Abstract
The current research investigates the utilization of laterite soil to adsorb the combined metals of Zn and Cu in batch mode. Laboratory experiments were conducted to analyze the adsorption isotherms at a fixed pH of 8, with an initial concentration of 10 mg/L, different adsorption doses ranging from 1 to 30 g/L, and an equilibrium contact time of 120 min. The isotherms were plotted, and the findings indicate that the Freundlich isotherm model is the most suitable fit. For Zn@20 g/L soil dose, the R2 value is 0.9906, and the RMSE is 0.054. Similarly, for Cu@20 g/L soil dose, the R2 value is 0.9912, and the RMSE is 0.053.The soil’s ability to adsorb Cu and Zn is demonstrated by its high adsorption capacity values (qZn = 1845 mg/kg, qCu = 1796.8 mg/Kg), indicating that it has excellent attenuative potential. To prove the heavy metal adsorption onto the soil sample and ascertain its mechanism, the natural soil sample and its heavy metal-loaded forms were characterized using scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Pollution due to anthropogenic activities, particularly due to untreated or ill-treated effluents when released to the lithosphere, creates nuisance to the soil and water. Pollution due to heavy metals is a major concern for the environment as they are toxic and non-biodegradable and have a high potential to pollute surface as well as underground water, making it unfit for plants and humans. The effluent containing Cu and Zn is normally discharged from mining, tanneries, metal plating, fertilizer industries [1, 2], galvanized industry, dyeing industries, paper industries [3], zinc processing units, etc. The main focus of this study is on Zn and Cu due to their high mobility and toxicity. Copper is more toxic for plants than animals and less impacted by other heavy metals [4].The mobility of Zn is significantly high in aqueous media, and it tends to be closely associated with the oxide and carbon-bound fractions [5]. Due to their toxicity, carcinogenic nature, persistence, and propensity to bioaccumulate [6], severe restrictions (such as those set forth by the WHO, US EPA, or EU) regarding the quality of wastewaters released have been developed. For instance, the WHO recommends that Cu and Zn concentrations in mining and electroplating wastewater be less than 2.0 and 5.0 mg/L, respectively, while the US EPA has established a limit of 1.3 and 1.7 mg/L, respectively. The presence of these substances in industrial wastewater makes it more harmful for the receiving habitats, which upsets the equilibrium of the aquatic fauna and flora and has a severe effect on human health [7]. To ensure the protection of surface water quality, it is necessary to remove or reduce heavy metals before they are released into aquatic habitats. Various methods can be used to treat wastewater, including solvent extraction [8, 9], ion exchange resins [10], complexation [11], membrane approaches [12], precipitation [13], and adsorption [13,15,16]. Among these methods, adsorption is commonly used due to its effectiveness and ease of use in removing heavy metals from liquid effluents. Adsorbents can be of mineral or organic origin, such as natural zeolite [17], calcium silicate powders [16], activated carbon, and bio-sorbents [14, 15, 18]. In this study, the adsorption process was chosen due to its low cost and simplicity. The study aims to remove heavy metals using a low-cost adsorbent, laterite soil, which is readily available in Durgapur.
2 Materials and Methods
2.1 Chemicals
The chemicals used were zinc sulfate (ZnSO4) and copper sulfate (CuSO4) for the preparation of stock solution. To prepare the stock solution of heavy metal ions, 1 g ZnSO4 and CuSO4 was dissolved in a 1 L volumetric flask filled with distilled water, resulting in a concentration of 1000 mg/L, which was then diluted to required concentration for working solution. For maintaining pH level, NaOH and HCl were used. All chemicals used were of analytical grade and were procured from Merck in India.
2.2 Instruments
A high-precision electrical balance (Sartorius GMBH) was used for weighing. Digital pH meter (DHP-500, SICO, India) was used for pH measurements. BOD shaker (Instrumentation, India) was used for all batch experiments. Atomic absorption spectrometer (PerkinElmer, PinAAcle 900 T) was used to measure the absorbance of zinc and copper solution.
2.3 Preparation and Characterization of the Adsorbent
The laterite soil sample was collected from a gravel quarry surroundings of Durgapur, West Bengal, and separated from different lumps of kankar. Then it was grounded and sieved. Finally it was kept in the oven for 24 hours at a temperature of 100 ± 2 °C. After drying properly it was kept in a desiccator before use. The BIS codes were followed to conduct tests on the physicochemical characteristics of the soil specimen. The physicochemical properties of the soil sample are listed in Table 1. Both natural and heavy metal-loaded soil samples were characterized as a confirmatory test using soil digestion, scanning electron microscopic energy-dispersive X-Ray (SEM-EDX), and instrumental analysis of Fourier transform infrared (FTIR). Soil digestion was done with the reference of US EPA (3050B) to determine the amount of naturally available heavy metals in the soil sample using flame atomic adsorption spectrophotometer (PerkinElmer). SEM images of soil sample with or without heavy metal loaded were taken using scanning electron microscope (ZEISS, SIGMA HD, and Germany) to determine the structural characteristics of the adsorbent. Scanning electron microscope (SEM) was employed to analyze the high-resolution surface morphology of the soil both before and after the adsorption of Zn+2 and Cu+2 ions. Fourier transform infrared (FTIR) spectroscopy (IRPrestige-21, SHIMADZU, Japan) was used to examine the functional groups of both the original adsorbent and the adsorbent that had absorbed heavy metals. The spectra data over 400–4000 cm−1 were studied.
2.4 Experimental Studies
To investigate the metal mobilization from soil, batch experiments were conducted at constant room temperature (32 ± 2 °C) and shaking speed of 150 rpm. The effects of various process parameters (effect of adsorbent dose, contact time, pH) were studied. The initial concentration of each heavy metal was selected (10 mg/L) for the entire batch test and pH adjusted from 4 to 12 by 1 N HCl and 1 N NaOH. The adsorbent dose was varied from 1 to 30 g/L for the batch study. The experiment was conducted in a BOD shaker at constant room temperature at 150 rpm. Following every test, the filtered substance was examined through AAS. The stock solution was also confirmed by AAS. The maximum adsorption of the soil was determined by Eq. (1).
C0 is the initial concentration of the heavy metal solution (mg/L), and Ce is the equilibrium concentration of the heavy metals in solution (mg/L). Isotherm studies were carried out to find out the adsorption capacity of soil sample.
3 Results and Discussion
3.1 Characterization Result of the Adsorbent
3.1.1 SEM Result
The SEM-EDX technique was employed to analyze the structural characteristics of the raw soil and soil after adsorption. The microstructure of soil formation and the state of individual particles were accurately determined by SEM-EDX. The SEM images in Fig. 1 depict the uneven and porous surface of the untreated adsorbent, while Fig. 2 displays a clear distinction in the surface features of the soil after adsorption. The SEM images in Fig. 2 were captured using a magnification of 26.79 KX and a scale bar of 5 μm and show the soil samples containing Zn+2 and Cu+2. The addition of Zn and Cu led to particle agglomeration and smoothening effect. In contrast, the SEM images of the raw soil mineral in Fig. 1 reveal several small discrete particles scattered across a rough surface. Figure 2 shows that the particle size slightly increased and became smoother after adsorption, which could be attributed to the introduction of Zn and Cu.
3.1.2 FTIR Result
To investigate the functional groups present in both the untreated soil and the soil treated with heavy metals, Fourier transform infrared (FTIR) analysis was conducted over a range of wavelengths from 400 to 4000 cm−1. The results showed that certain peaks had shifted in wave number after the adsorption process, suggesting that these functional groups were involved in binding heavy metals to the soil. The surface functional groups of the untreated and treated soils are depicted in Figs. 3 and 4, respectively. The Y-axis represents % transmittance, while the X-axis represents wave number (cm−1).
In the virgin soil, peaks at wave numbers of 485.1, 789.1, 930.5, 1026.5, 1620.2, 2943.4, and 3610.5 cm−1 representing C-I (stretch), C-Cl (stretch), =C-H (bending), C-F (stretch), C=C (stretch), C-H (stretch), and O-H (stretch, free), respectively, are found. After being loaded with heavy metals, one of the bonds disappears and appears, and the initial wave numbers are shifts from their original position. The wave number of C-I (stretch) shifts from 485.1 to 479.6 cm−1 as evident from Fig. 3 to Fig. 4. Based on Fig. 4, a strong peak at 1886.5 cm−1 and 670.5 cm−1 shows the presence of C-O (stretch, carbonyl) and C-Cl (stretch, alkyl halide), respectively, indicating the mentioned group appears new into the adsorbed soil and in the same time 2943.4 cm−1 indicating C-H (stretch) disappears, and the other peaks are found to shift from IR spectra of soil loaded with Zn+2 and Cu+2. Soil is an organic material, and it is likely to contain chloride in the natural form. In the heavy metal-loaded sample, the peaks which were in the virgin soil shifted from its original position and changed to 479.6, 670.5, 787, 928.9, 1014.6, 1627.7, 1886.5, and 3637.7 cm−1 indicating C-I (stretch), =C-H (bending), C-Cl (stretch), C-H (stretch), C-F (stretch), C=C (stretch), C=O (stretch), and O-H (stretch, free). It seems that these functional groups have a significant impact on the adsorption of heavy metals, particularly zinc and copper. The spectra undergo an evident alteration in both shape and position as a result of the adsorption of Zn and Cu, causing a shift in the peaks of the original sample. All these changes of peak positions with the appearance of C=O (stretch) and C-Cl (stretch) bonds and disappearance of C-H (stretch) bonds are surely a conclusive evidence of adsorption into the soil due to heavy metals.
3.1.3 EDX Result
In contrast, the EDX assessments were carried out in a high-vacuum environment, utilizing a 15 Kev accelerating voltage. This method revealed the X-ray spectrum characteristic of several elements including carbon (C), oxygen (O), magnesium (Mg), aluminum (Al), potassium (K), silicon (Si), calcium (Ca), phosphorous (P), sulfur (S), and iron (Fe). An analysis was conducted on the proportion of all the components. The elemental analysis and mapping are illustrated in Figs. 5 and 6, respectively, for raw as well as adsorbed soil sample.
Based on Table 2, C, O, Si, and Al have a high content, and for the same sample Fe, Ti, K, and Mg are having a lesser percentage. The major components as determined by EDX are C, O, Si, and Al, with percentage compositions of 6.55%, 58.13%, 18.51%, and 9.82%, respectively. There was also the presence of heavy metals in the virgin soil but in a lesser extent.
The EDX study of heavy metal-loaded soil with Zn and Cu is also tabulated in Table 3, which shows that soil with heavy metals also contains a higher percentage of oxygen, carbon, silicon, and aluminum and has a reasonable percentage of adsorbed heavy metals. The Zn and Cu peaks could be seen in the spectra of the Zn- and Cu-loaded soil and the unloaded soil from Fig. 5. It implies that the process of adsorption has effectively attached heavy metals onto the surface of soil particles.
3.2 Effect of Contact Time
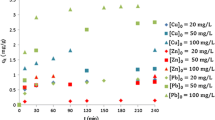
To identify the removal efficiency of the adsorbent accurately, it is important to consider sufficient contact time. Consideration of optimum time for the best removal efficiency is done by a time-dependent batch test. It has been observed that the removal efficiency increases gradually up to 120 min and then it becomes constant for both Zn and Cu (initial concentration of Cu & Zn = 10 mg/L, dose = 20 g/L) as shown in Fig. 7. The reason behind this is that at the beginning, there were no adsorbate molecules occupying the adsorbent sites, resulting in a rise in the concentration gradient between the adsorbate present in the solution and the adsorbent surface. Hence, during a contact time of 2 hours, the adsorbent is effective to remove the most.
3.3 Effect of Soil Dose
In Fig. 8, the quantity of heavy metal removal from the synthetic solution is displayed as a function of adsorption dosage. The adsorbent dose varies from 1, 2, 3, 5, 7.5, 10, 12, 15, 20, 25 to 30 g/L for initial concentrations of 10 mg/L. The heavy metal removal efficiencies of lateritic soil have been seen to increase with an increase in adsorption dose from 1 to 20 g/L, and then it decreases in 25 g/L as shown in Fig. 8, for both Zn and Cu. The increase in percentage removal with the increase in adsorbent dose is attributed to the increase in adsorbent sites available for the same amount of heavy metal cations.
3.4 Effect of pH
Figure 9 shows the variation in removal of heavy metals, and it is found that the removal efficiency can increase up to 90% with the pH varying from 4 to 12, and then it becomes constant for both the heavy metals (Zn and Cu). As the pHzpc of soil was found to be 7.2 and it is well known that below pHzpc (pH < pHzpc) the adsorbents are positively charged, the repulsive force between the metal cations and adsorbent surface becomes dominant resulting in reduced adsorption of the metal cations. On the other hand, at a higher pH (pH > pHzpc), the surface charges become negative, and they can effectively adsorb both Zn and Cu cations [19].
3.5 Isotherm Studies
The investigation of adsorption isotherm is beneficial in evaluating the capability of soil to adsorb heavy metals from wastewater. Through batch adsorption experiments, the manner in which the adsorbate attaches to the adsorbent was determined. A graph displaying the correlation between the quantity of heavy metal adsorbed per unit mass of soil and the equilibrium concentration of heavy metals was created. Typically, the Langmuir (Fig. 10) and Freundlich isotherm (Fig. 11) models are employed to describe the adsorption isotherm. Table 4 provides a summary of the R2 and RMSE values, as well as the coefficients of the models.
Table 4 shows that the test results were reasonably suited by the Freundlich and Langmuir isotherm models, which had greater coefficients of determination and smaller error values. Moreover, multiple binding and heterogeneity for heavy metal adsorption in soil media are indicated by the Freundlich isotherm model which was close fitting to the experimental batch adsorption results [5]. For the Freundlich isotherm studies, it shows higher R2 and lower RMSE values, and also the values of Kf and “n” signify the favorable adsorption capacity of the soil. In the present investigation, “n” values of 0.59 and 0.55 for Zn and Cu both clearly indicate a favorable adsorption process for heavy metals in soil. Due to higher R2 values and better RMSE values than Langmuir isotherm, we have taken Freundlich isotherm as satisfactory.
4 Conclusion
The study aimed to investigate the capacity of lateritic soil to eliminate two types of heavy metals (Zn+2 and Cu+2) from an aqueous solution. According to the batch adsorption study, the most effective removal of heavy metals occurred when the pH was set to 8, the soil dose was 20 g/L, and the contact time was 120 min. When the aforementioned optimal conditions are met, the lateritic soil exhibits the ability to eliminate over 90% of both Zn and Cu. It was observed that the laterite soil had a higher tendency to absorb Cu+2 compared to Zn+2. The results of the adsorption isotherm studies indicated that there was a good match between the Langmuir and Freundlich isotherm models. The test results revealed that Freundlich model is best fit with R2 = 0.9906 and RMSE = 0.054 for Zn and R2 = 0.9912 and RMSE = 0.053 for Cu.
Abbreviations
- AAS:
-
Atomic adsorption spectrophotometer
- BOD:
-
Biochemical oxygen demand
- EDX:
-
Energy-dispersive X-ray spectroscopy
- EU:
-
European Union
- FTIR:
-
Fourier transform infrared
- RMSE:
-
Root mean square error
- SEM:
-
Scanning electron microscope
- SEM-EDX:
-
Scanning electron microscopic energy-dispersive X-ray
- US EPA:
-
United States Environmental Protection Agency
- WHO:
-
World Health Organization
References
A. Alcolea, M. Vázquez, A. Caparrós, I. Ibarra, C. García, R. Linares, R. Rodríguez, Heavy metal removal of intermittent acid mine drainage with an open limestone channel. Miner. Eng. 26, 86–98 (2012)
H. Davies, P. Weber, P. Lindsay, D. Craw, J. Pope, Characterisation of acid mine drainage in a high rainfall mountain environment, New Zealand. Sci. Total Environ. 409, 2971–2980 (2011)
T.G. Chuah, A. Jumasiah, I. Azni, S. Katayon, S.Y. Thomas Choong, Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: an overview. Desalt 175, 305–316 (2005)
C.W. Daniel, C.L.O. Tsangandirenem, Competitive Cu and Cd sorption and transport in soils: a combined batch kinetics, column, and sequential extraction study. Environ. Sci. Technol. 40, 6655–6661 (2006)
W.S. Wan Ngah, M.A.K.M. Hanafiah, Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour. Technol. 99, 3935–3948 (2008)
M. Syrynskyy, Solid–liquid–solid extraction of heavy metals (Cr, Cu, Cd, Ni and Pb) in aqueous systems of zeolite–sewage sludge. J. Hazard. Mater. 161, 1377–1383 (2009)
R. Leyma, S. Platzer, F. Jirsa, W. Kandioller, R. Krachler, B.K. Keppler, Novel thiosalicylate-based ionic liquids for heavy metal extraction. J. Hazard. Mater. 314, 164 (2016)
D. Kołodyńska, J. Krukowska-Bąk, J. Kazmierczak-Razna, R. Pietrzak, Uptake of heavy metal ions from aqueous solutions by sorbents obtained from the spent ion exchange resins. Microporous Mesoporous Mater. 78, 102–114 (2017)
C. Zhang, X. Liu, X. Lu, M. He, Complexation of heavy metal cations on clay edges at elevated temperatures. Chem. Geol. Microporous Mesoporous Mater. 49, 852–860 (2017)
K. Sunil, G. Karunakaran, S. Yadav, M. Padaki, V. Zadorozhnyy, R.K. Pai, Al-Ti2O6 a mixed metal oxide based composite membrane: a unique membrane for removal of heavy metals. Chem. Geol. 47, 410–418 (2018)
P. Yuan-Zhen, H. Yong-Ming, Y. Dong-Xing, L. Yan, G. Zhen-Bin, Rapid analysis of heavy metals in coastal seawater using preconcentration with precipitation/co-precipitation on membrane and detection with X-Ray fluorescence. Chin. J. Anal. Chem. 40, 877–882 (2012)
Y. Huanga, X. Zenga, L. Guoa, J. Lanc, L. Zhanga, D. Cao, Heavy metal ion removal of wastewater by zeolite-imidazolate framework. Sep. Purif. Technol. 194, 462–469 (2018)
A.E. Burakov, E.V. Galunin, I.V. Burakova, A.E. Kucherova, S. Agarwal, A.G. Tkachev, V.K. Gupta, Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol. Environ. Saf. 148, 702–712 (2018)
H. Es-sahbany, M. Berradi, M. Belfaquir, H. Nassali, M.S. Elyoubi, Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. ICMES 10, 421–431 (2018)
J. Ma, G. Qin, Y. Zhang, J. Sun, S. Wang, L. Jiang, Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 14, 547–555 (2018)
L. Mao, H. Guo, W. Zhang, Addition of waste glass for improving the immobilization of heavy metals during the use of electroplating sludge in the production of clay bricks. Constr. Build. Mater. 163, 875–879 (2018)
K. Hanna, L. Lassabatere, B. Bechetn, Zinc and lead transfer in a contaminated roadside soil: Experimental study and modeling. J. Hazard. Mater. 161, 1499–1505 (2009)
A.Z. Al-Hamdan, K.R. Reddy, Surface speciation modeling of heavy metals in Kaolin: implications for electro kinetic soil remediation processes. Adsorption 11, 529–546 (2005)
I. Vázquez, J.R. Iglesias, E. Marañón, L. Castrillón, M. Alvaeez, Removal of residual phenols from coke wastewater by adsorption. J. Hazard. Mater. 147, 395–400 (2007)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Shome, S., Mondal, B., Das, S. (2023). Removal of Heavy Metals by Laterite Soil. In: Mazumder, D. (eds) Sustainable Advanced Technologies for Industrial Pollution Control. ATIPC 2022. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-37596-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-37596-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37595-8
Online ISBN: 978-3-031-37596-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)