Abstract
In the present study, the feasibility of soil used as a low-cost adsorbent for the removal of Cu2+, Zn2+, and Pb2+ ions from aqueous solution was investigated. The kinetics for adsorption of the heavy metal ions from aqueous solution by soil was examined under batch mode. The influence of the contact time and initial concentration for the adsorption process at pH of 4.5, under a constant room temperature of 25 ± 1 °C were studied. The adsorption capacity of the three heavy metal ions from aqueous solution was decreased in order of Pb2+ > Cu2+ > Zn2+. The soil was characterized by Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopic-energy dispersive X-ray (SEM-EDX), and Brunauer, Emmett, and Teller (BET) surface area analyzer. From the FTIR analysis, the experimental data was corresponded to the peak changes of the spectra obtained before and after adsorption process. Studies on SEM-EDX showed distinct adsorption of the heavy metal ions and the mineral composition in the study areas were determined to be silica (SiO2), alumina (Al2O3), and iron(III) oxide (FeO3). A distinct decrease of the specific surface area and total pore volumes of the soil after adsorption was found from the BET analysis. The experimental results obtained were analyzed using four adsorption kinetic models, namely pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion. Evaluating the linear correlation coefficients, the kinetic studies showed that pseudo-second-order equation described the data appropriable than others. It was concluded that soil can be used as an effective adsorbent for removing Cu2+, Zn2+, and Pb2+ ions from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The problems of the ecosystem are growing with developing technology and heavy metal pollution is one of the main problematic issues (Uzun and Güzel 2000). The widespread existence of heavy metal in water environment either from natural or anthropogenic sources, has deposited considerable public and political discussion relating to potential impacts on human health and wildlife as well as aquatic habitats (Orisakwe et al. 2012).
Prolonged exposure to heavy metals such as cadmium, copper, lead, nickel, and also zinc can cause deleterious health effects in humans (Singh et al. 2011). Significant worldwide research efforts are stimulated toward developing methods for heavy metal removal from drinking water due to the heightened awareness of the toxicity and regulatory modification (Tilson 2013).
Each of the current technologies has its own merits and demerits besides playing an important role in preventing widespread heavy metal toxicity in nature. Adsorption has been widely used for purification, separation, and compliance purposes in many industries at large scales (Shah et al. 2013). The fact that adsorption can be used with liquid process stream with high efficiency, low cost, and friendly operating conditions have made it a usual process in various industries is undeniable. Activated carbons (ACs), polymeric adsorbents, and zeolites are the main categories of adsorbents used to adsorb long range of adsorbates with varying concentrations (Shah et al. 2013).
Properties that make an adsorbent effective in removing certain species from water are high specific surface area and with an affinity for a desired sorbate (Payne and Abdel-Fattah 2005). Consideration on using soil as viable process for heavy metal removal is discussed in this study. The feasibility of soil component, kaolinite used as a low-cost adsorbent for the removal of heavy metal ions from aqueous solution was studied (Shahmohammadi-Kalalagh et al. 2011).
In order to make sound decisions regarding heavy metal removal from water environments by using soil, it is necessary to have a thorough understanding of the adsorption mechanisms of heavy metal adsorption over extended periods to comprehend the migration of heavy metals in the soil matrices. The distribution of chemicals in soils is governed by chemicals and soil properties (Dube et al. 2000).
Therefore, knowledge of heavy metal ion adsorption is essential for estimating the fate and behavior of heavy metal in the soil environments. It is vital to fully understand the metal-binding properties of the soil, and develop and validate procedures for metal speciation in soils before deciding to employ appropriate models for the evaluation of adsorption kinetic data. Various parameters of the adsorption process, the mechanism for heavy metals removal along with the kinetics of adsorption with the modeling aspects, are discussed.
Accordingly, the main aim of this work was to conduct kinetic studies at different initial concentrations at constant pH. Confirmatory tests using scanning electron microscopic-energy dispersive X-ray (SEM-EDX) and instrumental analyses of Fourier transform infrared (FTIR) and Brunauer, Emmett, and Teller (BET) surface area to study the properties, for example, mineral structure of soil were performed. The kinetic adsorption data can be processed to understand the dynamics of the adsorption reaction in terms of the order of the rate constant. Furthermore, the prediction of adsorption rate is essential in providing important information modeling the processes.
Materials and methods
Preparation of adsorbent
The soil samples were collected around the waterfall area in Kampung Assum. The village was located in the remote area of Padawan, approximately 80 km from the city center, Kuching. The site was included in one of the proposed development projects by the Ministry of Infrastructure Development and Communications. Apart from that, the sampling point was in areas away from industrial site, without apparent anthropogenic influence.
Few soil analysis tests were carried out for soil data collection. Surface treatment was conducted by flushing the soil samples using distilled water before carrying on the tests. Soil testing in this study was comprised of three steps: collection of a representative soil sample; laboratory analyses of the soil sample; and interpretation of analytical results.
Firstly, the soil samples were spread out in a clean, warm, dry area, and dried for 3 days. The air-dried soils were then broken into smaller sizes and ground to pass through 1-mm stainless steel sieve. The sieving was done to ensure that the soil was of uniform grade throughout the experiment. Next, the soil samples were stored in reagent bottles for subsequent analysis and experiments.
Chemicals
All chemicals used were of analytical reagent grade. The adsorbates, copper(II) sulfate (CuSO4), zinc sulfate (ZnSO4), and lead(II) nitrate (Pb(NO3)2) are purchased from Sigma-Aldrich Co. LLC., Malaysia. The stock solutions of 1000 mg/L of the heavy metal ions are prepared daily by dissolving a certain amount of CuSO4, ZnSO4, and Pb(NO3)2 in 1-L volumetric flask with deionized water and diluted to the mark. All working solutions are prepared by diluting the stock solution with deionized water to the desired concentration.
Adsorption kinetic studies
Kinetic batch experiments were conducted to determine adsorption isotherms for heavy metal ion to the soil samples at constant room temperature of 25 ± 1 °C under aerobic conditions. To study the kinetics of heavy metal adsorption on soil, initial heavy metal concentrations were selected to meet the following criteria: (i) the desired reaction pH should be within the range of 4 to 8, which is a common range for natural soils, to promote heavy metal ion as the predominant species of heavy metal in solution and (ii) the concentration of heavy metal in solution must be high enough to be in the detection range for analysis via AAS. With reference to the soil profile and groundwater contamination, a low pH for solution was chosen.
Three initial concentrations, C 0 (20, 50, and 100 mg/L) of each heavy metals, Cu2+, Zn2+, and Pb2+, were prepared using CuSO4, ZnSO4, and Pb(NO3)2, respectively. The batch experiments were initiated by mixing 0.5 g of air-dried soil with 50 mL of desired heavy metal solution in several 250-mL conical flasks. The experiments were conducted at initial pH 4.5 which is adjustable with 1 M hydrochloric acid (HCl) or 1 M sodium hydroxide (NaOH) solution. These acid or base additions changed the total volumes by <2 %. The final solid-solution ratio was 10 g/L.
The mixtures were shaken at 150 rpm in a refrigerated incubating shaker (Thermo Scientific 491, Japan) to prevent any possible sedimentation and were sampled at definite time intervals from the start of the experiment. After each certain time interval, the mixture was filtered using Whatman grade 1, cellulose filter paper, and the filtrate was analyzed for remaining metal ion concentration using atomic absorption spectrometry (AAS). Stock solution concentrations were also confirmed via the AAS. The tests were performed in triplicate for each input concentration C 0 in order to better understand the system.
The average amount of heavy metal adsorbed was analyzed. The amount of metal adsorbed by each soil was calculated by the mass balance equation of heavy metal ions before and after the adsorption as expressed by Eq. (1).
where q is the amount of adsorbed species (mg/g), C 0 is the initial concentration of the species in solution (mg/L), C e is the equilibrium concentration of the species in solution (mg/L), V is the solution volume (L), and M is the weight of air-dried soil (g).
Results and discussion
Effect of contact time
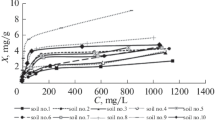
The contact time is an important parameter to identify the possible rapidness of binding and removal processes of the metal ions by the adsorbent and also to obtain the optimum time for complete removal of the target metal ions. The time profiles of heavy metal ions adsorption were plotted as shown in Fig. 1.
It can be seen that the adsorbed concentrations were zero at the beginning. Within a short time period, the amount of adsorbed species was varied considerably and then ended up with a very gradual and quite narrow variation range. There was an increase in adsorption with increase in contact time and maximum adsorption takes place at 120, 30, and 150 min contact time for Cu2+, Zn2+, and Pb2+, respectively. The equilibrium was reached after the contact period mentioned.
This may be due to the fact that initially, all adsorbent sites were vacant and thus leading to increase in concentration gradient between adsorbate in solution and the adsorbent surface. As time proceeds, the heavy metal ions uptake rate by adsorbent was decreased significantly due to the decrease in number of adsorption sites as well as ions concentration.
Moreover, in most agitation periods, Pb2+ ion adsorption rate was higher than that of Cu2+ and Zn2+ ions, demonstrating higher capability of Pb2+ ion in competition with Cu2+ and Zn2+ ions for the occupation of adsorption sites in the studied soils.
Effect of initial concentration
The rate of adsorption is a function of the initial concentration of the adsorbate, which makes it an important factor to be considered for effective adsorption. The time of reaching equilibrium also depends on the initial concentration of the metal. The lower was the initial concentration, the shorter the equilibrium time interval.
It was also shown that increases in initial metal ions concentration increased the amount of metal ions uptake per unit weight of soil (mg/g), as was expected. However, the adsorptivity was decreased with the increasing of initial heavy metal ion concentration. This was due to the fact that as concentration was increased, more ions were available for adsorption on surfaces; hence, retention becomes more difficult as competition from other ions was decreased for adsorption on the same sites. The removal capacity of heavy metal ions decreased in the order as follows: Pb2+ > Cu2+ > Zn2+.
The fast adsorption rate at the initial stage may be explained by an increased availability in the number of active binding sites on the adsorbent surface. The adsorption was rapidly occurred and normally controlled by the diffusion process from the bulk to the surface.
With the lapse of time, the surface adsorption sites were exhausted. The remaining vacant sites were difficult to be occupied by the cations due to repulsive forces between adsorbate present in solid and bulk phases. The adsorption was likely an attachment-controlled process due to less available adsorption sites. Similar results have been reported in literature (Das et al. 2013).
In addition, the adsorptivity of Pb2+ was higher compared to Cu2+ and Zn2+ significantly. This may be due to the phenomenon that phosphate did not form strong complexes with Cu2+ and Zn2+, but it was strongly adsorbed to soil surfaces, thus, physically suppressing the adsorption of Cu2+ and Zn2+ by blocking the specific adsorption sites of Cu2+ and Zn2+ (McLean and Bledsoe 1992).
Characterization of adsorbent
FTIR spectroscopy
The functional groups of the virgin adsorbent and adsorbents loaded with heavy metals were analyzed by Fourier transform infrared (FTIR) spectroscopy (Shimadzu IRAffinity-1, Japan). The spectra data are collected in the IR wavelength of 400 to 4000 cm−1. After the adsorption, some peaks are shifted in terms of wavenumber denoting that these functional groups are involved or participated in the binding of heavy metals on the soil. The surface functional group of virgin soil and soils loaded with heavy metals are shown in Fig. 2.
In the spectra of virgin soil, a peak at 3608.81 cm−1 is present in the hydroxyl stretching region at 3700–3200 cm−1. This is likely due to the presence of H2O in the soil minerals. In fact, heavy metals are adsorbed by a variety of soil phases with hydroxyl groups on their surfaces and edges, where adsorption reactions are often more rapid (Fonseca et al. 2009). The oxygen on each hydroxyl group may act as a strong Lewis base due to the presence of its vacant doublet electrons that enable a complex coordination with the electron-deficient metal ions (Paliwal 2006). The peak corresponds to the hydroxyl-based group is altered after adsorption, indicating the involvement in binding of the metal and adsorbent.
Based on Fig. 2a, a strong peak at 1508.33 cm−1 shows the presence of carbonate group and the peak is found to shift from IR spectra of soil loaded with Pb2+. A strong band carbonyl group is observed in the region 1850–1640 cm−1 where the peak at 1695.43 cm−1 is assigned to the appearance of C = O. Peak abundance at 684.73 and 781.17 cm−1 has been used to establish the relative amount of carboxyl relative to aromatic structures. Besides, there is a large peak on the multi spectra around 2300 cm−1 that corresponds to the asymmetrical stretch of carbon dioxide (CO2), indicating CO2 in atmosphere is dissolved into the sample.
Soil minerals are represented by silicate, SiO3 2− and orthosilicate, SiO4 4− stretch at 499.56 cm−1 and 1026.13 cm−1. These functional groups appear to play an important role in the adsorption process of all three heavy metals studied, especially of Pb2+, since these bands tend to overlap for the contaminated samples (Fonseca et al. 2009). There is an obvious change on the shape of the spectra where the peaks at 1022.27, 1028.06, and 1018.41 cm−1 are observed after adsorption of Cu2+, Zn2+, and Pb2+ ions, respectively. Moreover, a slight change of peak at 497.63 cm−1 is noticed from Fig. 2c.
In addition, the band at 990–1050 cm−1, present in the virgin soil sample, can be assigned to the stretching vibrations of Si(Al)-O. This band is sensitive to the content of structural Si and Al (Shin et al. 2014). The analysis has proved that the Si(Al)-O bond is involved in heavy metal adsorption.
Scanning electron microscopic-energy dispersive X-ray
Determination of the structural characteristics of the adsorbent, soil was done by means of a JEOL JSM-6390LA scanning electron microscope (SEM) equipped with an energy dispersive X-ray (EDX) analyzer. The high resolution surface morphology of soil before and after Cu2+, Zn2+, and Pb2+ ions adsorption were characterized by SEM. On the other hand, the elemental compositions of soil were determined by randomly selecting areas on the solid surfaces and analyzing by EDX. SEM-EDX system was employed to quantify and map elemental spatial distributions on the surfaces of soil (Shin et al. 2014).
SEM-EDX determined the microstructure of soil formation and particle intact condition well in all soil samples. The soil particles in spherical shape (Fig. 3a) were shown in the SEM micrograph. Soil structure is resulted from the binding together of soil particles into aggregates or clumps of vary sizes and shapes (Yadav and Tyagi 2006). The aggregation of small spherical particle allowed maximum space for air and water movement. This was proved by the micrograph of soil obtained from SEM, representing a large surface area for heavy metal ions adsorption. The porous and irregular surface structure of the adsorbent can be clearly observed in the SEM images displayed in Fig. 3a, b.
There was clear demarcation in the surface morphology of soil after adsorption as can be observed from Fig. 3c–h. As illustrated in Fig. 3d, f, h, SEM images of soil loaded with Cu2+, Zn2+, and Pb2+ were taken at a magnification capacity of ×5000 and a scale bar of 5 μm. It can be seen that agglomeration of particles has taken place due to the addition of copper through precipitation, adsorption, and ion exchange reactions. It can be seen that, physically, the pore or air spaces have reduced and the mixture has gained strength.
Meanwhile, the SEM of soil minerals that was shown in Fig. 3c, e, f revealed numerous small discrete particles scattered among the micro aggregate. It was found that the soil minerals have a rough surface. The additions of Cu2+, Zn2+, and Pb2+ have increased the particles size slightly without significant changes in the surface morphology.
On the contrary, the EDX analyses were conducted under high vacuum conditions using an accelerating voltage of 15 kV, a probe current of 1 nA to minimize the risk of sample damage. Based on Fig. 4, EDX revealed the energy spectrum of the X-ray character emitted from the element of carbon (C), oxygen (O), magnesium (Mg), aluminum (Al), potassium (K), silicon (Si), calcium (Ca), phosphorus (P), sulfur (S), and iron (Fe). The percentage of all elements was investigated in the following order: C > O > Si > Al > Fe > K > Mg > P > Ca > S. All elements were readily observed and mapped using Smiling program.
Based on Table 1, C, Si, and O have a high content. For the same sample, Al and Fe were presented in lesser quantities whereas K, Mg, P, Ca, and S having a lowest content. EDX has shown the dominant elements to be C, O, and Si whose percentage compositions are 31.65, 28.02, and 26.4 %, respectively. This analysis indicated that the mineral composition in the study areas were silica (SiO2), alumina (Al2O3), and iron(III) oxide (FeO3). Moreover, no detectable presence of any heavy metals was shown in EDX spectra of virgin soil.
The result from SEM-EDX showed that the concentration and distribution of elements were different. High percentage of elements (C, O, Si, Al, and Fe) may distribute in cluster. Though, the low percentage of elements (Mg, K, P, Ca, and S) has widely distribution.
Comparing the spectra of the soil loaded with Cu2+, Zn2+, and Pb2+ with that of unloaded one from Figs. 5, 6 and 7, the Cu, Zn and Pb peaks could be observed accordingly. It was suggested that the heavy metals have been adsorbed on the surface of soil successfully.
Surface area analysis
Brunauer, Emmett, and Teller (BET) surface area is a measurement of the accessible surface area per unit mass of soil minerals and equals to the sum of internal and external surface area (Heggy et al. 2013). The BET isotherm is a basis for determining the extent of nitrogen adsorption on the particle surface. The BET specific area and total pore volume of the adsorbent were determined by standard multipoint techniques of nitrogen adsorption, using the BET surface area analyzer (Quantachrome Autosorb-iQ, USA) at the temperature of 77 K. The analysis is tabulated in Table 2.
The surface area of the virgin soil was determined to be 287.171 m2/g by N2-BET analysis. However, the surface area of soil loaded with Cu2+, Zn2+, and Pb2+ were found to be 2.144 m2/g, 2.329 m2/g, and 1.312 m2/g, respectively. Additionally, a distinct decrease of the total pore volumes of the soil after adsorption was observed. It was believed that the decrease in total adsorption surface area available to heavy metal ions was resulting from overlapping or aggregation of adsorption sites. Hence, it can be concluded that the heavy metal ions were almost completely adsorbed onto the surface of adsorbent throughout the introduced dosage of soil.
Kinetic modeling
Pseudo-first-order kinetic model
The pseudo-first order equation has been adequately employed to describe reactions. However, it is typically expressed in a range of reaction only. The data for the adsorption of heavy metal by using soil was plotted as in Eq. (2), and the results are shown in Fig. 8.
where q e and q t (mg/g) are the adsorption capacities at equilibrium and time t (min), respectively. Meanwhile, the pseudo-first-order rate constant for the kinetic model is represented by k 1 (min−1).
The plot log (q e–q t) versus t should give a linear relationship; k 1 and q e can be calculated from the slope and intercept of the plot, respectively. The linearity of the plots were not so high (correlation coefficients, R 2 = 0.7238–0.9976). The pseudo-first-order theoretical q e values, obtained from the intercept of the linear plots, were also compared with the experimental q e values (Table 3).
It was shown that the pseudo-first-order kinetic model suffered from inadequacies when applied to heavy metals adsorption by using soil at varying heavy metal ion concentrations. The experimental q e values differed from the corresponding theoretical values. Owing to the low linearity of the plots obtained from this study and the discrepancies noted in the theoretical and experimental values of q e, chemisorption pseudo-second-order kinetic model was also used to test the data obtained from the same studies.
Pseudo-second-order kinetic model
If pseudo-second order kinetics are applicable, the plot of t/q t against t should give a linear relationship. The plots of t/q t against t from Eq. (3) are shown in Fig. 9 for the same data. Besides, the pseudo-second-order parameters, q e and k 2, obtained from the pseudo second order plot are presented in Table 3. The correlation coefficients, R 2 values, at different concentration are much higher (>0.99) compared to the pseudo-first-order.
where the second-order constants k 2 (g/mg.min) can be determined experimentally from the slope and intercept of plot t/q t versus t.
The pseudo-second-order equation was based on the adsorption capacity on the solid phase (Ho and McKay 1998). The behavior over the whole range of studies was predicted, and it was in agreement with chemisorption being the rate controlling step. It can be inferred from the plot that the possibility of the chemisorption playing a significant role in the rate-determining step cannot be rule out. This fact was attested to by the very high linearity of the plot.
Elovich kinetic model
The data obtained from the adsorption experiments were also tested with the Elovich kinetic model based on Eq. (4). The linearity values of the plots of q t versus In(t) and the Elovich parameters are presented in Table 3. The results showed that this kinetic model described the adsorption process better than the pseudo-first-order model but not as well as the pseudo-second-order kinetic model.
where α and β are the Elovich coefficients: α represents the initial sorption rate (mg/g.min) and β is the desorption constant (g/mg) during any one experiment. The Elovich coefficients can be obtained from the linear plot of q t versus In(t).
If the adsorption of heavy metals by using soil fits the Elovich model, a plot of q t versus In(t) should yield a linear relationship with a slope of (1/β) and an intercept of 1/βIn(αβ) (Oladoja et al. 2008). From Fig. 10, correlation coefficients obtained by Elovich model showed good linearity. It can also be observed that Elovich model gave an account of the occurrence of desorption process. It can be seen that as the initial concentration was increasing, the desorption constant, β was decreasing. In other words, adsorption was increasing. At lower concentrations, many other ions with higher energy of adsorption may compete and desorb the metal ion, whereas at higher concentration, the number of metal ions for adsorption is more, and hence, desorption decreases.
The correlation coefficients obtained were almost linear which shown the Elovich model fitted well. The model gave a good correlation for adsorption on highly heterogeneous surfaces like soil. Besides, it is also shown that along with surface adsorption, chemisorption was also a dominant phenomenon taking place.
Intraparticle diffusion kinetic models
Weber–Morris diffusion kinetic model
When intraparticle diffusion plays a significant role in controlling the kinetics of the adsorption process, the plots of q t versus t 1/2 based on Eq. (5) yield straight lines passing through the origin, and the slope gives the rate constant k id. In addition, the larger the intercept, c, the greater is the contribution of the surface adsorption in the rate controlling step.
where k id is the intraparticle diffusion rate constant (g/mg.min1/2) and c is the intercept in the plot of q t against t 1/2 that related to the thickness of the boundary layer.
The R 2 values given in Table 4 did not confirm that the rate-limiting step is actually the intraparticle diffusion process for the data analyzed. However, the linearity of the plots (Fig. 11) demonstrated that intraparticle diffusion played a role in the uptake of the heavy metal ions by soil. It can be observed that both surface adsorption and intraparticle diffusion were involved in the rate-limiting step.
Furthermore, there was no sufficient indication about which of the two steps was the rate-limiting step. It has been reported that if the intraparticle diffusion was the sole rate-limiting step, it was essential for q t versus t 1/2 plots to pass through the origin, which was not the case in this (Mohammed 2012).
Fractional attainment of equilibrium (FAE) and liquid film diffusion kinetic model
It was established that the kinetic theory behind FAE was that it may be used to explain the adsorption process in terms of adsorption being controlled by film-diffusion (Horsfall and Vicente 2007). When the transport of the solute molecules from the liquid phase up to the solid phase, boundary plays the most significant role in adsorption; the kinetic model equation may be applied as Eq. (6), where F as the fractional attainment of equilibrium is corresponded to q t/q e from Eq. (7).
where F is the fractional attainment of equilibrium. It is the ratio of the amounts of metal ion removed from solution after a certain time (Ct) to that removed when adsorption attained equilibrium (Ce).
where q t and q e are the amounts of metal adsorbent at time (t) and at equilibrium. The overall rate constant is represented by k 4 and can be determined from the gradient of the graphs of In (1-q t/q e) versus t.
A linear plot of In(1-F) versus t with zero intercept would suggest that the kinetics of the adsorption process is controlled by diffusion through the liquid film surrounding the solid adsorbent. Nevertheless, the deviation of the straight lines from the origin (Fig. 12) may be due to the difference in the rate of mass transfer in the initial and final stages of adsorption.
A non-linear relationship for the plot of In(1-F) versus t indicated that the diffusion of adsorbate onto the adsorbent surface is film-diffusion controlled (Horsfall and Vicente 2007). In such condition, an external factor such as high agitation speed may be needed to break the diffusion barrier and increase adsorbate-adsorbent interaction. The plots in Fig. 12 were non-linear and showed more than one step. The data shown that film diffusion-controlled process may possibly be a factor in the ability of metal ions to migrate to the soil surfaces.
Moreover, the degree of fitting of the data to the model gives an indication of the evolution of the porous texture of the soil. The data (Table 4) revealed that pore diffusion-controlled rate-limiting mechanism into the porous structure of the soil was inadequately applicable for the three metal ions investigated.
Comparison between kinetic models
Adsorption of metal ions on soil particles follows a three-step process: (1) film diffusion, (2) intra-pore diffusion, and (3) adsorption. Metals added to soil were normally retained at the soil surface. The extent of movement of a metal in the soil system was intimately related to the solution and surface chemistry of the soil. The retention mechanisms for metals added to soil included the adsorption of the metal by the soil solid surfaces and precipitation.
By evaluating the correlation coefficient, R 2, of every kinetic model, the results from Table 4 have shown that the adsorption of heavy metals by using soil was better described by the pseudo-second-order model with correlation coefficients, R 2 > 0.99. Intraparticle diffusion model has also proved the surface adsorption and intraparticle diffusion operated concurrently during adsorbent and adsorbate interaction.
The experimental results indicated that increasing soil concentration increased the uptake of heavy metal ions from aqueous solution. This agreed with previous results on the adsorption of copper metal ion in selected contaminated red soil samples in Palestine (Jodeh and Abu-Obaid 2011).
Furthermore, based on a research related to the adsorption of Pb2+ ions from aqueous system by kaolinite, which is a clay mineral, recorded at the a higher adsorptivity than Cu2+ and Zn2+; the kinetic studies also showed that a pseudo-second-order model was more suitable than the pseudo-first-order model (Shahmohammadi-Kalalagh et al. 2011). In addition, the experimental results obtained from the adsorption of heavy metal ions from aqueous solution by using soil have been correlated well to the four kinetic models studied (Mohammed 2012; Merlain et al. 2013).
Conclusions
In this study, the removal capacity of heavy metal, Cu2+, Zn2+, and Pb2+ ions from aqueous solution increased with increase in contact time before equilibrium point while it decreased with an increase in initial concentration. The adsorption of heavy metals onto soil was a very rapid process and a major fraction of the total metal concentration was removed from the aqueous phase in early stage for a wide range of initial concentration of adsorbate. The relative adsorption capacity of the three heavy metals for the kinetics study by using soil shown to be increased in the order of Zn2+ < Cu2+ < Pb2+.
The properties of soil and adsorption characteristics of heavy metals onto soil were reviewed through several instrumental analyses. Studies of the structure of soil after adsorption process by FTIR shown that some peaks are shifted in terms of wavenumber, indicating some functional groups are involved or participated in the binding of heavy metals onto soil. From SEM micrograph showed the soil particles in spherical shape and there was a large surface area for heavy metal ions adsorption. In term of surface morphology, it was also observed that the clogging of pores of soil occurred after the adsorption. The mineral composition in the study areas were determined to be silica (SiO2), alumina (Al2O3) and iron(III) oxide (FeO3) based on EDX analysis. Additionally, a distinct decrease of the specific surface area and total pore volumes of the soil after adsorption was found from BET analysis.
In the batch mode adsorption studies, the amount of Cu2+, Zn2+, and Pb2+ ions uptake (mg/g) was found to increase in contact time and initial concentration. The kinetic data was analyzed using four adsorption kinetic models, namely Pseudo-first-order, Pseudo-second-order, Elovich, and intraparticle diffusion.
In order to obtain parameters related to each kinetic model, experimental data have been tested with linear forms of these models. Correlation coefficients obtained adequately fitted well for all models but the ranking was in the order of Pseudo-second-order > Elovich > Pseudo-first-order > intraparticle diffusion. The agreement of experimental data and the pseudo-second order equation was much better than those for the other equations, with correlation coefficients, R 2 > 0.99.
This has implied that Cu, Zn, and Pb adsorption on soil may occur through a chemical process involving the valence forces of the shared or exchanged electrons. The resulting heavy metal adsorption values obtained from this model were almost the same as compared to the maximum adsorption capacity experimentally obtained. This was meant by the chemisorption reaction, or an activated process becomes more predominant in the rate-controlling step in Cu, Zn, and Pb systems.
As a result of this study, it may be concluded that soil was feasible for removal of heavy metal pollution from wastewater since it was of low-cost abundantly available adsorbent.
References
Das B, Mondal NK, Bhaumik R, Roy P, Pal KC, Das CR (2013) Removal of copper from aqueous solution using alluvial soil of Indian origin: equilibrium, kinetic and thermodynamic study. J Mater Environ Sci 4(4):392–408
Dube A, Zbytniewski R, Kowalkowski T, Cukrowska E, Buszewski B (2000) Adsorption and migration of heavy metals in soil. Pol J Environ Stud 10(1):1–10
Fonseca B, Maio H, Quintelas C, Teixeira A, Tavares T (2009) Retention of Cr(VI) and Pb(II) on a loamy sand soil: kinetics, equilibria and breakthrough. Chem Eng J 152:212–219
Heggy SEM, Komy ZR, Shaker AM, El-Sayed MEA (2013) Kinetics of zinc adsorption on soil minerals in the absence and presence of humic acid. J Am Sci 9(4):523–533
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Horsfall M, Vicente JL (2007) Kinetic study of liquid-phase adsorptive removal of heavy metal ions by almond tree (Terminalia catappa L.) leaves waste. Bull Chem Soc Ethiop 21(3):349–362
Jodeh S, Abu-Obaid AA (2011) The kinetic study of adsorption of copper metal Ion in selected contaminated red soil samples in Palestine. J Chem Eng Data 5:873–879
McLean JE, Bledsoe BE (1992) Behavior of Metals in Soils. Available from: http://www.epa.gov/superfund/remedytech/tsp/download/issue14.pdf. Accessed on 24 Oct 2013
Merlain TG, Nsami NJ, Mbadcam KJ (2013) Adsorption of copper (II) ions from aqueous solution onto synthetic goethite and two naturally available red soils from Yaoundé –Cameroon. Br Biotechnol J 3(3):221–235
Mohammed SAS (2012) Retention capacity of soils and amended soils for heavy metal ions. Phys Chem News 66:1–16
Oladoja NA, Aboluwoye CO, Oladimeji YB (2008) Kinetics and isotherm studies on methylene blue adsorption onto ground palm kernel coat. Turk J Eng Environ Sci 32:303–312
Orisakwe OE, Nduka JK, Amadi CN, Dike DO, Bede O (2012) Heavy metals health risk assessment for population via consumption of food crops and fruits in Owerri, South Eastern, Nigeria. Chem Cent J 6:77–83
Paliwal V (2006) Study of Lead Sorption on Magnetite at High Temperatures. Available from: http://digital.library.unt.edu/ark:/67531/metadc5445/. Accessed on 9 Apr 2014
Payne KB, Abdel-Fattah TM (2005) Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: effects of pH, temperature, and ionic strength. J Environ Sci Health 40:723–749
Shah IK, Pre P, Alappat BJ (2013) Steam regeneration of adsorbents: an experimental and technical review. J Chem Sci 2(4):1078–1088
Shahmohammadi-Kalalagh S, Babazadeh H, Nazemi AH, Manshouri M (2011) Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J Environ Sci 9(2):243–255
Shin WS, Kang K, Kim YK (2014) Adsorption characteristics of multi-metal ions by Red Mud, zeolite, limestone, and oyster shell. Environ Eng Res 19(1):15–22
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246–253
Tilson HA (2013) Disease Outcomes, Exposures, and Methodologies and Populations. Available from: http://ehp.niehs.nih.gov/wp-content/uploads/2013/10/CH_2013_final.pdf. Accessed on 19 Dec 2013
Uzun İ, Güzel F (2000) Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk J Chem 24:291–297
Yadav PR, Tyagi R (2006) Crop Biotechnology. Discovery Publishing House, New Delhi
Acknowledgments
The authors would like to thank Universiti Malaysia Sarawak for the use of laboratory facilities and equipments. This research was funded by Fundamental Research Grant Scheme (FRGS/02(17)/738/2010(24)) from the Ministry of Higher Education, Malaysia and CoERE Grant (CoERE/Grant/2013/04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Lim, SF., Lee, A.Y.W. Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ Sci Pollut Res 22, 10144–10158 (2015). https://doi.org/10.1007/s11356-015-4203-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4203-6