Abstract
Angina with no obstructive coronary arteries (ANOCA) is highly prevalent in contemporary populations, shows a relatively higher risk for cardiac events compared to reference subjects, and its management is often challenging. The heterogeneity of the underlying mechanisms of disease is one of the recognized causes of unsatisfactory response to management. There are multiple ANOCA phenotypes and mechanisms of the disease recognized by stress echo. Epicardial artery vasospasm can be elicited by exercise, dobutamine, or dipyridamole after antidote administration or selectively with a hyperventilation test. A similar pattern of regional wall motion abnormality due to true ischemia can be due to angiographically occult coronary artery disease or early-stage cardiomyopathy. Coronary small vessel inappropriate vasoconstriction is identified with reduction (instead of normal increase) in coronary flow velocity after hyperventilation. A fourth pattern is a reduction in coronary flow velocity reserve following vasodilators, suggestive of coronary microvascular disease and accompanied by a subnormal increase in global longitudinal strain during stress. The fifth biomarker is dynamic intraventricular obstruction, best unmasked by upright exercise. The sixth biomarker is altered heart rate reserve, a nonimaging index of abnormal cardiac autonomic function. Each biomarker is a mechanism of disease, a phenotypic hallmark, a risk modulator, and a selective therapeutic target.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary flow velocity reserve
- Coronary microcirculation
- Global longitudinal strain
- Normal coronary arteries
1 Definitions and Epidemiology
The clinical syndrome of “angina and no obstructive coronary artery disease” (ANOCA) is defined by two features: (1) Stable, chronic (several weeks or longer) symptoms suggesting ischemic heart disease such as chest discomfort with both classic and atypical features in terms of location, quality, and inciting factors. (2) Absence of flow-limiting obstruction by coronary angiography (invasive or computed tomographic angiography) as defined by any epicardial coronary artery diameter reduction ≥50% or fractional flow reserve <0.8 [1].
This clinical definition is different from ischemia with no obstructive coronary artery disease (INOCA) which requires as a third, obligatory criterion the objective evidence for myocardial ischemia from the ECG or a cardiac imaging study (echocardiography, nuclear imaging, or magnetic resonance imaging) at rest or during stress (exercise, mental, or pharmacological) [1].
A different entity is a myocardial infarction with angiographically normal coronary arteries (MINOCA), which also requires the absence of obstructive coronary artery disease but in presence of the universal acute myocardial infarction criteria with an elevated cardiac biomarker, typically a cardiac troponin >99th percentile of the upper reference level with a rise and fall in the level on serial assessment [1].
The main criteria for differential diagnosis are shown in Table 32.1.
MINOCA is found in 10% of all myocardial infarctions, ANOCA in >50% of patients referred to coronary angiography, and INOCA can be documented in about one-third of patients with ANOCA. Many patients with ANOCA suffer from cardiac extraischemic causes such as pericarditis, or noncardiac causes of chest pain, such as gastroesophageal reflux, asthma, psychiatric causes such as anxiety and panic attack, or osteoarticular disease with costochondritis [2]. In INOCA, the pathophysiology is heterogeneous. A significant central role is possibly played by coronary microvascular disease, coronary vasospasm, and altered cardiac autonomic function with disordered sympathetic innervation. Dynamic intraventricular obstruction increases coronary extravascular resistances and therefore can be considered a cause of functional coronary microvascular disease [3].
2 Coronary Microvascular Disease in ANOCA
Coronary microcirculation is a fundamental portion of the coronary artery tree, as it contains most of the coronary blood volume and represents the main regulator of coronary blood flow. Arterioles, capillaries, and venules originating from the major coronary artery branches and extending inside the myocardium, with a diameter of less than 300 μm, constitute the whole coronary microcirculation. Coronary microvascular impairment greatly contributes to the pathophysiology and outcome of many cardiac diseases. Different degrees of coronary microvascular impairment can be found both with and without epicardial obstructive atherosclerosis. Several conditions can be clustered together in the syndrome of microvascular disease (Table 32.2) [3].
Coronary microvascular alterations can be structural, functional, extravascular, and intravascular. In some of these conditions, the abnormalities of the microvasculature represent markers of risk and may determine myocardial ischemia, thus becoming important therapeutic targets [4].
3 INOCA: Not Only Coronary Microvascular Disease
The definition of INOCA has been used to encompass a broad range of conditions (Table 32.3). All ANOCA patients with the documented coronary microvascular disease have INOCA, but INOCA patients can recognize causes of ischemia differently from coronary microvascular disease.
Clinical history, electrocardiogram, and resting transthoracic echocardiogram are therefore essential for identifying patients with true coronary microvascular dysfunction (“cardiac syndrome X”) that probably represents no more than 30% of all INOCA patients [5]. The term “syndrome X” (originally the Group X in the 1973 paper by Arbogast and Bourassa) was coined to stress the uncertainty over the pathophysiology of chest pain [6]. It remains unclear whether the chest pain in these patients is ischemic or nonischemic in nature. However, since the unknown factor (X factor) in the original definition was clarified by the evidence of a reduced coronary flow velocity reserve (CFVR) with angiographically normal coronary arteries, we can dismiss the term Syndrome X and define “coronary microvascular disease” for a subset of INOCA patients with reduced (≤2.0) CFVR [7].
Takotsubo cardiomyopathy is clinically indistinguishable from an acute coronary syndrome, but myocardial involvement completely and rapidly recovers in a few days or weeks, making takotsubo cardiomyopathy a unique model of transient and completely reversible myocardial dysfunction, in the absence of significant coronary artery disease [8].
4 The Ischemic Cascade in Microvascular Disease
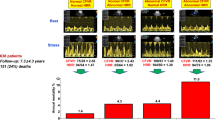
The typical pattern of microvascular disease during stress testing is the frequent induction of chest pain, ST-segment depression, and perfusion abnormalities without regional wall motion abnormality (RWMA) (Fig. 32.1) [9].
The features of microvascular disease consist of normal epicardial coronary arteries (even when observed by intravascular ultrasound: lower row) and reduced coronary flow reserve (by Doppler tracing showing a spectrum of coronary hyperemic responses, from normal—left to abolished—far right). Chest pain and ECG changes are frequent during stress, especially when flow reserve is reduced, whereas echocardiography changes (dashed lines) are only rarely observed. (Modified from Picano E et al. [9])
The sequence of events is therefore strikingly different from the ischemic cascade found during stress testing in the presence of coronary artery stenosis (Table 32.4).
In these patients with small coronary vessel dysfunction, focal ischemia in small myocardial regions scattered throughout the myocardium and caused by prearteriolar dysfunction, might explain the paradox of angina and ST-segment depression provoked by physical or pharmacological stress. The site of abnormally elevated resistances (in patients with reduced coronary flow reserve) might be intramural, upstream from the endocardium–epicardium branching point, which is not visualized by coronary angiography [10].
The evidence that, despite ischemic-like stress-induced chest pain and ST-segment changes, left ventricular function remains normal during SE in INOCA patients, is not incompatible with true myocardial ischemia: indeed, it is well known that the presence or absence of abnormal wall motion is related to the amount of a critical mass of ischemic subendocardial tissue, and minor degrees of patchy strictly subendocardial myocardial ischemia [11] are less likely to produce regional wall motion abnormality. A regional dysfunction by two-dimensional echocardiography requires a critical ischemic mass of at least 20% of transmural wall thickness and about 5% of the total myocardial mass. For minimal flow reductions, abnormalities of regional systolic function are subtle and certainly below the threshold of detection by echocardiography. Indeed, even under ideal imaging conditions, a subendocardial infarction can be accompanied in 20% of cases by a normal/hyperkinetic regional and global wall thickening [12], for hypercontraction and tethering from subepicardial layer and contiguous segments which may compensate for a limited subendocardial dysfunction.
5 SE Patterns in INOCA
In coronary microvascular disease, the peculiar pattern during SE is the regional and global left ventricular hyperkinesia with ST-segment depression and chest pain, consistently observed during dipyridamole [13], exercise [14], and dobutamine [15, 16].
In some patients (<10%) however, an RWMA appears and is not an innocent finding. It is due to angiographically occult coronary artery disease (detectable with intracoronary ultrasound) or initial latent cardiomyopathy which will become manifest in subsequent years [17, 18]. These false-positive results are more frequent in patients with left ventricular hypertrophy and high values of systolic blood pressure during stress, and usually involve the apical region [19].
With last-generation ultrasound technology and advanced expertise, dual imaging (function and flow) SE provides simultaneous insight into regional and global left ventricular function and coronary flow reserve. Coronary flow reserve can be best measured during Doppler transthoracic vasodilator or dobutamine SE in the mid-distal left anterior descending coronary artery, semi-simultaneously with wall motion imaging. A normal (>2.0) CFVR is found in 70% of ANOCA patients (Fig. 32.2).
Example of wall motion, coronary flow velocity reserve, and heart rate reserve assessment in patients with normal coronary arteries. Upper panels: end-systolic frames of apical four-chamber and two-chamber views showing normal function at rest and peak stress. Middle panels: Visualization of coronary flow in the mid-distal portion of the left anterior descending artery using pulsed-wave Doppler. Peak diastolic flow velocity was 54 cm s−1 under basal conditions (middle left panel) and 116 cm s−1 after dipyridamole infusion (middle right panel), with a normal CFVR value of 2.15. Heart rate reserve (lowest panels) is abnormal (rest = 52 beats/min; peak 58 beats/min; heart rate reserve = 58/52 = 1.11, normal values >1.22). (Courtesy of Dr. Lauro Cortigiani)
A reduced (≤2.0) CFVR can be found in 20% of ANOCA patients, in the absence of RWMA. However, the absence of RWMA does not necessarily imply that regional and global left ventricular mechanics are normal since subnormal increases in global longitudinal strain and subendocardial to subepicardial strain ratio can be observed in patients with coronary microvascular disease. Regional thickening and motion express radial function, which can be still normal when global longitudinal strain is impaired during less severe ischemia. When deformation imaging is applied to INOCA patients, an abnormal global longitudinal strain at rest or a reduced strain reserve during stress is observed in concomitance with the reduction in CFVR, suggesting that true ischemia occurs during stress [20,21,22,23,24,25,26].
In patients with INOCA, the cause of underlying ischemia can be coronary vasospasm of epicardial coronary arteries, unmasked by exercise, dobutamine, or dipyridamole stress, especially at the interruption of exercise or antidote administration. In these conditions, the diagnosis of vasospasm is easy and obtained by serendipity while testing the patient for coronary artery stenosis [27,28,29]. Other times, the test can be specifically targeted at vasospasm with ergonovine [30] or hyperventilation [31], eliciting coronary artery vasospasm and transmural ischemia detectable as RWMA.
In patients with left ventricular hypertrophy or young athletes, experiencing symptoms such as chest pain or syncope typically during exercise [32,33,34], a significant hyperdynamic contraction pattern with left ventricular intraventricular gradient during SE has been observed.
Due to the heterogeneity of underlying mechanisms, it is not surprising that the population of INOCA patients studied mostly under beta-blockers (which may exacerbate vasospasm) and with exercise or dobutamine stress (which may induce vasospasm) showed spontaneous improvements in angina and SE results, but the symptomatic improvement was not correlated with SE findings [35].
6 Prognostic Stratification
The prognosis of ANOCA patients is very heterogeneous, and this is not surprising considering the heterogeneity of underlying mechanisms. At long-term (9 years) follow-up, hard events are ten times more frequent in patients with inducible RWMA during SE than in those with negative SE results [36,37,38]. Within the lower-risk subset of patients with negative SE by wall motion criteria, the risk is higher in patients with reduced CFVR [39, 40].
In patients with ANOCA systematically tested for coronary vasospasm, the prognosis is worse in patients with vasospastic positivity during ergonovine echocardiography despite angiographically normal coronary arteries [41]. Only when all causes of true ischemia have been excluded, the patient can be assigned to a benign, likely noncardiac cause of chest pain. Interestingly, SE can identify an abnormal parameter also through nonimaging heart rate reserve since an abnormal heart rate reserve identifies a reduced cardiac autonomic balance [42, 43] and offers prognostic information independent and incremental over RWMA and CFVR [44].
7 Current Guidelines and Perspectives
Current European Society of Cardiology 2019 and American College of Cardiology/American Heart Association 2021 guidelines on the management of stable coronary artery disease recommend performing SE for the detection of inducible RWMA in association with angina and ischemic ECG changes. Thus, in every patient with sufficiently typical chest pain in whom, despite abnormalities of the electrocardiogram and/or stress test results indicative of myocardial ischemia, coronary angiography fails to show fixed or dynamic obstructions in epicardial coronary arteries, the existence of primary coronary microvascular disease should be suspected. Noninvasive stress testing of CFVR in the left anterior descending artery is now an established option recommended (class 2b) by both European and US guidelines [45, 46]. A CFVR <2.0 strongly suggests coronary microvascular disease. If such criteria are satisfied, more invasive investigations can usually be avoided (Table 32.5).
Although guidelines suggest that testing in the catheterization laboratory may be more comprehensive, it requires separate testing for epicardial artery stenosis (fractional flow reserve <0.8), coronary artery vasospasm (intracoronary acetylcholine testing), coronary microcirculation (coronary flow reserve <2.0), and completely misses the possibility to detect left ventricular obstruction. Noninvasive testing outside the catheterization laboratory by hyperventilation or ergonovine echocardiography testing [41] is by far a more feasible and possibly safer option to identify vasospasm, but not endorsed by current guidelines’ recommendations.
References
Ya’qoub L, Elgendy IY, Pepine CJ. Syndrome of nonobstructive coronary artery diseases: a comprehensive overview of open artery ischemia. Am J Med. 2021;134:1321–9. https://doi.org/10.1016/j.amjmed.2021.06.038.
Meeder JG, Hartzema-Meijer MJ, Jansen TPJ, Konst RE, Damman P, Elias-Smale SE. Outpatient management of patients with angina with no obstructive coronary arteries: how to come to a proper diagnosis and therapy. Front Cardiovasc Med. 2021;8:716319. https://doi.org/10.3389/fcvm.2021.716319.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40.
Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11.
Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–92.
Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Comparison with patients having significant coronary artery disease. Am J Cardiol. 1973;32:257–63.
Crea F, Lanza GA. Angina pectoris and normal coronary arteries: cardiac syndrome X. Heart. 2004;90:457–63.
Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Takotsubo Syndrome. Eur Heart J. 2010;31:1319–27.
Picano E, Palinkas A, Amyot R. Diagnosis of myocardial ischemia in hypertensive patients. J Hypertens. 2001;19:1177–83.
Epstein SE, Cannon RO. Site of increased resistance to coronary flow in patients with angina pectoris and normal coronary arteries. J Am Coll Cardiol. 1986;8:459–61.
Lieberman AN, Weiss JL, Jugdutt BI, Becker LC, Bulkley BH, Garrison JG, et al. Two-dimensional echocardiography and infarct size: relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation. 1981;63:739–46.
Carpeggiani C, L’Abbate A, Marzullo P. Multiparametric approach to the diagnosis of non-Q-wave acute myocardial infarction. Am J Cardiol. 1989;63:404–8.
Picano E, Lattanzi F, Masini M, Distante A, L’Abbate A. Usefulness of dipyridamole-echocardiography test for the diagnosis of syndrome X. J Am Cardiol. 1987;0:508.
Nihoyannopoulos P, Kaski JC, Crake T, Maseri A. Absence of myocardial dysfunction during stress in patients with syndrome X. J Am Coll Cardiol. 1991;19:1463–70.
Lanzarini L, Previtali M, Fetiveau R, Poli L. Results of dobutamine stress echocardiography in patients with syndrome X. Int J Card Imaging. 1994;10:145–8.
Panza JA, Laurienzo JM, Curiel RV, Unger EF, Quyyumi AA, Dilsizianet V, et al. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301.
Guerreiro RA, Fazendas P, Pereira AR, Marques A, Pais J, Alegria S, et al. Clinical and echocardiographic characterization of false-positive results from stress echocardiography. J Cardiovasc Imaging. 2020;28:123–33.
Labovitz AJ. The “myth” of the false-positive stress echo. J Am Soc Echocardiogr. 2010;23:215–6.
From AM, Prasad A, Pellikka PA, McCully RB. Are some false-positive stress echocardiograms a forme fruste variety of apical ballooning syndrome? Am J Cardiol. 2009;103:1434–8.
Ikonomidis I, Tzortzis S, Paraskevaidis I, Triantafyllidi H, Papadopoulos C, Papadakis I, Trivilou P, Parissis J, Anastasiou-Nana M, Lekakis J. Association of abnormal coronary microcirculatory function with impaired response of longitudinal left ventricular function during adenosine stress echocardiography in untreated hypertensive patients. Eur Heart J Cardiovasc Imaging. 2012;13:1030–40. https://doi.org/10.1093/ehjci/jes071.
Michelsen MM, Pena A, Mygind ND, Bech J, Gustafsson I, Kastrup J, et al. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography. 2018;35:196–203.
Rodriguez-Zanella H, Arbucci R, Fritche-Salazar JF, Ortiz-Leon XA, Tuttolomondo D, et al. On Behalf Of The Stress Echo Study Group Of The Italian Society Of Echocardiography And Cardiovascular Imaging Siecvi. Vasodilator strain stress echocardiography in suspected coronary microvascular angina. J Clin Med. 2022;11:711. https://doi.org/10.3390/jcm11030711.
Tagliamonte E, Sperlongano S, Montuori C, Riegler L, Scarafile R, Carbone A, et al. Coronary microvascular dysfunction affects global longitudinal strain response to dipyridamole stress echocardiography: a pilot study. Heart Vessel. 2023;38:470–7.
Cadeddu C, Nocco S, Deidda M, Pau F, Colonna P, Mercuro G. Altered transmural contractility in postmenopausal women affected by cardiac syndrome X. J Am Soc Echocardiogr. 2014;27:208–14.
Jovanovic I, Tesic M, Giga V, Dobric M, Boskovic N, Vratonjic J, Orlic D, Gudelj O, Tomasevic M, Dikic M, Nedeljkovic I, Trifunovic D, Nedeljkovic MA, Dedic S, Beleslin B, Djordjevic-Dikic A. Impairment of CFVR and global longitudinal strain in women with cardiac syndrome X and slow coronary flow. J Cardiol. 2020;76:1–8.
Zhao L, Wang Q, Xu P, Su X, Luo Q, Ding Y. Evaluation of left ventricular function in ischemia with normal coronary arteries. A research based on adenosine stress myocardial contrast echocardiography. Int J Card Imaging. 2023;39:349–57.
Picano E, Lattanzi F, Masini M, Distante A, L’Abbate A. Aminophylline termination of dipyridamole stress as a trigger of coronary vasospasm in variant angina. Am J Cardiol. 1988;62:694–7.
de Servi S, Falcone C, Gavazzi A, Mussini A, Bramucci E, Curti MT, et al. The exercise test in variant angina: results in 114 patients. Circulation. 1981;64:684–8.
Aboukhoudir F, Rekik S. Coronary artery spasm and dobutamine stress echocardiography in patients without known coronary disease: prevalence, predictors, and outcomes. Acta Cardiol. 2016;71:435–41.
Song JK, Lee SJ, Kang DH, Cheong SS, Hong MK, Kim JJ, et al. Ergonovine echocardiography as a screening test for diagnosis of vasospastic angina before coronary angiography. J Am Coll Cardiol. 1996;27:1156–61.
Morales MA, Reisenhofer B, Rovai D, Moscarelli E, Distante A, L'Abbate A. Hyperventilation-echocardiography test for the diagnosis of myocardial ischemia at rest. Eur Heart J. 1993;14:1088–93.
Cotrim C, Almeida AG, Carrageta M. Clinical significance of intraventricular gradient during effort in an adolescent karate player. Cardiovasc Ultrasound. 2007;5:39.
Christiaens L, Duplantier C, Alla J, Donal E, Nanadoumgar H, Barraine R, et al. Normal coronary angiogram and dobutamine-induced left ventricular obstruction during stress echocardiography: higher hemodynamic responsiveness to dobutamine. Echocardiography. 2001;18:285–90.
Madaric J, Bartunek J, Verhamme K, Penicka M, Van Schuerbeeck E, Nellens P, et al. Hyperdynamic myocardial response to beta-adrenergic stimulation in patients with chest pain and normal coronary arteries. J Am Coll Cardiol. 2005;46:1270–5.
Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA study. Circulation. 2021;144:1008–23. https://doi.org/10.1161/CIRCULATIONAHA.120.046791.
Sicari R, Palinkas A, Pasanisi E, Venneri L, Picano E. Long-term survival of patients with chest pain syndrome and angiographically normal or near-normal coronary arteries: the additional prognostic value of dipyridamole-echocardiography test. Eur Heart J. 2005;26:2136–41.
From AM, Kane G, Bruce C, Pellikka PA, Scott C, McCully RB. Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J Am Soc Echocardiogr. 2010;23:207–14.
Rachwan RJ, Mshelbwala FS, Dardari Z, Batal O. False-positive stress echocardiograms: predictors and prognostic relevance. Int J Cardiol. 2019;296:157–63.
Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103:626–31.
Schroder J, Michelsen MM, Mygind ND, Suhrs HE, Bove KB, Bechsgaard DF, et al. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive CAD: results from the power study. Eur Heart J. 2021;42:228–39. https://doi.org/10.1093/eurheartj/ehaa944. PMID: 33477168.
Om SY, Yoo SY, Cho GY, Kim M, Woo Y, Lee S, et al. Diagnostic and prognostic value of ergonovine echocardiography for noninvasive diagnosis of coronary vasospasm. JACC Cardiovasc Imaging. 2020;13:1875–87.
Frøbert O, Mølgaard H, Bøtker HE, Bagger JP. Autonomic balance in patients with angina and a normal coronary angiogram. Eur Heart J. 1995;16:1356–60.
Lanza GA, Giordano A, Pristipino C, Calcagni ML, Meduri G, Trani C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I] metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96:821–6.
Cortigiani L, Carpeggiani C, Meola L, Djordjevic-Dikic A, Bovenzi F, Picano E. Reduced sympathetic reserve detectable by heart rate response after dipyridamole in anginal patients with normal coronary arteries. J Clin Med. 2021;11:52. https://doi.org/10.3390/jcm11010052.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC Scientific Document Group. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;144:e368–454. https://doi.org/10.1161/CIR.0000000000001029. Erratum in: Circulation. 2021;144:e455
Acknowledgments
The authors would like to acknowledge the contributions of Prof. Leda Galiuto for her contribution to the previous editions of this chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Palinkas, A., Picano, E. (2023). Stress Echocardiography in Angina with Nonobstructive Coronary Arteries. In: Picano, E. (eds) Stress Echocardiography. Springer, Cham. https://doi.org/10.1007/978-3-031-31062-1_32
Download citation
DOI: https://doi.org/10.1007/978-3-031-31062-1_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31061-4
Online ISBN: 978-3-031-31062-1
eBook Packages: MedicineMedicine (R0)