Abstract
Bioremediation is important in reducing the load of toxic by-products in industrial effluents which has dramatically got increased due to industrialization and urbanization. In this study, we have used mixed bacterial culture (MBC) to convert the model pollutant cresol into a less toxic form. A kinetic study for bacterial growth and substrate removal showed that maximum MBC growth rate and cresol degradation rate took place in 800 ppm of initial cresol concentration at pH 7 and in an aerated condition. The maximum specific growth rate (μmax) and maximum specific degradation rate (qmax) in the above condition were found as 0.119 h−1 and 0.011 h−1, respectively. The optimized condition of process parameters for maximum percentage removal efficiency (%RE) of 83% in 24 h as obtained by response surface methodology was under 1095 mg/L of initial cresol concentration at pH 7 and in the presence of both shaking and aeration. The quadratic model was found to be the best-fit model. The R2 value of 0.9272 showed that the model is well fit. The C.V.% of 14.78 showed the precision of the data. The model is significant with a p-value of less than 0.0001 and an F value of 41.41. Two bacterial strains showing maximum cresol degradation have been identified as Stenotrophomonas sp. (Gram-negative) and Bacillus cereus (Gram-positive). Both of them showed positive responses in catalase, citrate, and triple sugar iron test.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Bioremediation
- Cresol biodegradation
- Maximum specific degradation rate
- Kinetic study
- Maximum specific growth rate
- MBC
- Optimization
- Percentage removal efficiency (%RE)
- Response surface methodology
- East Calcutta Wetlands
1 Introduction
The surge of industries and rapid urbanization has resulted in accumulation of toxic pollutants in industrial effluents [1]. Several of those compounds are recalcitrant and persistent pollutant causing serious health effects. The most common strategies employed for the removal of phenolics from industrial discharge include ozonation, adsorption, photolytic degradation, ion exchange, and membrane-based separation. But these processes are not only expensive employing hazardous reagents but also cause generation of secondary byproducts. On the other hand, bioremediation of wastewater, i.e., the use of microbes to degrade the pollutants, is gaining popularity over chemical treatment methods due to a lot of factors. It is a simpler, cost-effective process leading to complete mineralization of the pollutants to carbon dioxide and water [2, 3].
In our study, we have isolated the mixed bacterial cultures (MBC) from the soil of East Calcutta Wetlands (ECW), which is a natural sewage treatment plant for the city. Its microbial richness attracted us to explore the various bacterial consortium needed to remediate the pollutants from the industrial effluent. Individual microorganisms can degrade only a limited number of organic pollutants, whereas a consortium of mixed bacterial populations with overall broad enzymatic spectrum can enhance the rate and range of biodegradation of the pollutants. In a mixed culture, mutualism and symbiotic association among different species ensure better degradation ability of the consortium as a whole. It is easy to maintain and subculture mixed microbial culture indefinitely by maintaining the environmental conditions such as temperature, pH, and nature of substrate [4, 5]. We have chosen cresol as the model pollutant. Cresols are organic methyl phenols and are widely occurring natural and anthropogenic products [6]. The industries such as petrochemical, textile, dye, pharmaceutical, pulp and paper industries, and petroleum refineries release cresol in the effluent. Chronic exposure to even low concentrations of cresol results in vomiting, diarrhea, loss of appetite, headache, fainting, dizziness, and skin rashes [7,8,9].
This study aims in optimizing the process parameters for cresol removal from the wastewater by MBC using response surface methodology (RSM). The usual methods of kinetic studies consist of varying only one parameter at a time while maintaining others constant. It becomes a prolonged process since it requires many experimental runs. RSM is a structured method where the output can be affected by changing various experimental domains concurrently. It can spot the components that can bring the change in the response. It is also used to examine a considerable number of variables in a limited number of experimental runs, reducing the time and cost. RSM helps in determining the interactive effect of the process parameters. It is also one of those mechanisms that can figure out the optimal operating conditions of a system [10, 11].
2 Materials and Methods
2.1 Kinetic Study of Bacterial Growth and Cresol Removal by the Mixed Bacterial Culture in Various Substrate Concentration and in Different Modes of Agitation: Agitation with External Aeration, Agitation Without External Aeration, and No Agitation
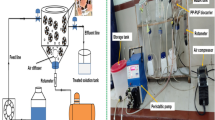
The soil of East Calcutta Wetlands (ECW) was chosen to isolate the mixed bacteria which were acclimatized to 1500 ppm of cresol as their sole carbon source. The MBC attuned to cresol were then used for batch kinetic study for bacterial growth and cresol removal in the presence of various substrate concentrations (100, 300, 500, 800, and 1000 ppm) and in different modes of agitation: agitation with external aeration, agitation without external aeration, and no agitation.
-
Agitation with external aeration: The bacterial cultures were made to grow in external aerated condition at room temperature till the cells reached their stationary phase or concentration of residual cresol dropped below the detection level.
-
Agitation without external aeration: The cultures were let to grow in the absence of external aeration and with an agitation speed of 60 rpm at room temperature.
-
No agitation: In this case, the MBC flasks were allowed to stand undisturbed without any agitation or aeration.
At regular interval, samples were collected, and bacterial concentration and residual cresol concentration was checked. The cell growth was measured by spectrophotometer (Labman visible spectrophotometer LMSP V320) by checking the optical density (OD) at 600 nm. The cell concentration was calculated with the help of a standard curve (absorbance at 600 nm vs. cell concentration (mg/L)). The cresol in the supernatant was checked by antipyrine assay, whose (OD) was checked at 490 nm. The cresol concentration was calculated from the absorbance at 490 nm with the help of a standard graph (absorbance at 490 nm vs. cresol concentration). The bacterial growth curve and cresol degradation curve in various substrate concentrations were plotted. The kinetic parameters such as maximum specific growth rate (μmax), maximum specific degradation rate (qmax), and percentage removal efficiency (RE%) were calculated for each concentration and compared in the presence of different modes of agitation.
The maximum specific growth rate (μmax) was calculated from the slope of another graph (ln(X/X0) vs. t) (Fig. 2) using Eq. (1).
where X is the bacterial cell concentration at time t in the log phase of bacterial growth and X0 is the bacterial cell concentration at time t0.
-
Cresol removal efficiency by mixed bacterial culture was calculated using Eq. (2).
$$ \%\mathrm{RE}=\left[\left({C}_{\mathrm{i}}-{C}_{\mathrm{f}}\right)/{C}_{\mathrm{i}}\right]\times 100 $$(2)where %RE is the percentage removal efficiency; Ci is the initial concentration of cresol at time t = 0; and Cf is the final concentration at time t = t.
-
The maximum specific degradation rate (qmax) of Cresol by MBC was calculated using Eq. (3).
$$ 1/q=1/{q}_{\mathrm{max}}+\mathrm{Ks}/\left({q}_{\mathrm{max}}.S\right) $$(3)
2.2 Optimization of Operating Conditions by Response Surface Methodology (RSM)
Response surface methodology was analyzed by Design Expert software (Version 13.0.5). Central composite design (CCD) was used to analyze the interaction between the operating conditions and output responses [11, 12]. Different process parameters such as concentration of cresol (100–800 mg/L), pH (3–11), aeration, and agitations together with their ranges in the system were fed into the software. The concentration of cresol and pH was considered to be the numeric factors as they can be varied within a defined range. Agitations and aerations were categorical factors as they were either present or absent in the runs. The tool then designed a set of 52 experimental runs with various combinations of the parameters. These tests were then performed in the laboratory followed by calculating the percentage removal efficiency (%RE). The results obtained were fed into the software which provided us with the optimum response. It also provided us with 3D graphs that showed the interactions of the different process parameters [13,14,15].
2.3 Isolation of Pure Bacterial Colonies
The cultures grown in optimized condition were chosen for the isolation of pure bacterial strains because the MBC in this condition showed maximum removal efficiency (%).
2.4 Characterization of Pure Strains
-
Gram Staining and Biochemical Characterization
Gram staining and biochemical profile such as catalase, citrate, and triple sugar iron test of these two isolates were performed.
-
Identification by 16s rRNA
The isolated bacterial strains showing maximum removal efficiency were outsourced for identification by the 16s rRNA method.
3 Results and Discussion
3.1 Kinetic Study of Growth of Mixed Bacterial Culture (MBC) in Presence of Different Concentrations of Cresol and Under Various Modes of Agitations
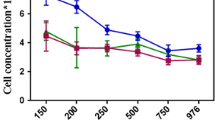
The bacterial cell concentration with time has been plotted as shown in Fig. 1a–c, in the presence of different cresol concentrations (100, 300, 500, 800, and 1000 ppm) and under three different modes of agitations (agitation with external aeration, agitation without external aeration, no agitated condition).
By plotting the graph, it has been observed that the growth rate was enhanced with the increase of the initial substrate concentration from 100 to 500 ppm. In spite of having a slight lag phase, the growth of bacteria was found to be maximum in the presence of 500 ppm of cresol in agitation without external aeration and no agitation condition. The growth of bacteria was observed to be maximum at 800 ppm of cresol in agitation with external aerated condition. This may be due to the induction of degrading enzymes which have resulted in more efficient utilization of cresol as the sole carbon source leading to increased bacterial growth [16]. Moreover, aeration may have some positive role in metabolism which has led to an increase in bacterial growth. In the presence of higher initial concentrations of cresol, after a prolonged lag phase, the growth rate was decreased markedly. This was probably due to the substrate inhibition caused by the toxic nature of high cresol concentrations which was intolerable by most of the bacterial strains in the consortium.
3.2 Comparative Study of Maximum Specific Growth Rate (μmax) of MBC in Different Concentrations of Cresol
The maximum specific growth rate (μmax) of mixed bacterial culture is obtained from the graph (ln(X/X0) vs. t) (Fig. 2a–c) using Eq. (1).
where X is the bacterial cell concentration at time t in the log phase of bacterial growth and X0 is the bacterial cell concentration at time t0.
The μmax of MBC in the presence of different concentrations of cresol was compared in Fig. 2a–c. The culture having 500 ppm of Cresol showed the highest maximum specific growth rate (μmax) of 0.0491 h−1 and 0.0296 h−1 in the presence of agitations with no aeration and no agitation condition, respectively. It was also noted that the (μmax) increased gradually from 100 to 500 ppm of cresol and then showed a decreasing trend till the concentration of cresol reached 1000 ppm in the culture media, thus indicating substrate inhibition beyond 500 ppm of initial cresol concentration in the growth media. On the other hand, (μmax) of 0.12 h−1 was found to be maximum at 800 ppm of cresol concentrations in agitations with external aerated condition.
3.3 Degradation Kinetics of Cresol by MBC in Different Cresol Concentrations and in Presence of Varying Modes of Agitations
The mixed bacterial culture (MBC) metabolized cresol completely from the growth media which was using it as the sole carbon source. A graph (residual cresol concentration vs. time) has been plotted as depicted in Fig. 3a–c, showing cresol degradation in the presence of different substrate concentrations and various modes of agitations.
From the comparative study of cresol degradation by the MBC shown in Fig. 3a–c, it was obtained that the time required for completely degrading cresol got enhanced with the increase of substrate concentration. The maximum specific degradation rate (qmax) in the presence of external aeration got increased with the increase of the initial substrate concentration to 800 ppm, after which it got decreased with the increase in cresol concentration in the growth medium (Figure). This was probably due to the expression of Cresol degrading enzymes induced by stress in response to the substrate concentration.
3.4 Comparative Study of Maximum Specific Degradation Rate (qmax) of MBC in Different Concentrations of Cresol
The maximum specific degradation rate (qmax) of the mixed bacterial culture in the presence of different concentrations of cresol and in various modes of agitations was calculated from Eq. (3) as stated above. The qmax as obtained in different conditions was compared in Fig. 4a–c.
The reduction of qmax in the presence of high substrate concentration (1000 ppm) was most likely due to the inhibitory effect of cresol on the metabolic enzymes. The maximum specific degradation rate (qmax) was found to be maximum at 500 ppm of initial cresol concentration in both agitations without external aerated and no aerated condition. The qmax at 800 ppm of cresol concentration was calculated to be 0.011 h−1, 0.0042 h−1, and 0.0019 h−1 in agitation with external aeration, agitation without external aeration, and no agitated condition, respectively. This also supports the theory that aeration not only accelerates degradation but also aids in mineralizing higher concentrations of cresol.
3.5 Study of Optimization of Operating Conditions by Response Surface Methodology (RSM)
The response surface methodology suggested 52 experimental runs with various combinations of process parameters as depicted in Fig. 5. These tests were performed in the laboratory followed by calculating the removal efficiency (%RE) in 24 h. The calculated (%RE) was fed into the software which then designed 3D graphs showing a change in response with a simultaneous change in cresol concentration and pH in different modes of agitations (Fig. 6a–c). In all the modes of agitations, the (%RE) was found to be maximum at pH 7 and decreases gradually with the increase of cresol concentration. Acidity and alkalinity of the medium reduce the growth of the bacteria resulting in substrate degradation. Percentage removal efficiency (%) was observed to be maximum in both aerated and agitated condition and was least in unaerated and unagitated condition. This showed that most of the strains in the consortium are aerobic in nature and aeration can enhance the mineralization of cresol.
3D graph representing a change in removal efficiency (%) with a simultaneous change in cresol concentration and pH in different modes of agitations: (a) without any agitations or aeration, (b) with agitations (shaking) and no aeration, (c) with external aeration but no agitations (shaking), and (d) both in the presence of external aeration and agitations (shaking)
The fit statistics depicted in Fig. 7a showed a reasonably high R2 value. The difference between the adjusted R2 and predicted R2 is also less than 0.2. The CV (%) was appreciably low. Adequate precision value was found to be greater than 4. The quadratic model was suggested by the software to be the best-fit model (Fig. 7b). Moreover p-value is less than 0.0001 (Fig. 7c). The above statistical results showed that the model is significant. The software has also designed an actual equation (Fig. 8) and optimized condition for maximum removal efficiency shown in Fig. 9.
3.6 Isolation of Pure Bacterial Colonies
The culture grown in 1095 mg/L of cresol concentration at pH 7 and under aerated and agitated condition was selected for bacterial colony isolation. Those single colonies were chosen based on the difference in size, shape, color, and transparency of the colonies. A total of 20 pure single colonies were selected. All the colonies were whitish in color, opaque in consistency, and fairly circular in shape with varying sizes.
Out of the 20 pure strains isolated from the MBC, the characterization of two strains (A and B) was done based on maximum removal efficiency.
3.7 Characterization of Pure Strains
-
Gram Staining
Gram staining of the two isolates showed that sample A was Gram-positive cocci whereas sample B was Gram-negative cocci (Fig. 10).
-
The biochemical tests with the results are shown below:
-
Catalase Test: Both the test cultures reacted with hydrogen peroxide to form effervescence as in Fig. 11 showing a positive test for catalase.
-
Citrate Test: Both the pure cultures could utilize citrate as their sole carbon source. When they metabolized citrate, the ammonium salts were broken down to ammonia, which lead to an increase in alkalinity. The shift in pH turned the bromothymol blue indicator in the medium from green to blue as depicted in Fig. 12.
-
Triple Sugar Iron Test: In the tube containing sample B (Fig. 13), the bottom part was observed to be turbid yellow and the slope red. We can infer that the strain in sample B has rapidly metabolized glucose only giving an acid slope and an acid bottom. The strain was not able to use lactose and sucrose as their carbon source within the said period. Sample A agar turned out to be turbid yellow indicating that the strain could metabolize the triple sugar simultaneously and produce CO2 and O2 gas seen by the detachment of the agar slant (Fig. 13).
-
-
Identification by 16s rRNA:
The pure bacterial strains were identified as Stenotrophomonas sp. (sample A) and Bacillus cereus (sample B) as shown in Fig. 14.
4 Conclusion
Our work emphasized the viability of using the isolated bacterial consortium to bioremediate cresol in industrial effluents. The optimized condition as obtained by response surface methodology for maximum percentage removal efficiency (%RE) of 83% was found to be 1095 mg/L of initial cresol concentration at pH 7 and in the presence of both shaking and aeration in 24 h. The biochemical characterization together with 16s rRNA gene sequencing helped to identify the bacterial strains present in mixed bacterial culture as Stenotrophomonas sp. and Bacillus cereus.
Abbreviations
- %RE:
-
Percentage removal efficiency
- C.V.:
-
Coefficient of variance
- CCD:
-
Central composite design
- ECW:
-
East Calcutta Wetlands
- MBC:
-
Mixed bacterial culture
- O.D.:
-
Optical density
- Ppm:
-
Parts per million
- q max :
-
Maximum specific degradation rate
- rRNA:
-
Ribosomal ribonucleic acid
- RSM:
-
Response surface methodology
- μ max :
-
Maximum specific growth rate
References
G. Durai, M. Rajasimman, Biological treatment of tannery wastewater – a review. J. Environ. Sci. Technol. 4(1), 1–17 (2011)
V. Pazhamalai, R. Ravi, Biodegradation of phenol using mixed cultures. Int. J. Bioprocess Technol. 1(1), 1–6 (2012)
K.K. Wong, B. Quilty, A. Hamzah, S. Surif, Phenol biodegradation and metal removal by a mixed bacterial consortium. Biorem. J. 9(2), 104–112 (2015)
V.S. Cerqueira, E.B. Hollenbach, F. Maboni, M.H. Vainstein, F.A.O. Camargo, M.C.R. Peralba, F.M. Bento, Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour. Technol. 102, 11003–11010 (2011)
S. Dey, S. Mukherjee, Performance and kinetic evaluation of phenol biodegradation by mixed microbial culture in a batch reactor. International Journal of Water Resources and Environmental Engineering 2(3), 40–49 (2010)
L.M. Tormoehlen, K.J. Tekulve, K.A. Nañagas, Hydrocarbon toxicity: a review. Clin. Toxicol. (Phila). 52(5), 479–489 (2014)
Z. Kyselova, Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 4(4), 173–183 (2011)
B. Chakraborty, Kinetic study of degradation of p-nitro phenol by a mixed bacterial culture and its constituent pure strains. Mater. Today Proc. 3(10), 3505–3524 (2016)
S. Bera, A. Sarma Roy, K. Mohanty, Biodegradation of phenol by a native mixed bacterial culture isolated from crude oil contaminated site. Int. Biodeterior. Biodegradation 121, 107–113 (2017)
S. Hazra Das, J. Saha, A. Saha, A.K. Rao, B. Chakraborty, S. Dey, Adsorption study of chromium (VI) by dried biomass of tea leaves. J. Indian Chem. Soc. 96, 447–454 (2019)
K.Y. Leong, S. See, J.W. Lim, M.J.K. Bashir, C.A. Ng, L. Tham, Effect of process variables interaction on simultaneous adsorption of phenol and 4-chlorophenol: statistical modeling and optimization using RSM. Appl. Water Sci. 7, 2009–2020 (2017). https://doi.org/10.1007/s13201-016-0381-8
M.J.K. Bashir, H.A. Aziz, M.S. Yusoff, M.N. Adlan, Application of response surface methodology (RSM) for optimization of ammoniacal nitrogen removal from semi-aerobic landfill leachate using ion exchange resin. Desalination 254(1–3), 154–161 (2010)
A. Shehzad, M.J.K. Bashir, S. Sethupathi, J.W. Lim, An insight into the remediation of highly contaminated landfill leachate using sea mango based activated bio-char: optimization, isothermal and kinetic studies. Desalination 57(47), 22244–22257 (2016)
S.C. Chua, F.K. Chong, M.A. Malek, M.R. Ul Mustafa, N. Ismail, W. Sujarwo, Optimized use of ferric chloride and sesbania seed gum (SSG) as sustainable coagulant aid for turbidity reduction in drinking water treatment. Sustainability 12(6), 2273 (2020)
B. Bhunia, A. Dey, Statistical approach for optimization of physiochemical requirements on alkaline protease production from Bacillus licheniformis NCIM 2042. Enzyme Res. 2012, 905804 (2012). https://doi.org/10.1155/2012/905804
B. Chakraborty, L. Ray, S. Basu, Study of phenol biodegradation by an indigenous mixed consortium of bacteria. Indian J. Chem. Technol. 22, 227–233 (2015)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Das, S.H. et al. (2023). Optimization of Process Parameters for Biodegradation of Cresol by Mixed Bacterial Culture Using Response Surface Methodology. In: Mazumder, D. (eds) Sustainable Advanced Technologies for Industrial Pollution Control. ATIPC 2022. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-37596-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-37596-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37595-8
Online ISBN: 978-3-031-37596-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)