-binding factors (CBFs/DREB1s), suggesting a potential connection between the clock, endogenous melatonin level, and plant defense (Shi et al. 2016). Phytomelatonin also controls its own production by increasing the expression of involved genes such as SNAT (serotonin N-acetyltransferase), ASMT (acetylserotonin O-methyltransferase), and COMT (caffeic acid 3-O-methyltransferase) (Mannino et al. 2021). Phytomelatonin controls the production of genes associated with and triggered by foliar senescence as well as genes linked to chlorophyll degradation (Hernández-Ruiz et al. 2021). In addition, during the rooting processes of primary, secondary, and adventitious roots, phytomelatonin controls the production of several factors such as PIN auxin transporters and AUX1 (Arnao and Hernández-Ruiz 2018). Phytomelatonin mentioned to improve plant health by slowing the harm and promoting systemic acquired resistance (SAR) in response to bacterial, fungal, and viral pathogen infections (Zeng et al. 2022a). Phytomelatonin was also known to control several enzymes in the cell wall, ethylene biosynthesis, and primary and secondary metabolisms. In addition, phytomelatonin enhanced the ethylene and lycopene concentration in fruits during post-harvest regulation along with maintaining the freshness of cut flowers (Xu et al. 2019).
1.3 Biosynthesis of Phytomelatonin
The chloroplast and mitochondria are hypothesized to be the major sites involved in the biosynthesis of melatonin since these cellular compartments are reported to have the highest melatonin levels in plants. Moreover, the localization of serotonin N-acetyltransferase (SNAT) in these organelles supports the above hypothesis. The serotonin N-acetyltransferase is a rate-limiting enzyme in the biosynthesis of melatonin. Phytomelatonin is synthesized from amino acid tryptophan. Plants can synthesize tryptophan de novo by the shikimate pathway. There are six enzymes known to be involved in the synthesis of melatonin (1) l-tryptophan decarboxylase (TDC), (2) tryptamine 5-hydroxylase (T5H), (3) serotonin N-acetyltransferase (SNAT), (4) acetylserotonin O-methyltransferase (ASMT), (5) caffeic acid 3-O-methyltransferase (COMT), and (6) a putative tryptophan hydroxylase (TPH) not yet identified. TDC, ASMT, and COMT are localized in the cytoplasm, while T5H lies in the endoplasmic reticulum and SNAT is expressed in chloroplasts (Back 2021; Zhao et al. 2021b).
The first step in melatonin biosynthesis is the conversion of tryptophan into serotonin. This conversion may occur via two different pathways (Back et al. 2016). Tryptophan is transformed into serotonin as the initial step in the production of melatonin. There are two different ways through which this conversion can take place. One method involves the decarboxylation of tryptophan to produce tryptamine. This step is catalyzed by l-tryptophan decarboxylase. Tryptamine is then converted into serotonin by the tryptamine 5- hydroxylase. Otherwise, the hydroxylation of tryptophan into 5-hydrotryptophan is followed by decarboxylation into serotonin. The above steps were catalyzed by tryptophan hydroxylase and l-tryptophan decarboxylase, respectively. Since, tryptophan decarboxylase has an affinity for both tryptophan as well as 5-hydoxytryptophan, both above-mentioned pathways are possible.
In plants, decarboxylation has been demonstrated as the first step followed by hydroxylation (Back et al. 2016). Furthermore, the synthesis of melatonin from serotonin is two-step pathway that involves three distinct enzymes, that is, serotonin N-acetyltransferase (SNAT), N-acetylserotonin methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT). SNAT catalyzes the reaction that changes serotonin into N-acetylserotonin, which is then converted into melatonin by either ASMT or COMT. Because SNAT has a substrate affinity for serotonin and 5-methoxytryptamine (SNAT), the process produces N-acetylserotonin, which is then O-methylated to melatonin by ASMT/COMT. Similarly, ASMT and COMT show substrate affinity for serotonin and N-acetylserotonin, with ASMT and COMT first methylating serotonin to 5-methoxytryptamine, followed by SNAT reaction to melatonin. In conclusion, SNAT and ASMT/COMT can catalyze the conversion of serotonin into N-acetylserotonin and 5-methoxytryptamine, which is followed by the creation of melatonin by ASMT/COMT and SNAT, respectively (Zhao et al. 2021b; Back et al. 2016) (Fig. 1.1).
1.4 Functions of Phytomelatonin
Phytomelatonin exhibit several important functions to improve growth and yield of plants such as improvement in photosynthetic activity, antioxidant defense system, regulates redox network, primary and secondary metabolism, defense against apoptosis, and stressful conditions as shown in Fig. 1.2. In the following section, the function of phytomelatonin in plant growth and yield are discussed in detail.
1.4.1 Impact of Phytomelatonin on Plant Growth
Following by the initial discovery of melatonin in plants, researchers have also linked it to the regulation of growth and development of variety of plants such as Arabidopsis thaliana, oat, mustard, cauliflower, cucumber, sunflower, barley, lupin, rice, Prunus, guava, soybean, tomato, wheat and corn plants (Gao et al. 2015; Arnao and Hernández-Ruiz 2017, 2018). Phytomelatonin is structurally quite similar to the IAA (Indole-3 Acetic Acid), a commonly occurring plant hormone of auxin class. According to a previous research, phytomelatonin and IAA might collaborate in several physiological processes relating to plant growth and development as their similar structures may serve related purposes. Both phytomelatonin and IAA also have the ability to act as antioxidants (Li et al. 2016). In St. John’s wort in vitro cultures, an increase in endogenous melatonin concentration considerably stimulated the root growth, whereas the build-up of serotonin, a precursor to melatonin, directs shoot production (Arnao and Hernández-Ruiz 2007).
Murch et al. (2009) presented the first encouraging study on the regulatory mechanism of melatonin in relation to auxin during plant growth and development. Researchers have identified potential interactions between melatonin and auxin using metabolic inhibitors of auxins, serotonin, and melatonin. The finding of this study emphasized on the possibility that melatonin and auxin interact to influence root architecture, hence promoting plant growth and development.
Till now, only a few genes and metabolites have been reported to be involved in the synthesis and degradation of phytomelatonin, which limit the understanding of melatonin-auxin-mediated interactions in plants. Thus, significant research should be conducted to identify the additional crucial elements involved in the regulation of phytomelatonin production and signaling. In addition, identification and characterization of novel gene(s) associated with phytomelatonin production and signaling will offer novel insights of the associated processes. It is also important to employ effective instruments and sophisticated methodologies for melatonin detection in plant samples.
1.4.2 Impact of Phytomelatonin on Seed Germination and Plant Performance
Seed germination is the most crucial stage of crop growth. The genetic makeup of a crop as well as its surroundings have a significant impact on its germination. Water is the one of these traits that has the greatest environmental impact on seed germination. Recent research has demonstrated that crop plants may experience severe hypoxic stress due to prolonged flooding or submersion, which will significantly reduce the crop yield. Phytohormones are among the main elements which regulates the seed germination. Studies have reported the activation of germination-related genes in the presence of plant hormones. Hence, phytohormone therapy is successfully employed to enhance seed germination. The widespread distribution of melatonin across fungi, algae, bacteria, mammals, and plants has led to the hypothesis that it may function as a photoperiodic and circadian rhythm regulator as well as a universal antioxidant (Paredes et al. 2009; Tan et al. 2012). Melatonin and a variety of its metabolites are known as endogenous free radical scavengers and powerful broad-spectrum antioxidants and reported to directly scavenge H2O2, which in turn maintain intracellular H2O2 concentrations (Reiter et al. 2007).
Previously, Korkmaz et al. (2017) found that melatonin treatment significantly reduced the H2O2 levels and MDA accumulation due to increased antioxidant enzyme activity and direct free radical scavenging by melatonin. In another study, Posmyk et al. (2009) showed that spraying melatonin on cucumber seed provides considerable protection to their membrane structures against peroxidation and MDA accumulation under cold stress. In this study, melatonin was reported to enhance the activity of antioxidant enzymes and demonstrate its critical role in providing antioxidant defense under cold stress circumstances. Cold-stressed pepper seeds and seedlings showed increased activity of SOD, CAT, and POX followed by melatonin treatment which revealed the involvement of melatonin in free radical scavenging. Similar findings were also reported by Zhang et al. (2013), who found that melatonin treatment significantly increased the activity of antioxidant free radical scavenging enzymes such as SOD, CAT, and POX in cucumber seeds germinating under drought stress conditions.
Zeng et al. (2022b) reported a significant increase in the seed germination, seedling growth, and early stand establishment of rice seed soaked in melatonin. Compared to the control, seeds soaked in melatonin presented a significant increase in the growth of rice seed shoots and roots, superoxide dismutase (SOD) activity, peroxidase (POD) activity (Posmyk and Janas 2009), and lower the content of malondialdehyde (MDA) which ameliorate the inhibitory effect of flooding stress on rice growth. Overall, soaking rice seeds in 100 μM melatonin would be a reasonable choice for bio stimulation. Melatonin treatment will be helpful in faster germination and survival of rice seedlings under flooding stress. Melatonin can also be used as seed coating to enhance the crop yield and their ability to withstand abiotic stress. An extensive research is required to explore the possibility of melatonin to be used as commercial seed coating treatment.
1.4.3 Impact of Phytomelatonin on Photosynthetic Efficiency of Plants
An essential plant metabolic process called Photosynthesis is an important metabolic process responsible to enhance crop output and carbon uptake. Cold stress is reported to significantly reduce the photosynthetic efficiency of plants. Melatonin, a novel plant growth regulator, can significantly regulates a variety of abiotic stress responses in plants. The molecular basis of melatonin-mediated photosynthetic control in plants under cold stress is not fully understood. According to a study by Altaf et al. (2022), spraying pepper seedlings with melatonin (200 μM) during low-temperature stress (15/5 °C for 7 days) improved gas exchange properties, amount of photosynthetic pigment and expression of their biosynthetic genes. Under cold stress conditions, photochemical activity of photosystems II (PSII) and I (PSI), specifically their maximum quantum efficiencies PSII (Fv/Fm) and PSI, was considerably reduced. In cold stress conditions, melatonin reported to significantly enhance the activity of photosynthetic enzymes (rubisco and fructose-1, 6-bisphosphatase), and quantity of starch, sucrose, soluble sugar, and glucose. In contrast, melatonin treatment significantly enhanced the photochemical activity of PSII and PSI. Melatonin treatment significantly reduced the effects of cold stress, which also resulted in significant reductions in actual PSII efficiency (ΦPSII), electron transport rate (ETR), and photochemical quenching coefficient (qP), while improving nonphotochemical quenching (NPQ). Overall, melatonin treatment significantly reduced the negative impacts of cold stress by maintaining photosynthetic efficiency, which may be useful when genotypes for CS tolerance are being explored.
Due to overuse of fertiliser and other anthropogenic activities, agricultural soil contains a significant amount of hazardous metals, which presented an adverse effect on crop plants, their yield and quality. The application of melatonin by Ayyaz et al. (2020) mentioned to enhance plant tolerance toward environmental stress and also increased plants growth and development under stress condition. Chromium stress mentioned to reduce energy trapping effectiveness by degrading oxygen evolving complex, however, the exogenous application of melatonin protects the oxygen evolving complex of PSII and assisted in maintaining PSII activity. Due to the beneficial effects of melatonin in plants, it is anticipated that in future it may play an important role in developing transgenic crops that exhibit potent photosynthetic activity under stress conditions.
1.4.4 Impact of Phytomelatonin on Antioxidant Defense System
According to Hardeland and Pandi-Perumal (2005), the kynurenine pathway’s direct scavenging of physiological melatonin concentrations by a non-enzymatic contribution and the subsequent activities of the metabolites generated become significant. But at normal levels, melatonin can always have signaling effects. Many antioxidant enzymes are upregulated by melatonin. This has most commonly been shown for glutathione peroxidase and occasionally glutathione reductase, most likely indirectly through GSSG. Occasionally, catalase and the superoxide dismutases Cu, Zn, and/or Mn are elevated in certain tissues. In both mammalian and avian brain, glutathione peroxidase appears to be widely distributed among tissues and is consistently stimulated; upregulations in other organs were less consistent. Melatonin’s effects on glutathione metabolism appear to go beyond those already discussed. By providing reducing equivalents (NADPH) for the activity of glutathione reductase and boosting the rate of glutathione synthesis, respectively, stimulation of glucose-6-phosphate dehydrogenase and -glutamylcysteine synthase indirectly enhances the function of glutathione peroxidase. Moreover, melatonin helps prevent the generation of radicals in a variety of separate ways. Prooxidant enzymes are downregulated by it, particularly NO synthases and the 5- and 12-lipoxygenases. In order to restrict the growth in the extremely prooxidant metabolite peroxynitrite and of the free radicals formed from it, namely, •NO2, carbonate (CO3•-), and hydroxyl (•OH) radicals, the commonly documented reduction of NO production is particularly crucial. Limits on inflammatory responses may also be imposed via inhibiting lipoxygenase and NO synthase, while the immunomodulatory effects of melatonin are undoubtedly more complicated and may also involve other effects of melatonin and AMK. Instead of focusing primarily on antioxidant actions for the removal of already created radicals, the effects of melatonin on the respiratory chain give new perspectives for reducing radical generation.
1.4.5 Impact of Phytomelatonin on Plant Hormones Interaction
Plant melatonin appears to be a multi-regulatory chemical with numerous distinct functions in plant physiology, comparable to those seen in animals. Studies on melatonin in plants have become much more prevalent in recent years. Melatonin’s effects on biotic and abiotic stress, including those brought on by drought, severe heat, salt, chemical pollution, and UV radiation, among others, are among its most researched effects on plants. Melatonin has a significant role in controlling the expression of genes associated to plant hormones, including auxin carrier proteins, indole-3-acetic acid (IAA), gibberellins, cytokinins, abscisic acid, and ethylene metabolism. The majority of studies have focused on melatonin’s auxin-like action, which works in a manner similar to IAA in that it can induce growth in shoots and roots and encourage the development of new lateral and adventitious roots. In addition to safeguarding photosynthetic systems and associated subcellular structures and processes, melatonin has the ability to delay senescence. Also important is its function as a gene regulator of ethylene-related elements during fruit ripening and post-harvest procedures. Its part in the pathogen–plant interaction is another important consideration. Melatonin, along with other well-known chemicals like nitric oxide and hormones like jasmonic acid and salicylic acid, appears to play a major role in the plant immune response. In this way, the finding of higher melatonin levels in endophytic organisms connected to plants has shed light on a potential novel method of communication between advantageous endophytes and host plants (Arnao and Hernández-Ruiz 2018).
One of the more specific auxin-like functions of melatonin is its ability to promote growth. In the aerial tissues of lupinus, phalaris, triticum, hordeum, arabidopsis, and cucumis, melatonin generated a three to fourfold increase in growth compared with control plants, and a less marked rise in others. Melatonin slows down the induced senescence process as well. It has been shown that melatonin treatment of barley leaves results in a concentration-dependent delay of dark-induced senescence, which reduces chlorophyll loss in detached leaves. The senescence process was also delayed by incubating leaf sections with various amounts of kinetin (a synthetic cytokinin), which resulted in treated leaves losing less chlorophyll and retaining higher levels of total chlorophyll than control leaves. Treatment with melatonin changed the levels of GAs and ABA. Melatonin induced the upregulation of GA biosynthesis genes in cucumber seedlings under salinity, resulting in a high concentration of active GAs like GA3 and GA4, which supported the salt-inhibited germination process. Moreover, melatonin treatment caused the downregulation of 9-cis-epoxycarotenoid dioxygenase (NCED), a crucial enzyme in ABA production, and the overexpression of ABA catabolism genes (two CYP707 monooxygenases), which led to a rapid drop in ABA levels during seed germination under salt stress. Similar findings were made in drought-stricken apple leaves, where melatonin pre-treatment reduced the ABA concentration by half by controlling the same ABA production and catabolism enzymes as previously indicated (Zhang et al. 2014).
The key aim is to identify a potential melatonin receptor in order to better understand the mechanisms that trigger the reactions in plants. Because it is an amphipatic molecule, its dual action as an antioxidant and a gene expression regulator appears obvious, but it still raises questions about its transport within cells (free or protein-assisted), as well as between tissues and organs (through the xylem and phloem), its potential conjugation with carbohydrates or amino acids, the signaling components involved, and its potential sharing with plant hormone mechanisms. Although there are many hints in this work that plant hormones and melatonin are related, there is still much research to be done before any conclusive findings can be made. Future research on melatonin and specific plant hormones, however, will surely shed light on the level of interaction present at the cellular and tissular level as well as any potential synergies or antagonistic interactions in the mediated responses.
1.4.6 Impact Phytomelatonin on Primary and Secondary Metabolism of Plants
The data on melatonin’s regulatory function in several plant metabolic pathways has come from numerous studies. Its crucial impact on enzyme transcripts and regulatory elements in many organelles (chloroplasts, mitochondria, endoplasmic reticulum), subcellular locations (cytosol, cell wall), and secondary metabolism stands out. Melatonin definitely has an impact on a number of critical metabolic activities, including photosynthesis, the pentose phosphate shunt (Sharkey 2021), gluconeogenesis, glycolysis, the Krebs cycle, and the production of amino acids and fatty acids. Although one of the most studied, there is still much to learn about carbohydrate metabolism. Melatonin appears to be a key player in the fate of carbohydrates made in the chloroplast and cytosol, from the regulation of Rubisco to the processes of glycolysis and fermentation. Melatonin controls the Calvin cycle’s generation of triose phosphate, its conversion to hexoses, the amount of starch in the chloroplastic stroma, and the amount of sucrose in the cytosol and cell walls. Melatonin generally promotes the primary metabolism of carbohydrates as well as that of other primary substances including lipids and amino acids. As a result, the metabolic turnover is activated and conditioned to be appropriate for the current physiological circumstances. Melatonin has a variety of regulatory effects. For instance, it affects photosynthesis, increasing the effectiveness of Rubisco and other Calvin cycle-related enzymes, Photosystem I and II, chlorophyll and carotenoid content, and stomatal complex, with the result being a higher net photosynthesis. Additionally, melatonin mobilizes some important pathways in specific carbohydrate metabolism, such as starch and sucrose biosynthesis, through upregulation of SPS.
Melatonin is a critical bio stimulator or plant growth regulator because it regulates numerous aspects of the metabolism of plant hormones, which, in conjunction with the modulation of the redox network, enable the plant to adapt to its environment in a way that minimizes negative effects and increases tolerance to stressors. Melatonin has the power to affect several stages of secondary metabolism, particularly in the phenolic chemical and terpenes biosynthesis, which explains why it can alter carbohydrate metabolism and enhance the levels of sugars in fruits as well as their organoleptic properties. The regulation of anthocyanins and other flavonoids, as well as carotenoids and essential oils, are some of its standout features. Moreover, melatonin treatment has a good impact on crop yield. For example, melatonin treatment has been shown to boost production of the following crops: rapeseed, cucumber, tomato, wheat, rice, and others (Arnao et al. 2021). There are a good number of questions that need to be answered regarding how melatonin affects carbohydrate metabolism, including: how genes in the nucleus, chloroplasts, and mitochondria are regulated; how it interacts with other plant hormones; how it functions in various organs (leaf, stem, root, flowers, fruits); how it affects the accumulation and breakdown of starch in amyloplasts; how it affects the metabolism of sucrose in source and sink tissues; and many. The equilibrium between carbs, fatty acids, and amino acids has yet to be fully understood.
1.5 Conclusion
Melatonin is an important phytohormone and participated in number of developmental activities of the plant. The information contained in this book chapter clarifies the chemical characteristics of phytomelatonin and the typical biosynthetic pathways occurring in plants. The various morpho-physiological functions regulated by phytomelatonin described in a systematic way. Moreover, the history of phytomelatonin was highlighted. In this chapter, plant hormones interaction, secondary metabolism influence, and antioxidant enzymes activity in respect to phytomelatonin give clear edge of this hormone as a beneficial plant growth regulator.
References
Altaf MA, Shu H, Hao Y, Mumtaz MA, Lu X, Zhiwei W (2022) Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants 11(12):2414. https://doi.org/10.3390/antiox11122414
Arnao MB (2014) Phytomelatonin: discovery, content, and role in plants. Adv Bot 2014:815769. https://doi.org/10.1155/2014/815769
Arnao MB, Hernández-Ruiz J (2006) The physiological function of melatonin in plants. Plant Signal Behav 1:89–95
Arnao MB, Hernández-Ruiz J (2007) Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 42(2):147–152. https://doi.org/10.1111/j.1600-079x.2006.00396.x
Arnao MB, Hernández-Ruiz J (2009a) Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res 46:295–299
Arnao MB, Hernández-Ruiz J (2009b) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res 46:58–63
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19:789–797
Arnao MB, Hernández-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150
Arnao MB, Hernández-Ruiz J (2017) Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta Physiol Plant 39(6):1–9. https://doi.org/10.1007/s11738-017-2428-3
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121(2):195–207. https://doi.org/10.1093/aob/mcx114
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48
Arnao MB, Hernández-Ruiz J (2020) Is phytomelatonin a new plant hormone? Agronomy 10(1):95. https://doi.org/10.3390/agronomy10010095
Arnao MB, Hernández-Ruiz J, Cano A, Reiter RJ (2021) Melatonin and carbohydrate metabolism in plant cells. Plan Theory 10(9):1917. https://doi.org/10.3390/plants10091917
Ayyaz A, Amir M, Umer S, Iqbal M, Bano H, Gul HT, Noor Y, Kanwal A, Khalid A, Javed MS, Athar HUR, Zafar ZU, Farooq M (2020) Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 6(7):e04364. https://doi.org/10.1016/j.heliyon.2020.e04364
Back K (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J 105:376–391
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61:426–437
Debnath B, Islam W, Li M, Sun Y, Lu X, Mitra S, Hussain M, Liu S, Qiu D (2019) Melatonin mediates enhancement of stress tolerance in plants. Int J Mol Sci 20:1040
Dubbels R, Reiter RJ, Klenke E et al (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18(1):28–31
Fan J, Xie Y, Zhang Z, Chen L (2018) Melatonin: a multifunctional factor in plants. Int J Mol Sci 19:1528
Gao B, Chu J, Yu T, Xu Q, Sun X, Yuan J, Xiong G, Wang G, Wang Y, Li J (2015) Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc Natl Acad Sci U S A 112(15):4821–4826. https://doi.org/10.1073/pnas.1503998112
Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Muñoz JL, Reiter RJ, Barriga C, Rodríguez AB (2010) Jerte valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A Biol Sci Med Sci 65:909–914
Hardeland R, Pandi-Perumal SR (2005) Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr Metab 2(1):22. https://doi.org/10.1186/1743-7075-2-22
Hattori H, Migitaka MI et al (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35(3):627–634
Hernández-Ruiz J, Arnao MB (2018) Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 8:33
Hernández-Ruiz J, Cano A, Arnao MB (2004) Melatonin: growth-stimulating compound present in lupin tissues. Planta 220:140–144
Hernández-Ruiz J, Cano A, Arnao MB (2005) Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res 39:137–142
Hernández-Ruiz J, Cano A, Arnao MB (2021) A phytomelatonin-rich extract obtained from selected herbs with application as plant growth regulator. Plan Theory 10(10):2143. https://doi.org/10.3390/plants10102143
Kanwar MK, Yu J, Zhou J (2018) Phytomelatonin: recent advances and future prospects. J Pineal Res 65:e12526
Kolar J (2003) Effects of melatonin on circadian rhythms and Photoperiodism in higher plants. PhD Thesis, Faculty of Natural Sciences, Charles University, Prague, Czechia
Kolar J, Machackova I, Illnerova H, Prinsen E, van Dongen W, van Onckelen H (1995) Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol Rhythm Res 26:406–409
Kolar J, Machackova I, Eder J, Prinsen E, van Dongen W, van Onckelen H, Illnerova H (1997) Melatonin: occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 44:1407–1413
Kolar J, Johnson C, Machackova I (2003) Exogenously applied melatonin affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant 118:605–612
Korkmaz A, Karaca A, Kocacinar F, Cuci Y (2017) The effects of seed treatment with melatonin on germination and emergence performance of pepper seeds. J Agric Sci 23:167–176
Kul R, Esringü A, Dadasoglu E, Sahin U, Turan M, Örs S, Ekinci M, Agar G, Yildirim E (2019) Melatonin: role in increasing plant tolerance in abiotic stress conditions. In: De Oliveira A (ed) Abiotic and biotic stress in plants. London, IntechOpen, pp 1–19
Lei XY, Zhu RY, Zhang GY, Dai YR (2004) Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res 36:126–131
Lerner A, Case JF, Heinzelman RV (1959) Structure of melatonin. J Am Chem Soc 81(22):6084–6085. https://doi.org/10.1021/ja01531a060
Li M, Hasan MK, Li C, Ahammed GJ, Xia X, Shi K, Zhou Y, Reiter RJ, Yu J, Xu M, Zhou J (2016) Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J Pineal Res 61(3):291–302. https://doi.org/10.1111/jpi.12346
Liu J, Zhai R, Liu F, Zhao Y, Wang H, Liu L, Yang C, Wang Z, Ma F, Xu L (2018) Melatonin induces parthenocarpy by regulating genes in Gibberellin pathways of ‘Starkrimson’ Pear (Pyrus communis L.). Front Plant Sci 9:946. https://doi.org/10.3389/fpls.2018.00946
Manchester LC, Coto-Montes A, Boga JA, Andersen LB, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59(4):403–419. https://doi.org/10.1111/jpi.12267
Mannino G, Pernici C, Serio G, Gentile C, Bertea CM (2021) Melatonin and phytomelatonin: chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—an overview. Int J Mol Sci 22(18):9996. https://doi.org/10.3390/ijms22189996
Murch SJ, Saxena PK (2002) Melatonin: a potential regulator of plant growth and development? In Vitro Cell Dev Biol Plant 38:531–536
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L.) plants. Plant Cell Rep 19:698–704
Murch SJ, Campbell SSB, Saxena PK (2001) The role of serotonin and melatonin in plant morphogenesis. Regulation of auxin-induced root organogenesis in in vitro-cultured explants of Hypericum perforatum L. in vitro cell. Dev Biol Plant 37:786–793
Murch SJ, Alan AR, Cao J, Saxena PK (2009) Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res 47:277–283
Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009) Phytomelatonin: a review. J Exp Bot 60(1):57–69. https://doi.org/10.1093/jxb/ern284
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45:24–31
Posmyk MM, Balabusta M, Wieczorek M, Sliwinska E, Janas KM (2009) Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J Pineal Res 46:214–223
Posmyk MM, Janas KM (2009) Melatonin in plants. Acta Physiol Plant 31(1):1–11. https://doi.org/10.1007/s11738-008-0213-z
Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol 54(1):1–9
Sharif R, Xie C, Zhang H, Arnao MB, Ali M, Ali Q, Muhammad I, Shalmani A, Nawaz M, Chen P et al (2018) Melatonin and its effects on plant systems. Molecules 23:2352
Sharkey TD (2021) Pentose phosphate pathway reactions in photosynthesizing cells. Cell 10(6):1547. https://doi.org/10.3390/cells10061547
Sharma A, Zheng B (2019) Melatonin mediated regulation of drought stress: physiological and molecular aspects. Plan Theory 8(7):190
Sharma A, Wang J, Xu D, Tao S, Chong S, Yan D, Zheng B et al (2020) Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci Total Environ 713:136675
Shi H, Wei Y, He C (2016) Melatonin-induced CBF/DREB1s are essential for diurnal change of disease resistance and CCA1 expression in Arabidopsis. Plant Physiol Biochem 100:150–155. https://doi.org/10.1016/j.plaphy.2016.01.018
Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2012) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot 63(2):577–597. https://doi.org/10.1093/jxb/err256
van Tassel D, O'Neill S (1993) Melatonin: identification of a potential dark signal in plants. Plant Physiol 102(supplement 1):659
Wang P, Yin L, Liang D, Li C, Ma F, Yue Z (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res 53:11–20
Wang Y, Reiter RJ, Chan Z (2018) Phytomelatonin: a universal abiotic stress regulator. J Exp Bot 69:963–974
Wei J, Li D, Zhang J, Shan C, Rengel Z, Song Z, Chen Q (2018) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J Pineal Res 65:e12500
Wolf K, Kolar J, Witters E, van Dongen W, van Onckelen H, Machackova I (2001) Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J Plant Physiol 158:1491–1493
Xu T, Chen Y, Kang H (2019) Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01388
Yu Y, Lv Y, Shi Y, Li T, Chen Y, Zhao D, Zhao Z (2018) The role of phyto-melatonin and related metabolites in response to stress. Molecules 23:1887
Zeng H, Bai Y, Wei Y, Reiter RJ, Shi H (2022a) Phytomelatonin as a central molecule in plant disease resistance. J Exp Bot 73(17):5874–5885. https://doi.org/10.1093/jxb/erac111
Zeng H, Liu M, Wang X, Liu L, Wu H, Chen X, Wang H, Shen Q, Chen G, Wang Y (2022b) Seed-soaking with melatonin for the improvement of seed germination, seedling growth, and the antioxidant defense system under flooding stress. Agronomy 12(8):1918. https://doi.org/10.3390/agronomy12081918
Zhan H, Nie X, Zhang T, Li S, Wang X, Du X, Tong W, Weining S (2019) Melatonin: a small molecule but important for salt stress tolerance in plants. Int J Mol Sci 20:709
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54(1):15–23. https://doi.org/10.1111/j.1600-079X.2012.01015
Zhang H, Zhang N, Yang R, Wang L, Sun Q, Li D, Cao Y, Weeda S, Zhao B, Ren S, Guo Y (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57(3):269–279. https://doi.org/10.1111/jpi.12167
Zhao D, Wang H, Chen S, Yu D, Reiter RJ (2021a) Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci 26(1):70–82. https://doi.org/10.1016/j.tplants.2020.08.009
Zhao D, Yao Z, Zhang J, Zhang R, Mou Z, Zhang X, Li Z, Feng X, Chen S, Reiter RJ (2021b) Melatonin synthesis genes N-acetylserotonin methyltransferases evolved into caffeic acid O-methyltransferases and both assisted in plant terrestrialization. J Pineal Res 71:e12737
Acknowledgments
This work is supported by MK Bhan young researcher fellowship, Department of Biotechnology, New Delhi, India.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Meenu, M. et al. (2024). Phytomelatonin: History, Biosynthesis, and Functions. In: Sharma, A., Ahammed, G.J. (eds) Melatonin in Plants: Role in Plant Growth, Development, and Stress Response. Plant Life and Environment Dynamics. Springer, Singapore. https://doi.org/10.1007/978-981-99-8051-2_1
Download citation
DOI: https://doi.org/10.1007/978-981-99-8051-2_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8050-5
Online ISBN: 978-981-99-8051-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)