Abstract
The present study was conducted to assess, for the first time, the effects of a 14 days experimental exposure to polyethylene (PE) based MPs (40–48 µm) on the clam Ruditapes decussatus. Clams were exposed to three different concentrations of MPs in controlled laboratory conditions: 10 µg/L (low), 100 µg/L (medium), and 1000 µg/L (high). The effects of MPs were assessed using a multi-marker approach, including the filtration rate, growth, and the integrity of immune cells (such as haemocyte numbers, viability, and lysosomal membrane destabilization). The results revealed that as the concentration of PE-MPs increased, the filtration rate decreased, indicating that PE-MPs hindered the clams’ ability to filter water. Furthermore, there was a noticeable decrease in the overall weight of the clams, particularly in the group exposed to 1000 µg/L. This decrease could be attributed to the impairment of their nutrient filtration function. In terms of immune system biomarkers, exposure to PE-MPs led to immune system disruption, characterized by a significant increase in the number of haemocytic cells, especially in the group exposed to the high concentration. Additionally, there was a notable reduction in the viability of haemocytes, resulting in the destabilization of their lysosomal membranes, particularly in the groups exposed to medium and high PE-MPs concentrations. The findings of this study indicate that the sensitivity of hemolymph parameter changes and filtration rate in R. decussatus exposed to PE-MPs (100 and 1000 µg/L), surpasses that of growth performance and can serve as reliable indicators to assess habitat conditions and contaminant levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine litter encompasses persistent waste materials, either manufactured or transformed into solid substances, which ultimately find their way into the ocean. Among the various materials falling under the category of marine litter, plastics have emerged as significant contaminants of concern (Barnes et al. 2009). Plastics are synthetic organic polymers that possess the ability to be molded into diverse shapes and products (Worm et al. 2017), exhibiting high durability and light weight. The continuous surge in synthetic plastic production, coupled with inadequate waste management practices, has led to a substantial accumulation of these materials in aquatic environments. It has been indicated that the annual entry of plastic waste into the ocean ranges from 4.8 to 12.7 million tons (Jambeck et al. 2015), with approximately 80% originating from land-based activities (Andrady 2011). Plastics production is undergoing an unparalleled surge, with an exponential growth that contrasts with its relatively modest levels from 1910 to 1950. During that period, global plastics production remained around 1.7 million tons. However, since the conclusion of World War II, the production has skyrocketed, culminating at approximately 390.7 million tons in 2021, according to PlasticsEurope (2022).
Microplastics (MPs), which vary in size from 100 nm to 5 mm (Ng et al. 2018), have garnered significant attention from the scientific community and society due to their emergence as a global contaminant. They are categorized into two groups based on their origin. Primary MPs, constituting approximately 15–31% of naturally occurring MPs, are manufactured for industrial purposes. On the other hand, secondary MPs, accounting for up to 69% of the total MPs, are formed through the fragmentation of larger plastic items due to exposure to UV rays, heat, biological activity, or mechanical forces such as waves and wind (Cole et al. 2011). These microparticles have become pervasive in the marine environment, being found across various locations such as water surfaces, water columns, sediments, and organisms worldwide (Qu et al. 2018).
Marine organisms have multiple pathways through which they uptake MPs, such as mistaking them for prey or ingesting them through filtration trophic transfer (Nelms et al. 2019; Rist et al. 2019). The potential ecotoxicological effects of MPs have been investigated in a wide range of organisms, revealing that the ingestion of these emerging pollutants by marine organisms can lead to detrimental consequences including physical damage, by blocking or wearing down the food organs and digestive tract, reducing feeding rates, inhibiting growth, causing starvation and death. Additionally, the presence of sharp MPs can inflict damage on the gills or intestinal tissues (Abidli et al. 2021b; Talvitie et al. 2015; Saud et al. 2023). Furthermore, MPs can pose direct toxicity to aquatic organisms through the plastic material itself, and they may also carry toxic substances to these organisms. These substances include toxic additives, heavy metals, and persistent organic pollutants (Al Marshoudi et al. 2023; He et al. 2023; Tiwari et al. 2019; Digka et al. 2018).
Emerging research indicates that MPs have been detected in human blood and organs, as indicated by recent studies (Revel et al. 2018; Leslie et al. 2022). Furthermore, there is a substantial body of literature reporting the harmful effects of MPs on various marine organisms, including bivalve species (Abidli et al. 2021b; Bringer et al. 2020; Sussarellu et al. 2016; Jiang et al. 2022; Rodrigues et al. 2022; Shi et al. 2020; Tang et al. 2020, 2022). In fact, due to their sedentary nature, high capacity for pollutant accumulation, widespread distribution, and ease of sampling (Stankovic et al. 2014), bivalves are frequently utilized as sentinel organisms in ecotoxicological investigations. These filter-feeding organisms are commonly employed as bioindicators in ecotoxicological studies due to their potential to ingest significant quantities of pollutants (Faggio et al. 2018).
The evaluation of the immune system is crucial in ecotoxicological studies focusing on bivalves due to their heightened sensitivity to pollutants (Fournier et al. 2005). Various factors, such as the type and concentration of contaminants, as well as the duration of exposure (acute or chronic), can influence the impact on this system (Fournier et al. 2005; Salo et al. 2005). Pollution-induced disruption of the immune system has been found to result in diverse effects, including immunosuppression, immunostimulation, and hypersensitivity, thereby increasing the risk of infection (Pipe and Coles 1995; Gagné et al., 2015; Shi et al. 2020; Tang et al. 2020, 2022), autoimmune diseases, or cancers (Brousseau et al. 2012). Notably, excessive disturbances can render bivalves susceptible to pathogens like Vibrio sp., which are typically harmless (Gagné et al. 2015). The immune responses of bivalves can manifest in cellular and humoral forms, depending on the encountered risk (Gueguen et al. 2003; Tanguy et al. 2013). Key parameters assessed following contamination include the overall count and viability of haemocytes, their phagocytic capability, and the release of degradative or lysosomal enzymes, cytokines, and reactive oxygen intermediates (Pipe and Coles 1995; Brousseau et al. 2012; Gagné et al. 2015).
The present investigation aimed to assess the potential toxicity of polyethylene (PE) MPs in the clam species Ruditapes decussatus. This particular bivalve species was chosen as a model organism due to its economic significance in the marine industry and previous evidence of MP accumulation in its body (Abidli et al. 2019). The selection of PE polymer for this study was based on prior findings, which revealed that the majority of MPs found in sediments and tissues of molluscs and fishes along the northern coast of Tunisia consist of this polymer type (Abidli et al. 2018, 2019, 2021a; Toumi et al. 2019).
The ecological and toxicological implications of pollutants are typically assessed by examining various physiological, biochemical, and immune parameters in aquatic organisms (Palanikumar et al. 2012). Among these parameters, the filtration rate holds significant importance as it reflects the bivalves’ ability to feed on suspended particles in the water column (Oliveira et al. 2018). Additionally, the measurement of immunological biomarkers is commonly employed in studies investigating the mechanisms of environmental toxicity in organisms exposed to pollutants (Regoli and Giuliani 2014; Faggio et al. 2016).
As MP concentrations in marine environments continue to rise, it is crucial to understand their impact on the biological systems of marine organisms. In this regard, a multi-marker approach was adopted to evaluate the effects of MPs on clams. Specifically, the filtration rate and several immunological biomarkers such as the number and viability of hemocytes and the lysosomal membrane’s stability were measured in the haemolymph of R. decussatus after 14 days of exposure to PE-MPs at concentrations of 10 μg/L, 100 μg/L, and 1000 μg/L.
Material and methods
Animal sampling
A total of 200 adults of the clam species R. decussatus were manually collected from the sandy sediments of the Menzel Jemil site in the Bizerte lagoon, located in Northern Tunisia (latitude 37° 22’ N and longitude 9° 93’ E) at a depth of 0.5 m. The collection took place on July 10th, 2020. To reduce stress on the clams, they were promptly transferred to the laboratory in an ice chest container immediately after sampling.
In the laboratory, the clams were placed in a large glass aquarium with a volume of 50 L. The aquarium was filled with filtered surface seawater obtained from the sampling site using Whatman® GF/C filters with a pore size of approximately 10 μm. To ensure optimal conditions, the seawater (T = 22 °C, S = 33.3 psu, and pH = 7.8) was continuously aerated. The clams were allowed to acclimate in this environment for a period of four days before the start of the experiment. After the acclimation period, biometric measurements were taken, revealing an average length of 20.45 ± 1.27 mm and an average weight of 4.62 ± 1.04 g for the clams. The decision to use filtered seawater was based on a preliminary analysis of MPs in the seawater at the sampling station indicated a concentration of 5 items per liter with MP sizes ranging from 0.5 to 2 mm in length (Unpublished data). Therefore, during the acclimation period, the seawater was filtered using 10 μm filters to remove MPs while allowing the passage of micro-algae such as Phaeodactylum tricornutum, Isochrysis galbana, and Tetraselmis sp, which were consumed by the clams. It is worth noting that no mortality was observed during the acclimation period.
Experimental design
Characterization of MPs in seawater from the Open Sea of Bizerte
Given the proximity of the laboratory to the Open Sea of Bizerte (latitude 37° 26′ N and longitude 9° 88′ E) and to avoid the need for frequent trips to the sampling site during the contamination period to bring seawater, an analysis of the level of MP pollution in the seawater from the Open Sea of Bizerte was carried out. To achieve this, three separate 1-liter replicates of surface seawater were collected in glass bottles and promptly transported to the laboratory. In the laboratory, the seawater samples underwent filtration using Whatman® GF/C filters with a pore size of 1.2 μm. A Millipore vacuum pump was employed for this filtration process. Subsequently, the filters were carefully placed in covered glass petri dishes and left to dry overnight at room temperature. Under a ZEISS KL 1500 LCD stereomicroscope, the characterization of MPs was conducted. Precautions were taken to prevent external contamination, especially from airborne particles. The equipment used for MP extraction was thoroughly rinsed with bi-distilled water prior to use. Furthermore, blank filters without seawater were tested to evaluate potential airborne contamination, and the analysis under the stereomicroscope did not reveal any signs of such contamination. The results of the MP characterization in the seawater from the Open Sea of Bizerte indicated a concentration of 12 MPs per liter, with sizes ranging from 0.2 to 3 mm.
Following the completion of the acclimatization period, on July 14th, 2020, a total of 45 adult clams per treatment (15 individuals per replicate) underwent measurements for total length and weight. Subsequently, they were transferred to separate glass aquaria with a capacity of 10 L. Each aquarium was filled with 5 L of filtered seawater obtained from the Open Sea of Bizerte (S = 34.7 psu, and pH = 8), using filters with a pore size of 1.2 μm. To ensure sufficient oxygen supply, continuous aeration was provided through air diffusers positioned in the middle of each aquarium. The temperature was maintained at 22 °C within a designated acclimatized room, with lighting following the natural photoperiod.
In this study, clams were exposed to PE-MPs, which are the most widely produced plastic polymer according to PlasticsEurope (2022), and also the most commonly encountered type in Tunisian seawater based on previous studies by Abidli et al. (2018, 2019, 2021a). The PE-MPs used in this study were commercially purchased from Sigma Aldrich, USA, and were free of additives. They had a particle size ranging from 40 to 48 μm and possessed an ultra-high molecular weight.
To prepare the stock solution of MPs, 100 mg of PE-MPs were added to 100 ml of filtered seawater (1.2 µm) and mixed for 30 minutes, resulting in a concentration of 1 g/L. Alongside a control group that consisted of filtered seawater without any added PE-MP, three different concentrations of PE-MP were selected for the experimental groups: 10 μg/L, 100 μg/L, and 1000 μg/L. Each concentration was replicated three times. The chosen concentrations were based on the consideration that concentrations below 23 μg/L were deemed representative of coastal areas. Therefore, in this study, a concentration of MPs (10 μg/L) lower than 23 μg/L was selected, as well as two higher concentrations (100 μg/L and 1000 μg/L). To minimize potential airborne contaminants, all aquariums were covered with glass lids throughout the experiment. The seawater in the aquariums was renewed and contaminated with the appropriate concentration of PE-MP three times per week. The physicochemical properties of the filtered seawater used in the experiment were assessed during each water renewal, and no significant changes were observed throughout the contamination period. To prevent interactions between microalgae and MPs, a protocol was implemented during each seawater renewal and contamination event. Specifically, one hour before each renewal, the clams were provided with a feeding of brown microalgae Isochrysis galbana, which has a particle size of 5 μm. The feeding amount was set at 40 µL/L of seawater, following the method described by Revel et al. (2019).
The overall exposure period for the clams lasted for 14 days. At the conclusion of the experiment, it is worth noting that no mortality was observed in either the exposed groups or the control groups across all treatment conditions.
Filtration rate
The determination of the filtration rate (FR) in clams was carried out individually for each clam immediately after the 14-day exposure period, following the method described by Coughlan (1969). This method relies on monitoring the removal of neutral red dye particles from the water column by the clams. In brief, a stock solution of neutral red (1 g/L) was prepared, and from this stock solution, diluted solutions (1 mg/L) were prepared in 100 ml bottles. Nine clams from each treatment (with 3 specimens per replicate, of confused sex) were randomly chosen from the aquaria. These selected clams were then placed individually in 100 mL beakers, each containing 100 µl of the prepared neutral red stock solution (1 g/L), and were left in darkness for a period of 2 hours. Prior to immersing the clams in the neutral red solution, a volume of 10 ml water was extracted from each beaker for the purpose of determining the initial concentration, C0 by measuring the optical density (OD) at 550 nm. After 2 h, the clams were taken out from the beakers, and both the remaining solutions (Ct) and the initial aliquot (C0) were acidified to a pH of 5 with HCl (5%). The concentrations of neutral red were then assessed by performing triplicate absorbance measurements at 550 nm using a Thermo Scientific™ Multiskan™ FC Microplate Spectrophotometer. To establish a standard curve for the dye concentrations, neutral red standards were measured alongside the samples, enabling the extrapolation of the dye concentrations. The FR was determined using the formula: FR = [M / nt] log (C0/Ct), where M represents the volume of the test solution (mL), n denotes the number of clams used, t indicates the duration in hours, and C0 and Ct represent the dye concentrations at the beginning and after 2 hours, respectively. The results were reported as mL/h/individual.
Immunomodulation
To collect haemolymph samples from clams, sterile needle syringes with a capacity of 10 mL were used. The posterior adductor muscles were accessed by puncturing the dorsal side of the shell using forceps. Haemolymph samples were withdrawn and pooled (~1 mL) in 1.5 mL Eppendorf tubes, with two specimens collected from each replicate. To prevent cell agglomeration, the Eppendorf tubes were kept on ice and stored at room temperature (20 °C).
For haemocyte counting, a Malassez counting cell covered by a coverslip was utilized. The haemocytes were counted under light microscope (Olympus CH20) in each replicate. The results were reported as the number of haemocytes per liter of haemolymph.
Haemocyte viability was assessed using the trypan blue staining method. A 30 μL sample of haemolymph taken from the posterior adductor muscle of the clams was placed on a slide and allowed to incubate in a dark room at a temperature of 20 °C for 30 minutes, facilitating cell adhesion to the slide. After removing the excess fluid from the slide, 30 μL of Trypan blue solution (1.2%) was added, and the mixture was left to stand for 5 minutes. The excess dye was then removed, and 30 μL of filtered seawater was added, followed by covering with a coverslip. Under a microscope, viable cells (clear) and dead cells (blue) were counted separately. The results were presented as the ratio of living cells to the total number of cells counted.
Lysosomal membrane stabilization was assessed through the neutral red retention time (NRRT) assay. Neutral red, a lipophilic dye capable of crossing both the cell membrane and lysosomal membrane, was used in this procedure. To begin, after haemolymph collection, a 30 μL drop of the sample was placed on a slide and allowed to incubate in a humid, dark room at a temperature of 20 °C for 30 minutes to enable cell adhesion to the slides. Following the incubation period, the slides were unloaded, and 30 μl of neutral red solution (prepared at a concentration of 144 μg/mL from a stock solution of 28.8 mg/mL dimethyl sulfoxide, DMSO) was added. The mixture was then incubated for 15 minutes. After the incubation period, the excess dye was removed, and 30 μL of filtered seawater was added. The slides were covered with coverslips and examined under a microscope every 15 minutes. The objective was to observe the percentage of cells that exhibited the release of the dye from the lysosomes. In each examination, 10 different fields, each containing 8–10 cells, were assessed. The completion of the test was determined by the time at which 50% of the cells displayed the release of the dye from the lysosomes.
Statistical analyzes
The percentages of cell viability were statistically compared among the different treatments using the Chi-square test with the R i386 3.3.0 software. For the comparisons of biometric parameters, filtration rate, and immunological biomarkers among the different treatments, one-way ANOVA was conducted followed by the HSD Tukey (THSD) post-hoc test using Statistica 8.0 software. Prior to analysis, all data were assessed for homogeneity of variances using Levene’s test in Statistica 8.0 software, and normality using Kolmogorov-Smirnov test in IBM SPSS version 29.0.1.0, and Table 1 provides the detailed findings of these tests for all the data. Statistical significance was considered at p < 0.05, and the results are presented as the mean ± S.D.
Results
Filtration rates
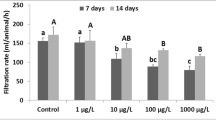
After a 14-day exposure to PE-MPs, the clams in the control group exhibited a higher filtration rate compared to the other treatment groups. The filtration rate of R. decussatus gradually decreased with increasing concentrations of the pollutant, indicating that PE-MPs hindered the clams’ ability to filter water. The difference in filtration rate between the control group (38.33 ± 7.59 mL/animal/h) and the group contaminated with 1000 μg/L (14.12 ± 2.31 mL/animal/h) was found to be statistically significant (F = 7.96; p = 0.001). Among the contaminated groups, a significant difference was observed only between the group exposed to the high concentration of PE-MPs (Fig. 1).
Effect of MPs on the growth of the clam R. decussatus
In order to assess the impact of MPs on the growth of R. decussatus clams, the total weight and total length were measured both at the start and end of the contamination experiment. The results revealed a significant reduction in total weight for clams exposed to 100 µg/L (F = 0.64, P = 0.003) and 1000 µg/L (F = 0.089, P = 0.01) of PE-MPs between the beginning and end of the experiment (Fig. 2). However, no significant difference in total length was observed for any of the treatment groups between the beginning and end of the experiment (Fig. 3).
Effect of MPs on immunological biomarkers in R. decussatus
Haemocytes count
After exposure for 14 days to PE-MPs, the number of haemocyte cells increased significantly (F = 0.06; P = 0.003) in R. decussatus exposed to the high concentration of 1000 µg/L Fig. 4.
Haemocytes viability
After exposing R. decussatus clams to PE-MPs for a period of 14 days, a significant decrease in haemocyte viability was observed among the different treatments. The reduction was particularly pronounced between the control group and the group exposed to the high concentration of 1000 µg/L (χ2 = 105.67, P = 2.2 10−16) (Fig. 5).
Lysosomal membrane stability test
Mean values of NRRT in the clam R. decussatus of all treatments are shown in Fig. 6. The results revealed a significant decrease in NRRT in all MP-treated groups compared to the Control group. The Control group exhibited the highest NRRT value, indicating the absence of lysosomal membrane disruption throughout the entire 120-minute observation period (Fig. 6). Conversely, the lowest NRRT value (15 minutes) was observed in the high concentration of PE-MPs (1000 µg/L) (F = 4.0; P = 0.0002).
Discussion
In recent decades, the release of emerging pollutants into the global environment has presented a significant ecotoxicological challenge. The widespread use of plastic products has resulted in the substantial generation of plastic waste. MPs, which have infiltrated aquatic ecosystems worldwide, necessitate focused ecotoxicological attention to enhance our understanding of the toxicity associated with this class of pollutants. In this study, three concentrations of MPs were chosen, including two environmental concentrations (10 μg/L and 100 μg/L) and one high concentration (1000 μg/L). Additionally, based on previous findings in the lagoon of Bizerte, where the three bivalve species M. galloprovincialis, R. decussatus, and C. gigas were examined, it was determined that the primary type of MPs detected in their tissues consisted of PE polymer (Abidli et al. 2019). Consequently, for this investigation, the same PE polymer was selected. A multi-marker approach was employed to evaluate the effects of PE-MPs on R. decussatus. Parameters assessed included filtration rate, growth, and specific immunological biomarkers, namely, haemocyte cell count, viability test, and lysosomal membrane stabilization (measured from haemolymph collected from the posterior adductor muscle). The findings of this study indicated that exposure to PE-MPs resulted in reduced filtration rates in R. decussatus. These results suggest that this particular species of bivalve is highly sensitive to MP contamination in its habitat. As a consequence of this impaired filtration function, the bivalve’s capacity to obtain nutrients through water filtration may be diminished.
The decrease in filtration rate observed in R. decussatus after exposure to MPs aligns with previous findings in various bivalve species, including Mytilus galloprovincialis (Abidli et al. 2021b), Corbicula fluminea (Oliveira et al. 2018), Atactodea striata (Xu et al. 2017), and Ruditapes philippinarum (Sıkdokur et al. 2020). Oliveira et al. (2018) suggested that this functional impairment could potentially result in starvation and a decrease in the condition index with prolonged exposure. It appears that R. decussatus employs a strategy of closing its valves to avoid contact with these emerging pollutants, thereby reducing the ingestion of PE-MPs. Exposure to PE-MPs caused a significant decrease in total weight in R. decussatus individuals exposed to 100 µg/L and 1000 µg/L concentrations over the course of the experiment. The ingestion of MPs by clams appears to disrupt nutrient uptake, leading to a loss of nutrient filtration function. This disruption can subsequently lead to a decrease in available energy, resulting in weight loss and impaired growth. A research conducted by Trestrail et al. (2021) demonstrated that the presence of MPs (PE-MPs at concentrations of 21.4 µg/L (20 μm), 1720.1 µg/L (75 μm), and 8600.3 µg/L (75 μm), as well as polystyrene MPs (PS-MPs) at a concentration of 21.4 µg/L (20 μm)) caused alterations in the activity of digestive enzymes in the mussel M. galloprovincialis. These changes, in turn, had the potential to disrupt the energy flow within the digestive system, which may explain the observed decrease in growth and reproductive output among invertebrates that have ingested MPs. In Manila clam, R. philippinarum, PS-MPs (5 and 10 μm) significantly increased the rates of respiration and excretion, while significantly reducing feeding and absorption efficiency. Consequently, this led to a substantial decrease in the available energy for growth, ultimately resulting in slower growth rates (Jiang et al. 2022). However, Sussarellu et al. (2016) found no significant difference in the condition index between control groups and those contaminated with PS-MPs (2 and 6 μm; 0.023 mg/L) in the oyster C. gigas. These findings suggest that different bivalve species, such as R. decussatus (in the present study) and C. gigas, may exhibit distinct responses to MP contamination in terms of avoidance strategies and impacts on their condition.
Bivalves rely on circulating haemocytes to perform various physiological functions, including wound repair, shell repair, nutrient digestion and transport, excretion, and internal defense (Donaghy et al. 2009). In the marine environment, bivalves face the challenge of maintaining a robust immune response to survive, as they are exposed to a wide range of pollutants (Avio et al. 2015; Détrée, Gallardo-Escárate (2018); Shi et al. 2019; Liu et al. 2016; Guan et al. 2019). However, it remains unclear whether immunological parameters in the clam R. decussatus are affected by contamination from PE-MPs, as no previous study has addressed this issue. Therefore, this study aimed to investigate the effects of exposure to PE-MPs on several immunological biomarkers in R. decussatus, including total haemocyte count (THC), haemocyte viability, and lysosomal membrane stabilization. The results revealed that after 14 days of exposure, PE-MPs significantly impacted the immune system of R. decussatus. The clam exhibited an increase in haemocyte production in response to MPs, with the magnitude depending on the concentration of the pollutant. The increase in THC could be attributed to either a cell proliferation process or may be indirectly induced by MPs. This indirect effect may be caused by the alteration of the physiological status of digestive cells and other tissues, inducing the multiplication of circulating haemocytes to repair and protect the entire organism. Additionally, there was an elevation in the number of dead haemocytes and a decrease in lysosomal membrane stabilization. These findings suggest that MPs have a detrimental effect on the immune system of R. decussatus, potentially increasing the susceptibility to infection (Pipe and Coles 1995; Gagné et al. 2015) and the risk of autoimmune diseases or cancers (Brousseau et al. 2012). These results highlight the vulnerability of R. decussatus to the immunotoxic effects of MPs. Moore et al. (2006) and Martínez-Gómez et al. (2008) have established criteria for assessing the health status of animals based on the Neutral Red Retention Time (NRRT) assay. According to their findings, animals are considered healthy if the NRRT is equal to or greater than 120 minutes. If the NRRT falls below 120 minutes but remains above 50 minutes, it indicates that the animals are stressed but able to compensate. However, if the NRRT is less than 50 minutes, it suggests that the animals are severely stressed and likely exhibiting pathology. In the present study, clams exposed to 100 and 1000 µg/L of PE-MPs should be categorized as severely stressed, while the 10 µg/L treatment can be considered moderately stressed. The immunotoxicity of MPs has also been investigated in other bivalve species. In the blood clam Tegillarca granosa, PS-MPs (0.5 and 30 μm) induced a significant reduction in haemocyte viability (Shi et al. 2020). This finding indicates that PS-MPs caused immunosuppression in T. granosa, making them highly susceptible to infections. Similarly, Tang et al. (2020, 2022) documented immunotoxicity in the same species, as evidenced by alterations in hematic indexes, disturbing of humoral immune responses, and suppression of haemocyte chemotactic activity. In the case of M. galloprovincialis, the stabilization of lysosomal membranes in haemocytes was assessed after incubation with different concentrations of PS-NH2 (1, 5, and 50 µg/mL). The results demonstrated a concentration-dependent decrease in lysosomal membrane stabilization, with a 50% reduction observed at the highest concentration of PS-NH2 (Canesi et al. 2015). Furthermore, Von Moos et al. (2012) reported significant destabilization of lysosomal compartments in the digestive tissue of Mytilus edulis following exposure to MPs at a concentration of 2.5 g/L. These studies highlight the adverse effects of MPs on the immune system and cellular health of bivalves, underscoring their vulnerability to immunosuppression and physiological disruptions caused by MP contamination.
Conclusion
For the first time, our experimental results highlight that environmentally relevant concentrations of PE-MPs (40–48 µm) are toxic for R. decussatus. Following a 14-day exposure, these emerging pollutants significantly reduced the filtration rate and weight of the clams, particularly in the group subjected to the high concentration of PE-MPs. Furthermore, in terms of immune system biomarkers, exposure to PE-MPs disrupted the immune system, evident by a notable increase in the number of haemocytic cells and a significant decrease in the viability of haemocytes, leading to the destabilization of their lysosomal membranes, particularly in the groups exposed to medium and high PE-MPs concentrations. Further investigations should consider exploring the effects of different MP polymers and prolonged exposure periods. Additionally, studying the combined effects of MPs with other contaminants, such as pharmaceuticals, would help to elucidate the role of MPs as potential vectors of pollutants.
References
Abidli S, Akkari N, Lahbib Y, Trigui El Menif N (2021a) First evaluation of microplastics in two commercial fish species from the lagoons of Bizerte and Ghar El Melh (Northern Tunisia). Reg Stud Mar Sci 41:101581
Abidli S, Antunes JC, Ferreira JL, Lahbib Y, Sobral P, Trigui El Menif N (2018) Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar Coast Shelf Sci 205:1–9
Abidli S, Lahbib Y, Trigui El Menif N (2019) Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar Pollut Bull 142:243–252
Abidli S, Pinheiro M, Lahbib Y, Neuparth T, Santos MM, Trigui El Menif N (2021b) Effects of environmnetally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): Filtration rate and oxidative stress. Environ Sci Pollut Res 28:26643–26652
Al Marshoudi M, Al Reasi HA, Al-Habsi A, Barry MJ (2023) Additive effects of microplastics on accumulation and toxicity of cadmium in male zebrafish. Chemosphere 334:138969
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364:1985–1998
Bringer A, Thomas H, Prunier G, Dubillot E, Bossut N, Churlaud C, Clérandeau C, Le Bihanic F, Cachot J (2020) High density polyethylene (HDPE) microplastics impair development and swimming activity of Pacific oyster D-larvae, Crassostrea gigas, depending on particle size. Environ Pollut 260:113978
Brousseau P, Pillet S, Frouin H, Auffret M, Gagné F, Fournier M (2012) Linking immunotoxicity and Ecotoxicological Effects at Higher Biological Levels. Ecological Biomarkers: Indicators of Ecotoxicological Effects, Amiard-Triquet C, Amiard JC & Rainbow PS (Édit.) CRC Press, Boca Raton. p 132–146.
Canesi L, Ciacci C, Bergami E, Monopoli MP, Dawson KA, Papa S, Canonico B, Corsi I (2015) Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar Environ Res 111:34–40
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Coughlan J (1969) Estimation of filtering rate from clearance of suspensions. Mar Biol 2:356–358
Détrée C, Gallardo-Escárate C (2018) Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol 83:52–60
Digka N, Tsangaris C, Torre M, Anastasopoulou A, Zeri C (2018) Microplastics in mussels and fish from the northern Ionian Sea. Mar Pollut Bull 135:30–40
Donaghy L, Lambert C, Choi KS, Soudant P (2009) Hemocytes of the carpet shell clam (Ruditapes decussatus) and the Manila clam (Ruditapes philippinarum): current knowledge and future prospects. Aquaculture 297:10–24
Faggio C, Pagano M, Alampi R, Vazzana I, Felice MR (2016) Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat Toxicol 180:258–265
Faggio C, Tsarpali V, Dailianis S (2018) Mussel digestive gland as a model tissue for assessing xenobiotics: an overview. Sci Total Environ 636:220–229
Fournier M, Blakley B, Boermans H, Brousseau P (2005) Toxicologicical considerations making the connection between toxicologic and immunotoxicologic studies as these relate to human and ecosystem health. Investigative Immunotoxicology, Tryphonas H, Fournier M, Blakley BR, Smits JEG & Brousseau P (Édit) CRC Press, Boca Raton. p 385–406.
Gagné F, Douville M, Fortier M, Fournier M (2015) Effects of a municipal effluent on freshwater mussel Elliptio complanata following challenge with Vibrio anguillarum. J Environ Sci 37:91–99
Guan X, Tang Y, Zha S, Han Y, Shi W, Ren P, Yan M, Pan Q, Hu Y, Fang J (2019) Exogenous Ca2+ mitigates the toxic effects of TiO2 nanoparticles on phagocytosis, cell viability, and apoptosis in haemocytes of a marine bivalve mollusk, Tegillarca granosa. Environ Pollut 252:1764–1771
Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguière C, Girardot AL, Garnier J, Hoareau A, Bachère E, Escoubas JM (2003) Immune gene discovery by expresses sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene 303:139–145
He W, Liu S, Zhang W, Yi K, Zhang C, Pang H, Huang D, Huang J, Li X (2023) Recent advances on microplastic aging: identification, mechanism, influence factors, and additives release. Sci Total Environ 889:164035
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryma M, Andrady A, Narayan R (2015) Plastic waste inputs from land into the ocean and Kara Lavender Law. Science 347:768–771
Jiang W, Fang J, Du M, Gao Y, Fang J, Jiang Z (2022) Microplastics influence physiological processes, growth and reproduction in the Manila clam, Ruditapes philippinarum. Environ Pollut 293:118502
Kolandhasamy P, Su L, Li J, Qu X, Jabeen K, Shi H (2018) Adherence of microplastics to soft tissue of mussels: a novel way to uptake microplastics beyond ingestion. Sci Total Environ 610:635–640
Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH (2022) Discovery and quantification of plastic particle pollution in human blood. Environ Int 163:107199
Liu S, Shi W, Guo C, Zhao X, Han Y, Peng C, Chai X, Liu G (2016) Ocean acidification weakens the immune response of blood clam through hampering the NF-kappa beta and toll-like receptor pathways. Fish Shellfish Immunol 54:322–327
Martínez-Gómez C, Benedicto J, Campillo JA, Moore M (2008) Application and evaluation of the neutral red retention (NRR) assay for lysosomal stability in mussel populations along the Iberian Mediterranean coast. J Environ Monit 10:490–499
Moore MN, Allen JI, McVeigh A (2006) Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar Environ Res 61:278–304
Nelms SE, Barnett J, Brownlow A, Davison NJ, Deaville R, Galloway TS, Lindeque PK, Santillo D, Godley BJ (2019) Microplastics in marine mammals stranded around the British coast: ubiquitous but transitory? Sci Rep 9:1075
Ng EL, Huerta Lwanga E, Eldridge SM, Johnston P, Hu HW, Geissen V, Chen D (2018) An overview of microplastic and nanoplastic pollution in agroecosystems. Sci Total Environ 627:1377–1388
Oliveira P, Gabriel L, Barboza A, Branco V, Figueired N, Carvalho C, Guilhermino L (2018) Effets des microplastiques et du mercure chez le bivalve d'eau douce Corbicula fluminea (Müller, 1774): Taux de filtration, biomarqueurs biochimiques et bioconcentration du mercure. Ecotoxicol Environ Saf 30:155–163
Palanikumar L, Kumaraguru AK, Ramakritinan CM, Anand M (2012) Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol Environ Saf 75:187–197
Pipe RK, Coles JA (1995) Environmental contaminants influencing immune function in marine bivalve molluscs. Fish Shellfish Immunol 5:581–595
PlasticsEurope (2022) Plastics – the Facts 2022, an analysis of European plastics production, demand, conversion and waste management. 81p
Qu X, Su L, Li H, Liang M, Shi H (2018) Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci Total Environ 621:679–686
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Revel M, Châtel A, Mouneyrac C (2018) Micro(nano)plastics: a threat to human health? Curr Opin Environ Sci Health 1:17–23
Revel M, Lagarde F, Perrein-Ettajani H, Bruneau M, Akcha F, Sussarellu R, Rouxel J, Costil K, Decottignies P, Cognie B, Châtel A, Mouneyrac C (2019) Tissue specific biomarker responses in the blue mussel Mytilus spp. exposed to a mixture of microplastics at environmentally relevant concentrations. Front Environ Sci 7:33
Rist S, Steensgaard IM, Guven O, Nielsen TG, Jensen LH, Møller LF, Hartmann NB (2019) The fate of microplastics during uptake and depuration phases in a blue mussel exposure system. Environ Toxicol Chem 38:99–105
Rodrigues FG, Vieira H, Campos D, Pires SFS, Rodrigues A, Silva AL, Soares A, Oliveira JMM, Bordalo M (2022) Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis. Toxics. 10:43
Salo H, Dautremepuits C, Smits J, Brousseau P, Fournier M (2005) Immune markers in ecotoxicology: a comparison across species. Investigative Immunotoxicology, Tryphonas H, Fournier M, Blakley BR, Smits JEG & Brousseau P (Édit) CRC Press, Boca Raton. p 147–162.
Saud S, Yang A, Jiang Z, Ning D, Fahad S (2023) New insights in to the environmental behavior and ecological toxicity of microplastics. J Hazardous Mater Adv 10:100298
Shi W, Han Y, Guan X, Rong J, Su W, Zha S, Tang Y, Du X, Liu G (2019) Fluoxetine suppresses the immune responses of blood clams by reducing haemocyte viability, disturbing signal transduction and imposing physiological stress. Sci Total Environ 683:681–689
Shi W, Han Y, Sun S, Tang Y, Zhou W, Du X, Liu G (2020) Immunotoxicities of microplastics and sertraline, alone and in combination, to a bivalve species: size-dependent interaction and potential toxication mechanism. J Hazard Mater 396:122603
Sıkdokur E, Belivermis M, Sezer N, Pekmez M, Bulan OK, Kılıç O (2020) Effects of microplastics and mercury on manila clam Ruditapes philippinarum: feeding rate, immunomodulation, histopathology andoxidative stress. Environ Pollut 262:114247
Stankovic S, Kalaba P, Stankovic AR (2014) Biota as toxic metal indicators. Environ Chem Lett 12:63–84
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A (2016) Oyster reproduction is affected by exposure to polystyrene microplastics. PNAS 113:2430–2435
Talvitie J, Heinonen M, Pääkkönen JP, Vahtera E, Mikola A, Setälä O, Vahala R (2015) Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci Technol 72:1495–1504
Tang Y, Han Y, Zhang W, Yu Y, Huang L, Zhou W, Shi W, Tian D, Liu G (2022) Bisphenol A and microplastics weaken the antimicrobial ability of blood clams by disrupting humoral immune responses and suppressing hemocyte chemotactic activity. Environ Pollut 307:119497
Tang Y, Zhou W, Sun S, Du X, Han Y, Shi W, Liu G (2020) Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ Pollut 265:115115
Tanguy M, McKenna P, Gauthier-Clerc S, Pellerin J, Danger JM, Siah M (2013) Sequence analysis of a normalized cDNA library of Mytilus edulis hemocytes exposed to Vibrio splendidus LGP32 strain. Results Immunol 3:40–50
Tiwari M, Rathod TD, Ajmal PY, Bhangare RC, Sahu SK (2019) Distribution and characterization of microplastics in beach sand from three different indian coastal environments. Mar Pollut Bull 140:262–273
Toumi H, Abidli S, Bejaoui M (2019) Microplastics in freshwater environment: the first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia). Environ Sci Pollut Res 26:14673–14682
Trestrail C, Walpitagama M, Miranda A, Nugegoda D, Shimeta J (2021) Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci Total Environ 779:146418
Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Janssen CR (2015) Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ Pollut 199:10–17
Von Moos N, Burkhardt-Holm P, Köhler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46:11327–11335
Worm B, Lotze HK, Jubinville I, Wilcox C, Jambeck J (2017) Plastic as a persistent marine pollutant. Annu Rev Environ Resour 42:1–26
Xu X-Y, Lee WT, Chan AKY, Lo HS, Shin PKS, Cheung SG (2017) Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar Pollut Bull 124:798–802
Acknowledgements
This work was supported by the Laboratory of Environment Bio-monitoring, Faculty of Sciences of Bizerte, University of Carthage, and by the Encouragement of Young Researchers project attributed to Sami Abidli (Reference: 19PEJC05-05) from the Directorate General for Scientific Research, Department of National Research Programs, the Ministry of Higher Education and Scientific Research in Tunisia. Finally, we acknowledge the Editor and the two anonymous reviewers for valuable comments and suggestions that greatly improved the manuscript.
Funding
The authors declare that a partial financial support was received from the Encouragement of Young Researchers project attributed to Sami Abidli (Reference: 19PEJC05-05) from the Directorate General for Scientific Research, Department of National Research Programs, the Ministry of Higher Education and Scientific Research in Tunisia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. SA: sampling of bivalves, analysis of microplastics in samples, contamination of clams in laboratory conditions, dissection of organs, filtration rate and biomarkers analyses and writting the first draft of the manuscript, SZ: dissection of organs, biomarkers analysis. RBY: immunological biomarker analysis and correction of the first draft of the manuscript. YL: sampling of bivalves and statistical analysis. NTM: correction of the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study did not require ethics approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abidli, S., Zaidi, S., Ben Younes, R. et al. Impact of polyethylene microplastics on the clam Ruditapes decussatus (Mollusca: Bivalvia): examination of filtration rate, growth, and immunomodulation. Ecotoxicology 32, 746–755 (2023). https://doi.org/10.1007/s10646-023-02683-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02683-2