Abstract
Bioaccumulation of waterborne pharmaceutical residuals may be influenced by the presence of microplastics in the surrounding water and therefore, this study investigated whether in vivo co-exposure to weathered polyethylene microplastics (PE) (nominal 1 mg/L) and bezafibrate (BZ) (500 ng/L) add-on effects on immune function in comparison to exposure to BZ alone, as it has been observed for other emergent contaminants. Mytilus galloprovincialis was used as model organism and the co-exposure study was carried out for 3 weeks. Pyrolysis–gas chromatography mass spectrometry (Py-GC/MS) technique was used for quantifying PE concentration in hemolymph. Lysosomal membrane stability (LMS) and phagocytosis efficiency of hemocytes, extracellular lysozyme activity and production of oxyradicals were determined as endpoints of immune function after 10 and 20 days. PE concentration in hemolymph of mussels treated with PE and PE+BZ were similar and had a high variability (hundreds of nanograms per milliliters) but values were one order of magnitude higher than mussels treated only with BZ, representing the first quantitative estimation of microplastic concentration that can be reached in this tissue under in vivo exposure conditions. However, still It remains to be determined what fraction of plastic particles were present in the plasma and what fraction carried by hemocytes. Mussels exposed to BZ and co-exposed to BZ+PE had significantly higher phagocytic rate than control mussels after 10 days, but this induction was transitory as phagocytosis efficiency was rather similar between treatments and control after 20 days. Lysosomal destabilization was found only after 20 days of exposure to BZ and BZ+PE, but not after exposure to PE. Overall, results obtained in this in vivo study showed that the BZ+PE co-exposure did not exert synergic immunotoxic impact on mussels and that effects of BZ on immune function prevailed over the effects caused by the PE microparticles itself.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
31.1 Introduction
The pharmaceuticals are introduced into surface waters and oceans due to incomplete removal of pharmaceuticals in wastewater treatment plants. Their occurrence in marine organisms raises concerns regarding toxic effects and development of drug-resistant genes [1, 2]. To date, pharmaceuticals detected in marine organisms and seafood include anti-inflammatories, antibiotics, oral contraceptives, and blood lipid regulators. Notably, drugs of the fibrate class are used as blood lipid regulators to treat hypertriglyceridemia. Fibrates act by stimulating nuclear receptors called peroxisome proliferator-activated receptors (PPARs). PPARs play an essential role in the general transcriptional control of numerous cellular processes, including lipid metabolism, glucose homeostasis, cell cycling, cell differentiation, inflammation, and extracellular matrix remodeling. Bezafibrate (BZ) is a lipid regulator that can be present as a contaminant in surface water in concentrations of ng/L [1, 3]. Some studies have revealed possible toxic effects in aquatic species caused by exposure to fibrates, ranging from general toxicity to endocrine disrupting effects and immune function [4,5,6].
The abundance of microplastics (MPs) in surface waters, sediments, and biota from the Mediterranean region are among the highest levels reported worldwide [7]. Therefore, bioaccumulation of waterborne pharmaceutical residuals can be influenced by the presence of microplastics in the surrounding water. The marine mussel Mytilus sp. presents some ecological and physiological characteristics that make it a good model for studying chemical contamination and contamination by microplastics, as well as an excellent model to investigate the effects of such contaminants on immune function [8]. The present study investigated whether in vivo co-exposure to weathered polyethylene microplastics and bezafibrate caused add-on effects on immune function in mussels (Mytilus galloprovincialis) exposed solely to bezafibrate, as it has been observed for other contaminants.
31.2 Experimental
31.2.1 Materials
Marine mussel Mytilus galloprovincialis [4–5 cm] were acquired from a Mediterranean myticulture farm located in Benalmádena (Málaga, Spain) (February 2021) and immediately transferred to the laboratory (Murcia, Spain), where they were acclimatized in a 500 L tank with aerated filtered natural seawater (FSW) (37 PSU and 19-20 ºC) until their use.
Polyethylene (PE) microparticles used in this study are produced for industrial purposes (commercially named MPP-635XF) and were acquired from Micro Powders, Inc. (USA). They have a crystalline (heterogeneous) form, a density of 0.96 g cm3 at 25 ºC and mean size of 4–6 µm. MPs were suspended in filtered seawater (37 PSU) and stored in closed quartz glass Erlenmeyer flasks for four weeks. During this period, flasks were exposed to natural sunlight (July, 14 h sunlight/day) and outdoor ambient temperatures (ranging from 18 to 36 ºC). Afterward, the MPs were filtered, rinsed twice with FSW, and filtered to recover. Bezafibrate (CAS Number 41859-67-0) was purchased from Sigma-Aldrich. A stock solution of 101.8 mg L−1 in methanol was prepared and stored in the dark at 4 °C until use.
31.2.2 Methods
Five days before the experiments, mussels were translocated to 15L to continue the acclimatization period. The volume of FSW per mussel in the aquariums was adjusted to a ratio 1:4. Bivalves were exposed by triplicate for 10 and 21 days to four treatments:
-
Control seawater (Filtered SW)
-
PE = Polyethylene microparticles in FSW (1 mg L−1)
-
BZ = Bezafibrate in FSW (500 ng L−1)
-
PE + BZ = Polyethylene microparticles (1 mg L−1) + Bezafibrate (500 ng L−1) in FSW
In the case of PE + BZ treatment, PE microparticles were previously incubated with BZ in 200 mL of FSW using a glass bottle (24 h at 15 ºC) to allow potential adsorption of BZ on the PE plastic surface. After incubation, the suspension was added to the volume of FSW in the aquarium to get a final concentration of 1 mg L−1. Aquarium water changes were performed 3 times a week, while PE load management and BZ dosage in the aquariums were performed every day and before feeding (Tetraselmis suecica/Tisochrysis lutea; adjusted to 1% of mussels dry weight). Bivalve mortality was checked daily. On days 0, 10, and 21, hemolymph samples were collected from mussels to measure immune parameters and concentrations of PE in the hemolymph.
31.2.2.1 Preparation of Samples
Hemolymph samples were obtained of a least 12 mussels for each treatment (four samples by triplicated). Hemolymph was withdrawn from the anterior adductor muscle. Individual primary cell culture samples (n = 12) were prepared for lysosomal membrane stability and phagocytosis analysis following the procedure described in [9]. Additionally, three hemolymph pooled samples (n = 4) by treatment were prepared to analyze extracellular lysozyme activity, extracellular reactive oxygen species (ROS) production, and PE concentration. Aliquots (250 µL) of pooled hemocyte suspension were diluted in an equal volume of TBS for ROS analysis. Aliquots of serum of hemocytes (centrifugation hemolymph pooled samples at 3000 g for 10 min at 4 ºC) were used for lysozyme activity analysis. We measured PE concentration in pellets obtained from pooled hemocyte suspensions after centrifugation.
31.2.2.2 Analytical Techniques
Lysosomal membrane stability (LMS) was measured by the neutral red retention assay (NRR) following the procedure described by [9]. Lysosomal enzyme release by mussel hemocytes was evaluated by measuring lysozyme activity spectrophotometrically at 450 nM in the extracellular medium as described by [4]. The decrease in absorbance was continuously recorded at 450 nm for 5 min at room temperature. The results were expressed as Units of lysozyme ·mg protein−1. Phagocytic efficiency (PhE) was measured using fluorescent microscopy (×400; Olympus BX43), following the methodology described by [9]. PhE was expressed as a percentage of cells that have internalized at least 3 fluorescent particles after counting at least 300 cells for each sample. The extracellular production of oxyradical by hemocytes was measured by the reduction of cytochrome c following the procedure described in [4]. Samples were read at 550 nm for 20 min using a TECAN SpectraFluor, and results were expressed as changes in optic density per mg protein.
Size exclusion liquid chromatography coupled to high-resolution mass spectrometry equipped with an Atmospheric pressure photoionization source (SEC-APPI-HRMS), working in negative conditions, was used to quantify PE concentration in the hemolymph. The separation of the polymer was based on toluene as a carrier liquid and separated in a polymeric size exclusion (APC) column. The acquisition was made in full scan mode working from 500 to 3,000 Da at a resolution of 17,500 FHWM. The quantification of PE was carried out by comparison with the calibration curve standard of a polymer of 1000 Da compared to samples. The quantification was based on equivalents of concentration.
31.3 Results and Discussion
31.3.1 Results
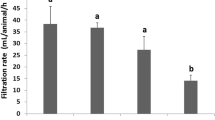
The control mussels had a low PE concentration at the start of the experiment (0.170 ± 0.087 ng mL−1). However, control mussels, after 10 and 20 days, had values of PE concentration in hemolymph within the same order of magnitude as mussels exposed to 10 mg L−1 of PE (confidence interval for the mean 95% = 506.4–772.9 ng mL−1). Low residual concentrations were found in mussels exposed only to BZ after 10 and 20 days (CI for the mean 95% = 13.1–69.0 ng mL−1). Overall, a significant increase in PE concentrations over time exposure was not observed in any treatment except the Control SW (Fig. 31.1).
Moderate effects on lysosomal membrane stability were only observed in mussels exposed during 20 days to BZ and co-exposed to BZ + PE. The effects of co-exposure BZ + PE on LMS were more pronounced than the effects of exposure to PE, but significant differences were not proven (Fig. 31.2). Stimulation of phagocytosis was found in all treatments after 10 and 20 days of exposure, but it was significantly higher in mussels exposed to PE than in the rest of the treatments. Nevertheless, stimulation of phagocytosis was higher in mussels co-exposed to PE + BZ than in mussels exposed only to BZ (Fig. 31.1). In control samples, lysozyme activity in the extracellular medium ranged from 33 to 85 U µg prot.−1. Overall, any treatment-related pattern was observed, although values close to zero activity were found after 20 days in mussels exposed to PE. Extracellular ROS production was similar in individual exposures (PE; BZ) and co-exposure conditions (PE + BZ) (Fig. 31.2). However, significantly higher ROS production was measured after 20 days than at time 0 in Control SW treatment.
Effect on immune function responses in mussel hemolymph (Mytilus galloprovincialis) exposed to control seawater (Control SW), waterborne polyethylene microparticles (PE) (1 mg L−1, nominal concentration), PE, and bezafibrate (BZ) (1 mg PE L−1 + 500 ng L−1 BZ) and BZ (500 ng L−1) during 10 and 20 days. Lysosomal membrane stability quantifies as neutral red retention time (NRRT). Data are the Mean ± Standard Error of three experiments (n = 4) by triplicate * = p-value ≤ 0.05 (significant difference against respective control). BAC = Background Assessment Criteria; EAC = Environmental Assessment Criteria
31.3.2 Discussion
Interpretation of immune responses after exposure and co-exposure conditions of PE and BZ of this study are limited given that chemical analysis pointed to a PE contamination in control treatments along the experimental exposure, and a significant increase of phagocytosis activity and extracellular ROS production in Control SW treatment after 20 days. One possible explanation could be a human error caused by the dosage of PE in the control aquariums. Another potential explanation could be the aerial crossed-contamination between adjacent aquariums caused by handling during cleaning tasks, and by the aerosols produced during the air bubbling in the aquariums. However, low PE concentration found in control mussels at the beginning of the experiment and small residual concentration in mussels exposed only to BZ after 10 and 20 days give robustness to the quantification methodology and allow specific comparisons. This is the first quantitative estimation of microplastic concentration that can be reached in this tissue under in vivo exposure conditions. However, it remains to be determined what fraction of plastic particles was present in the plasma and what fraction carried by hemocytes.
Previous studies have proven that in vitro exposure to BZ stimulates phagocytosis in hemocytes of the freshwater mussel Elliptio complanata and marine mussel Mytilus galloprovincialis [10, 11], which is in agreement with our results. Furthermore, marine mussels in vivo injected with BZ showed a concentration-dependent lysosomal destabilization and extracellular lysozyme release [11]. In this sense, our results pointed to a cumulative effect of BZ on the lysosomal membrane over time, requiring an exposure of 500 ng/L for at least 20 days to observe moderate cellular stress on lysosomal destabilization (NRRT > 50 min). Co-exposure of mussels to PE + BZ stimulated phagocytosis response and decreased LMS in hemocytes to a greater extent than single exposure to BZ, suggesting some add-on effects on immune function, which were however not statistically proven. One possible explanation for the pattern observed could be non-linear bioaccumulation of BZ in mussels, as suggested by [11], and higher bioaccumulation of BZ in co-exposed mussels as a consequence of BZ sorption on PE particle’s surface. In any case, chemical bioaccumulation data of this drug in mussels (currently ongoing process) are needed to fully elucidate the behaviour and kinetics of accumulation. However, longer exposure times would be necessary to fully understand if co-exposure to weathered polyethylene microplastics and bezafibrate caused add-on effects on immune function compared to exposure to bezafibrate alone.
31.4 Conclusions
The occurrence of PE in mussel hemolymph is a direct consequence of its presence in the surrounding waters. Overall, this in vivo short-term exposure study showed that co-exposure to BZ + PE does not significantly alter the immune function of mussels in comparison to exposure to bezafibrate alone, and that the effects of BZ on immune function outweighed the effects induced by the PE microparticles themselves. However, results point that in vivo, at longer exposure times, the additive effects of BZ + PE on hemocyte parameters could be significant, and further research is necessary.
References
Metcalfe C, Miao X-S, Koenig BG, Struger J (2003) Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes Canada. Environ Toxicol Chem 22:2881–2889
Madikizela LM, Ncube S (2022) Health effects and risks associated with the occurrence of pharmaceuticals and their metabolites in marine organisms and seafood. Sci Total Environ 155780
Daughton C, Ternes T (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Persp 107:907–938
Canesi L, Ciacci C, Betti M, Fabbri R, Canonico B, Fantinati A, Pojana G (2008) Immunotoxicity of carbon black nanoparticles to blue mussel hemocytes. Environ Int 34(8):1114–1119
Rosal R, Rodea-Palomares I, Boltes K, Fernández-Piñas F, Leganés F, Gonzalo S, Petre A (2010) Ecotoxicity assessment of lipid regulators in water and biologically treated wastewater using three aquatic organisms. Environ Sci Pollut R 17(1):135–144
Velasco-Santamaría YM, Korsgaard B, Madsen SS, Bjerregaard P (2011) Bezafibrate, a lipid-lowering pharmaceutical, as a potential endocrine disruptor in male zebrafish (Danio rerio). Aquat Toxicol 105(1–2):107–118
Papadimitriu M, Allinson G (2022) Microplastics in the Mediterranean marine environment: a combined bibliometric and systematic analysis to identify current trends and challenges. Microplast Nanoplast 2(1):1–25
Canesi L, Ciacci C, Balbi T (2016) Invertebrate models for investigating the impact of nanomaterials on innate immunity: the example of the marine mussel Mytilus spp. Curr Bionanotechnol (Discontinued) 2(2):77–83
Martínez-Gómez et al (2019) In vitro effects of mercury (Hg) on the immune function of mediterranean mussel (Mytilus galloprovincialis) are enhanced in presence of microplastics in the extracellular medium. In: Cocca MC, Di Pace E, Errico ME, Gentile G, Montarsolo A, Mossotti R, Avella M (eds) Proceedings of the 2nd international conference on microplastic pollution in the Mediterranean sea, pp 27–33. Springer, Cham
Gagné F, Blaise C, Fournier M, Hansen PD (2006) Effects of selected pharmaceutical products on phagocytic activity in Elliptio complanata mussels. Comp Biochem Physiol C: Toxicol Pharmacol 143(2):179–186
Canesi L, Lorusso LC, Ciacci C, Betti M, Regoli F, Poiana G, Marcomini A (2007) Effects of blood lipid lowering pharmaceuticals (bezafibrate and gemfibrozil) on immune and digestive gland functions of the bivalve mollusc, Mytilus galloprovincialis. Chemosphere 69(6):994–1002
Acknowledgements
The Spanish Inter-Ministerial Science and Technology Commission sponsored this study (PLAS-MED project; CICYT, CTM2017-89701-C3-3-R).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Martínez-Gómez, C., Llorca, M., Oporto, T., Rapuano, S., del Mar García-Pimentel, M., Farré, M. (2023). Quantification of Polyethylene in Mussel Hemolymph and Its Limited Additive Effect on Immune Function Induced by Bezafibrate. In: Cocca, M., Ambrogi, V., Avolio, R., Castaldo, R., Errico, M.E., Gentile, G. (eds) Proceedings of the 3rd International Conference on Microplastic Pollution in the Mediterranean Sea. ICMPMS 2022. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-031-34455-8_32
Download citation
DOI: https://doi.org/10.1007/978-3-031-34455-8_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34454-1
Online ISBN: 978-3-031-34455-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)