Abstract
The management of Chiari 1 malformation (CM1) and Syringomyelia (Syr) has shown many changes in surgical indications and techniques over time. The dedicated neurosurgical and neurological community recently planned to analyze the state of the art and find conduct uniformity. This led to international consensus documents on diagnostic criteria and therapeutic strategies. We aimed to evaluate, in a large, monocentric surgical series of adult and children CM1 patients, if the daily clinical practice reflects the consensus documents. Our series comprises 190 pediatric and 220 adult Chiari patients submitted to surgery from 2000 to 2021. The main indications for the treatment were the presence of Syr and symptoms related to CM1. While there is great correspondence with the statements derived from the consensus documents about what to do for Syr and symptomatic CM1, the accordance is less evident in CM1 associated with craniosynostosis or hydrocephalus, especially when considering the early part of the series. However, we think that performing such studies could increase the homogeneity of surgical series, find a common way to evaluate long-term outcomes, and reinforce the comparability of different strategies adopted in different referral centers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Chiari malformation type 1 (CM1) represents a heterogeneous group of congenital malformations affecting children and adults. Since the first description of Chiari malformation in 1891 [1], the spectrum of the disease has been dramatically expanded. The main characteristic of CM1 is the caudal cerebellum ptosis through the foramen magnum, leading to obstruction of cerebrospinal fluid (CSF) outflow. Whereas the estimated prevalence of cerebellar tonsils descent at magnetic resonance imaging (MRI) has been estimated between 1.9 and 8.4/100,000 [2, 3], most individuals with radiologically defined CM1 remain asymptomatic. However, various neurological signs and symptoms can be reconducted to the malformation. Despite often being nonspecific, the clinical presentation of CM1 comprises headache, ocular and otoneurologic alterations, ataxia, and lower cranial nerves disturbances [4].
Moreover, CM1 could determine or be associated with Syringomyelia (Syr) or syringobulbia, represented by single or multiple fluid-filled cavities within the spinal cord and/or the bulb. The degree of clinical manifestation of a Syr is variable, but many patients present neurological damages and progressive disability, according to the extent, location, and severity of spinal cord compression. The therapeutic management of CM1 and Syr often comprises suboccipital craniectomy and foramen magnum opening, associated with posterior C1 arch laminectomy, with or without enlargement duraplasty, arachnoid dissection, and tonsillar reduction [5,6,7,8,9]. Nevertheless, considering the wide range of clinical presentations and the surgical strategies proposed during the past years, it has been challenging to evaluate the best outcome according to the techniques performed. More recently, the neurosurgical and neurological community committed to CM1 management planned to analyze the contemporary state of the art and find conduct uniformity. In 2021, according to the “experts” agreement obtained at the Chiari and Syringomyelia Consensus Conference held in Milan in 2019, an international document was produced to reach a consensus on controversial topics in children and adult patients with CM1 [10, 11]. The results of a Delphi process allowed the authors to summarize as a consensus document (CD) some indications about the management of CM1.
Some debate points with less accordance among experts were the role of bone decompression alone, the integrity of the arachnoid membrane, and the use of autologous and allograft dural patches instead of artificial grafts. This study aims to evaluate, in a large, monocentric surgical series of adult and pediatric CM1 patients, if the daily clinical practice reflects what was achieved in the CD. To strengthen the value of international recommendations, we would support and highlight the degree of consensus or difference based on a homogenous series analysis, using evaluation grids derived CD to quote the accordance of a large series with the indications for surgery and reoperation, and calculating a comparable outcome rate.
2 Materials and Methods
The main statements about diagnosis, indications for treatment, surgical technique, evaluation of surgical results, and indication for reoperation were summarized (Tables 1, 2, and 3). A retrospective analysis was performed on a prospectively collected database, reporting data about consecutive CM1 adults and children admitted at our Institution between 2000 and 2021. The malformation was documented, in all patients, with a sagittal midline T1-weighted MRI showing the ptosis of one or both cerebellar tonsils for more than 5 mm beyond the basion-opisthion, or McRae, line. In all cases, a whole spine MRI was also performed to detect syrinx, and a flow study in the posterior fossa was done to confirm the constraint to CSF passage posteriorly. All patients had neurological symptoms attributable to the CM1 (i.e., Chiari-type headache, bulbar or cerebellar dysfunction) as evaluated preoperatively by a pediatric or adult neurologist or harbored progressive Syr. The data collected were related to the tonsil descent measured in cervical vertebra level (C1, C2, C3), the syrinx presence, and extension (medullar, cervical, dorsal, holochord), the association with hydrocephalus or previous CSF shunting. The clinical presentation, the headache characteristics (typical, atypical, both), and the presence of other associated diseases were also analyzed. Finally, we evaluated all results for the accordance with the CD, using specific grids.
2.1 Surgery and Postoperative Management
All patients were submitted to a standard preoperative evaluation, including blood tests, general and neurological clinical assessment. At our center, posterior fossa decompression with duraplasty (PFDD) is the treatment of choice on all patients, except rare cases, mainly children, in which the associated malformation (hydrocephalus, craniosynostosis, tethered cord) was treated before/concomitantly/after. After general anesthesia, the patients were placed prone with the head secured in a Mayfield clamp, with a 15–30-degree flexion. A midline incision was made from inion to the spinous process of C2. After the suboccipital bone and C1 arches were exposed, the bony decompression was performed with a high-speed drill and bony rongeurs. There was no standard rule regarding the extent of the resection, but we tend to set the bone removal to avoid a cerebellar slump. The arch of C1 was always removed. The dura is then sharply dissected with a longitudinal incision, starting caudally and proceeding cranially. The arachnoid may be left intact or opened depending on the intraoperative findings, such as important adhesions or CSF flow obstruction. The same thought process was applied to whether or not to perform the tonsillar coagulation via bipolar cautery. After assessing cerebellar pulsations as a sign of restored CSF flow, the duraplasty was performed by using allograft patches, such as bovine or equine pericardium. A watertight closure was the dural closure goal, which was reinforced by collagen or fibrin sheets. Postoperatively, patients were closely monitored for complications, such as CSF leaks, hematomas, wound infections and dehiscence, hydrocephalus, pseudomeningocele, or general complications. A non-contrast CT scan was also performed before the patients’ discharge. A clinical and neuroradiological follow-up (brain MRI and, when necessary, spinal MRI) was indicated at 3 months and 1 year from surgery. Afterward, the follow-up was repeated every 1–3 years, depending on the outcome.
3 Results
3.1 Pediatric Series Characteristics
From 2000 to 2021, 190 pediatric Chiari patients underwent surgery at our Institution, mainly due to the first author. There was a slight predominance of females (53.2%) on males (46.8%). The mean age at surgery was 10.8 years, ranging from 9 months to 18 years, whereas 23.2% of the whole cohort (44 patients) were younger than 6 years. The indication for surgery was mainly represented by the presence of Syr, as it happened in 132 patients (69.5%); 29% of patients had, nevertheless, symptoms related to CM1. Only in 3 patients (1.5%), the surgical indication was headache only. Considering that aspect, our series presents accordance with the CD in 100% of the surgically treated cases (Table 1). There were no CM0, and in 35 cases, the radiological diagnosis was CM 1.5 (18.4%).
A total of 25 patients (13.1%) presented associated hydrocephalus. The CD showed an agreement greater than 90% in treating hydrocephalus first and CM1 later if symptoms do not disappear. In our series, 15/25 children received a specific treatment before surgery, 3 afterward, while 7 were never treated.
Finally, the CD indicates to treat craniosynostosis before CM1 (agreement >90%): among our pediatric series, 40 children (21%) presented associated craniosynostosis, largely isolated forms (39 children); only 11 of them were submitted to cranioplasty before CVD, mainly the complex forms. A smaller percentage of children (2.1%) presented an associated tethered cord, with the conus lying lower than L3; an untethering procedure was performed according to the neurourological presentation (specifically, 2 cases before and another 2 after the PFD). It is worth noting that we never performed any extradural sectioning of the filum terminalis, with 100% accordance with the CD.
3.2 Adult Population
The cohort of 220 adult patients was composed of 65 males (29.5%) and 155 females (70.5%), thus with a significant predominance of females. The mean age was of 41 ± 9.9 years (range 18–77 years). Also, in this case, as it happens for the pediatric cohort, the main indication for surgical treatment was the Syr, affecting 171 (77.2%) patients; among the 220 patients in total, 46 (20.9%) presented symptoms, but headache alone was the indication for surgery only in 3 patients. Again, the concordance with the CD indications was 100%.
Preoperative MRI findings showed that out of 220 patients, 193 (87.7%) had CM1, 22 (10%) had CM1.5, and 5 were diagnosed as CM0. Every patient had a tonsillar herniation >5 mm, apart from the 5 CM0 patients; the average tonsil descent was 12.6 ± 4.45 mm, ranging from 5 to 33.85 mm. CM1-associated hydrocephalus occurred in 18 (8.1%) patients; however, only 13 underwent a preoperative shunting procedure (Table 1).
3.3 Surgical Findings and Long-Term Outcome
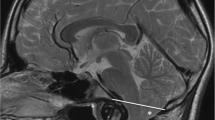
Among children, 178 patients underwent PFDD, while the other 12 were submitted to bone decompression only. In 188 children, C1 laminectomy was also performed. In particular, 7 out of 12 bone decompression only were indicated in craniosynostosis patients. The posterior arch of C2 was never removed. Almost all these findings were in total accordance with the CD indications (Table 2). In the adult population, PFDD was performed on 217 patients (98.6%); only 3 patients underwent posterior fossa decompression without duraplasty (PFD); only 1 patient received craniovertebral junction fixation. Every patient underwent C1 laminectomy; the C2 arch was partially eroded in 130 patients due to significant tonsillar descent. Nevertheless, we tried to preserve the muscular and ligamentous system to guarantee stability in such cases (Fig. 1).
Regarding the whole series, isolated or bilateral tonsil coagulation, due to severe stenosis or tonsils’ herniation, was carried out in 125 cases (31.8%), mainly upon intact arachnoid. The average length of stay was 4.5 days. The overall complication rate was 10.9%, principally due to CSF leakage and collection, but a surgical treatment for this specific issue was necessary only in 9 cases (2.3%). Other minor complications, such as partial wound dehiscence, were rapidly resolved. No deaths, respiratory failures, or intracranial infections occurred.
The average follow-up duration was 4.5 ± 3.3 years, with a minimum of 0.33 years (4 months) and a maximum of 21 years. Symptoms disappeared early in 314 cases, and Syr showed a favorable evolution (progressive and persistent reduction or disappearance) in 216 affected patients. However, a late second surgery was proposed in 52 cases due to the persistence of symptoms or Syr secondary to reactive arachnoiditis. Among these patients, 36 represented a re-do surgery of patients previously treated at different centers (Table 3). These results overcome the agreement obtained about the statement indicating that CM1 adults with persistent symptoms and Syr, and without MRI improvement at 6- or 12-month follow-up, should be submitted to a second surgery.
4 Discussion
Our retrospective analysis showed homogeneity in the management and treatment of CM1 patients, both for adults and children. Nevertheless, we aimed to evaluate whether our results were comparable to other reported series and experts’ opinions [6, 12]. Considering the difference among neurosurgeons involved in CM1, the CM1-Syr Consensus Conference (Milan, 2019) proposed a CD. This document reported shared indications by 47 international experts, coming from 27 centers pooling 27,000 cases, based on the experience of high-volume referral units for the CM1-Syr associations’ network. The CD produced, by using the Delphi method in 3 rounds, 121 detailed recommendations on CM1-Syr regarding diagnosis, treatment, outcome, and follow-up. Besides answering the patients’ questions on the correct therapeutic and diagnostic pathway, the main advantage of CD was to create a common language between clinicians for CM1-Syr diagnosis, symptoms, surgical outcomes, and failures, also allowing a comparison between series treated by different techniques. Some statements obtained a very high consent among experts. The first one is related to adult CM1 with syringomyelia [10]: despite the presence of symptoms, with or without symptoms, patients should undergo surgery if a holocord syringomyelia is present, or in case of clinical or radiologically worsening progression over time, or if the Vaquero index is greater than 0.5 (defined by syrinx/canal ratio) [13], or in syringobulbia [14, 15]. This statement had an agreement higher than 95% among experts, and our series fully reflect this statement. Almost all adults with Syr underwent PFDD, except for one case belonging to the early period of the series. Moreover, there is a complete agreement about treating symptomatic CM1 in adult patients without Syr in the case of persistent headache (typical, invalidating, and resistant to therapy) associated with cerebellar, auditory, bulbar, or spinal signs. The surgical statements were more complex in pediatric CM1 [11]. In symptomatic CM1 children without Syr, there was 80% agreement to suggest bony decompression only (but after a detailed discussion with patients and relatives), with the perspective of a possible second surgery; on the other hand, the agreement was always >80% in CM1 with Syr children to perform PFDD. The CD had a higher consent >90% on the need for a pediatric neurology definition of CM1 symptoms to indicate surgery; our series shows a complete agreement with all such statements.
The management of CM1 associated with craniosynostosis is not following the CD, which suggests (with an agreement >90%) to treat the craniosynostosis before the CM1. Our series comprises a high percentage of associated craniosynostosis (21%), mainly isolated (31 children), usually discovered after CM1 because actively searched by 3D-CT. As a result, only in 12.9% of cases, surgery for craniosynostosis was performed before CM1 treatment. During the last years, we paid more attention, also in older children, to the association among the two diseases to better recognize secondary CM1 [16], and we experienced no need for PFD after Cranial Vault expansion in two cases. On the contrary, in the nine cases with complex synostosis, well known before CM1, the accordance with CD was good
In the case of concomitant hydrocephalus, our current operative approach includes a shunting procedure such as VPS or ETV prior to PFDD; however, it is not a firm rule, and the decision depends on the clinical decision and radiological presentation. PFDD is then accomplished in patients who have not improved after the shunting operation. Despite these premises, the series analysis shows that associated hydrocephalus in children was present in 25 cases (13.1%), but only 15 of them underwent a shunting procedure before posterior fossa decompression. This low agreement (60%) with CD can present some speculative hypotheses, mainly related to the previous hypothesis that PFDD may solve a ventricular dilatation, improving CSF circulation; the higher complication rate experienced in these cases led us recently to a stricter adherence to this Consensus statement.
Four children underwent an intradural untethering procedure (two children before and two after PFD) for a true tethered cord, associated with CM1. Patients submitted to untethering depicted no relief of CM1 symptoms and no radiological improvement of tonsil descent, reinforcing the hypothesis that this quite rare association has a polimalformative rather than a causative relation. Some groups have advocated this procedure as a potential, less invasive approach to the CM1; however, considering the lack of robust scientific evidence for this procedure, especially in case of the absence of tethered cord syndrome, such technique plays no role in the treatment of CM1, due to complete different pathophysiology [17, 18]. There is a total agreement among experts about the lack of usefulness of untethering procedures to manage a possible CM1 syndrome, and our series followed and confirmed this statement.
About the surgical technique, our series shows a significant concordance with the CD statements but considering that the general indications should be shaped around the single institution or surgeons. For example, it is accepted that an excessive craniectomy may cause cerebellar ptosis, but we do not follow a strict measurement rule, and we determine the extension of the decompression based on intraoperative findings, mainly occurrence of dural pulsations. C1 laminectomy was always carried out, with a partial C2 posterior arch erosion due to significant tonsillar herniation and obstruction. The issues regarding C2-laminectomy are secondary to the possible postoperative onset of kyphotic deformity and secondary craniovertebral junction (CVJ) instability [19]; however, no such complications occurred in our study. This may depend on the careful preservation of the C2 muscular and ligamentous complex.
Concerning the risks and benefits of duraplasty or tonsil coagulation, the surgical experience and the results of some meta-analyses [6, 7, 9, 20,21,22,23] led us to utilize PFDD in CM1 patients with Syr due to the better symptomatic and syrinx results. We did not run into any complications such as scar tissue or neurological deficits by cerebellar tonsils reduction. PFDD is also associated with a higher risk of CSF-related complications: in a recent meta-analysis [22], CSF leak rates were reported to be around 8%, ranging from 3.44% to 11.23%; our results did not differ significantly, with CSF leaks occurring in 10% of the patients, despite that it drops down to 2.3% considering just the cases deserving surgical revision.
Regarding the type of duraplasty, we tend to use pericardium-based heterologous products, mainly equine or less frequently bovine pericardium. The patch is then sewn in a waterproof fashion with non-resorbable stitches to prevent CSF leaks. This point was reached during the years, while previously we used adsorbable sutures, and most of the CSF leaks or collection requiring surgery were related to the old approach. Furthermore, we prefer to leave the arachnoid membrane intact in the absence of significant arachnoid scarring. In contrast, spontaneous arachnoid interruption with arachnoiditis was identified after dural opening, mainly in patients with the longest history or extended descendants of tonsils [10].
Finally, about the postoperative management, we agree with the statement indicated by CD: in case of effective surgery, the long-term postoperative follow-up in children and adults is performed by a clinical examination and whole neuraxis MRI for at least 10 years, or until the end of growth, with a timetable depending on clinical and MRI patterns.
In conclusion, the CM1 has been known for more than 100 years; however, many debates are still ongoing, in the present time, about the exact definition and treatment options. When, if, and how to treat CM1 patients often depends on the different surgeons and centers’ thinking, but the introduction of the CD (based upon experts’ agreement) could reduce this variability and obtain comparable series. The present series analysis on 446 consecutive patients, treated according to the CD, confirms that PFD is a safe surgical option for the treatment of CM1, in particular for Valsalva-exacerbated symptoms; the addition of duraplasty, in our hands, showed an overall better outcome, especially in patients with Syr, at the low cost of CSF-related complications rate (2.3%). This effectiveness was confirmed by the few cases (7.6%) requiring additional surgeries due to recurrent symptoms, postoperative complications, or Syr persistence.
References
Chiari H. Ueber Veränderungen des Kleinhirns infolge von Hydrocephalie des Grosshirns. Dtsch Medizinische Wochenschrift. 1891;17(42):1172–5.
Kahn EN, Muraszko KM, Maher CO. Prevalence of Chiari I Malformation and syringomyelia. Neurosurg Clin N Am. 2015;26(4):501–7.
Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, Speer MC. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–17.
Beretta E, Vetrano IG, Curone M, Chiapparini L, Furlanetto M, Bussone G, Valentini LG. Chiari malformation-related headache: outcome after surgical treatment. Neurol Sci. 2017;38(Suppl 1):95–8.
Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008;2(1):42–9.
Förander P, Sjåvik K, Solheim O, Riphagen I, Gulati S, Salvesen Ø, Jakola AS. The case for duraplasty in adults undergoing posterior fossa decompression for Chiari I malformation: a systematic review and meta-analysis of observational studies. Clin Neurol Neurosurg. 2014;125:58–64.
Hankinson T, Tubbs RS, Wellons JC. Duraplasty or not? An evidence-based review of the pediatric Chiari I malformation. Childs Nerv Syst. 2011;27(1):35–40.
Hoffman H, Bunch KM, Paul T, Krishnamurthy S. Comparison of pericranial autograft and AlloDerm for duraplasty in patients with Type I Chiari malformation: retrospective cohort analysis. Oper Neurosurg. 2021;21(6):386–92.
Lin W, Duan G, Xie J, Shao J, Wang Z, Jiao B. Comparison of results between posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari Malformation Type I: a systematic review and meta-analysis. World Neurosurg. 2018;110:460–474.e5.
Ciaramitaro P, Massimi L, Bertuccio A, et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: international consensus document. Neurol Sci. 2022;43(2):1327–42.
Massimi L, Peretta P, Erbetta A, et al. Diagnosis and treatment of Chiari malformation type 1 in children: the International Consensus Document. Neurol Sci. 2022;43(2):1311–26.
Saletti V, Farinotti M, Peretta P, Massimi L, Ciaramitaro P, Motta S, Solari A, Valentini LG. The management of Chiari malformation type 1 and syringomyelia in children: a review of the literature. Neurol Sci. 2021;42(12):4965–95.
Vaquero J, Martinez R, Arias A. Syringomyelia-Chiari complex: magnetic resonance imaging and clinical evaluation of surgical treatment. J Neurosurg. 1990;73(1):64–8.
Chavez A, Roguski M, Killeen A, Heilman C, Hwang S. Comparison of operative and non-operative outcomes based on surgical selection criteria for patients with Chiari I malformations. J Clin Neurosci. 2014;21(12):2201–6.
Ciaramitaro P, Garbossa D, Peretta P, et al. Syringomyelia and Chiari Syndrome Registry: advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann Ist Super Sanita. 2020;56(1):48–58.
Valentini LG, Saletti V, Erbetta A, Chiapparini L, Furlanetto M. Chiari 1 malformation and untreated sagittal synostosis: a new subset of complex chiari? Childs Nerv Syst. 2019;35(10):1741–53.
Massimi L, Peraio S, Peppucci E, Tamburrini G, Di Rocco C. Section of the filum terminale: is it worthwhile in Chiari type I malformation? Neurol Sci. 2011;32(Suppl 3):S349–51.
Milano JB, Barcelos ACES, Onishi FJ, et al. The effect of filum terminale sectioning for Chiari 1 malformation treatment: systematic review. Neurol Sci. 2020;41(2):249–56.
Rocque BG, Oakes WJ. Surgical treatment of Chiari I malformation. Neurosurg Clin N Am. 2015;26(4):527–31.
Chai Z, Xue X, Fan H, Sun L, Cai H, Ma Y, Ma C, Zhou R. Efficacy of posterior fossa decompression with duraplasty for patients with Chiari Malformation Type I: a systematic review and meta-analysis. World Neurosurg. 2018;113:357–365.e1.
Lu VM, Phan K, Crowley SP, Daniels DJ. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation Type I: a systematic review and meta-analysis of surgical and performance outcomes. J Neurosurg Pediatr. 2017;20(5):439–49.
Xu H, Chu LY, He R, Ge C, Lei T. Posterior fossa decompression with and without duraplasty for the treatment of Chiari malformation type I—a systematic review and meta-analysis. Neurosurg Rev. 2017;40(2):213–21.
Yahanda AT, Simon LE, Limbrick DD. Outcomes for various dural graft materials after posterior fossa decompression with duraplasty for Chiari malformation type I: a systematic review and meta-analysis. J Neurosurg. 2021;1–14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflicts of interest to declare.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Valentini, L.G. et al. (2023). Evaluation of Adult and Pediatric Chiari Type 1 Malformation Patients: Do Consensus Documents Fit Everyday Practice?. In: Visocchi, M. (eds) The Funnel: From the Skull Base to the Sacrum. Acta Neurochirurgica Supplement, vol 135. Springer, Cham. https://doi.org/10.1007/978-3-031-36084-8_24
Download citation
DOI: https://doi.org/10.1007/978-3-031-36084-8_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36083-1
Online ISBN: 978-3-031-36084-8
eBook Packages: MedicineMedicine (R0)