Abstract
The photocatalytic degradation approach for industrial dyes in the presence of appropriate nanocatalyst offers a possible way for the complete removal of various pollutants from the aqueous environment. Therefore, in this study, we have made an attempt on use of bio-synthesized copper oxide (CuO) nanoparticles harnessed by neem extract for the degradation of reactive orange M2R (RO-M2R). The significant catalytic degradation of dye was found to be pH10, catalytic dosage at 10 ppm, and dye concentration at 3 gL−1 and irradiation time at 5 h through one factor at a time (OFAT). Further, Box Behnken design was applied to optimize and investigate the interaction between these four variables for the degradation of dye. It was predicted that maximum degradation was 84.04% under optimum conditions of pH at 11.84, catalytic dosage at 2.20 gL−1, dye concentration at 7.49 ppm and irradiation time of 4 h. The validity of the optimal level predicted under the RSM was established by an independent experiment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Water pollution has become a very sensitive issue in the world due to inorganic and organic pollutants. The primary source of contamination of water is the organic dyes discharged by textile industries. There are more than 10,0000 commercially available dyes with over 7 × 105tons of dyestuff produced. In which, concerning 36,000 dye/year are consumed by textile industries across the world. 20% of the dyes in the world production are lost during the dyeing process and is discharged in the textile wastewater (Reza et al. 2017; Esplugas et al. 2002; Houas et al. 2001). In particular, the principal and most adaptable classes of azo dyes are presently utilized in the dyeing of different materials such as textiles, leather, plastics, and cosmetics. The azo dyes containing effluents are released into rivers, lakes, and ground waters for the period of dying process that contains mutagenic and carcinogenic health hazards. As a result, it leads to severe environmental problems due to their good stability under ambient conditions (Mathur et al. 2012).
Organic pollutants are challenging to biological degradation; also, conventional treatment processes are not succeeded to attain their complete elimination of dye molecules. Even though, for a decade, researchers have attempted various methodologies such as membrane filtration, chemical coagulation/flocculation, ion exchange, precipitation, adsorption, biological degradation, and ozonation, etc. Still, these methods are not capable enough and have more limitations. UV light is generally applied to excite a semiconductor surface to produce photoinduced holes or reactive oxygen species (ROS), such as hydroxyl and superoxide radicals in the photocatalytic advanced oxidation technology. Then, the ROS interrelate with organic compounds and guides to their oxidation and overall degradation (Jantawasu et al. 2009). Moreover, for a decade nanocatalyst development has highly attracted researchers to work towards the degradation of industrial wastewater pollutants. Apart from dye degradation studies by nanoparticles, Magnetic Moringa Oleifera (MMO) have also been used as a coagulant for palm oil waste water treatment (Noor et al. 2022).
There are various studies reported on dye degradation by metallic nanoparticles. Chaibakhsh et al. (2016) have reported on photocatalytic degradation of neutral red dye using titanium oxide (TiO2) nanocatalyst and its optimization by Box-Behnken design. Recently, Uddin and Baig (2019) have studied on the removal of methyl orange dye using cobalt oxide (Co3O4) nanoparticles and Dawoud et al. (2020) have also reported on photocatalytic degradation of organic dye of Rhodamine B by using silver (Ag) doped Zirconium oxide (ZrO2) nanoparticles. Recently, CuO NP`s have been prepared from various sources such as Canthium coromandelicum leaves extract (Selvam et al. 2022), Punica granatum (Kaur et al. 2022), Pistacia vera L. hull (Ltaief et al. 2021), Dictyota dichotoma endophytic fungi (Kumar et al. 2022), Elaeagnus indica (Indhira et al. 2022), Solanum tuberosum (Hamami and Javanbakht 2021) were used as a catalyst for textile dye degradation.
Most of the reports on dye removal by magnetic nanoparticles have been performed by ‘one variable at a time’ (OFAT) approach that perfectly presumed that all variables are independent and do not represent the combined effect of all the variables involved. Moreover, this method is a prolonged one and also requires a higher number of experiments to determine optimum levels, which may or may not be trustworthy (Farooq et al. 2017). These limitations of the conventional methods can be reduced by response surface methodology (RSM), using this statistical design of experiment.
Thus, herein, biological route synthesized CuO nanoparticles assisted photocatalytic degradation of RO-M2R dye, and their optimization studies were performed using Box-Behnken design to maximize the percentage of dye degradation.
12.2 Materials and Methods
12.2.1 Photocatalytic Degradation Confirmation
RO-M2R was obtained from Precision Scientific Co. Coimbatore, India. The photocatalytic degradation experiment of RO-M2R was performed under sunlight irradiation. The photocatalyst CuO NP`s 2 gL−1was added to 50 ppm of RO-M2R dye solution at neutral pH to establish the adsorption equilibrium between the dye molecule and CuO NP`s. The resulting solution was exposed to sunlight for 4 h, and control was maintained without addition of nanoparticles. These suspensions were measured against absorbance at 495 nm. The percentage of degradation was calculated from the following equation;
where, Co = Initial concentration of dye, C = Final concentration of dye.
12.2.2 Preliminary Experiment
Preliminary experiments were conducted by one factor at a time (OFAT) approach to identify the ranges of four parameters such as pH, dye concentration, catalytic dosage and irradiation time for the degradation of RO-M2R. Statistical design of experiment consists of a variation of several trials at a time when compared to one factor at a time approach in which only one factor is changed and keeping all other factors constant.
12.2.2.1 Evaluation of Process Parameters by OFAT
The dye solution was taken out in reaction vessel, and the pH (3, 4, 5, 6, 7, 8, 9, 10, 11 and 12) was adjusted by using a pH meter (Elico LI 120, India). Similarly, the different concentrations of RO-M2R working solutions (10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 ppm) were prepared and kept the irradiation time for 4 h to study the effect of dye concentration on dye degradation. Likewise, the different concentrations of CuO nanoparticles (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5 and 5 g/L) were varied and the reaction vessel was kept under sunlight for 4 h. Similarly, the different reaction periods (0, 50, 100, 150, 200, 250, 300, 350, 400 and 450 min) were chosen to check the percentage of degradation. Finally, 2 g/L of CuO nanoparticles was added to various ranges of pH and concentration of RO-M2R dye solution to establish the adsorption equilibrium between the dye molecule and CuO nanoparticles.
12.2.3 Response Surface Methodology for Optimization
Response surface methodology (RSM) was utilized to optimize the four parameters were catalytic dosage, pH, dye concentration and irradiation time. The four parameters were selected as independent variables, and the degradation percentage of RO-M2R was the response variable. Box-Behnken model was preferred to study the combined effect of four independent variables, and Design-expert 11 version was used to explain the response surface. The model was statistically examined. Analysis of variance (ANOVA) employed Fischer’s F- test to evaluate the overall significance of the model, correlated probability values, and coefficient of determination to estimate the goodness of fit of the regression model. The apt polynomial equation was further conveyed the three-dimensional plots (3D) form, which depicted the interactions graphically. Finally, the central and interaction effects, the 3D plots were analyzed to check the accuracy of the model (Kanmani et al. 2013).
The following second-order polynomial equation explains the connection between the dependent and independent variables:
Here the Y is the predicted response, β0 is the intercept, β1, β2, β3, are the linear coefficients, β11, β22, β33, are the squared coefficients, and β12, β13, β23 are the interaction coefficients.
12.3 Results and Discussion
12.3.1 Confirmation of Photocatalytic Degradation
The previously synthesized CuO nanoparticles (Thirumurugan et al. 2017) were used for the photocatalytic degradation of RO-M2R under sunlight. These nanoparticles were already proved its photocatalytic degradation of 4-nitrophenol as a model (Ramya et al. 2019) and photocatalytic degradation of reactive red 120 (Thirumurugan et al. 2017). Thus, in this study, the photocatalytic degradation of RO-M2R was studied by using green synthesized CuO NP`s under sunlight, as shown in Fig. 12.1. After the addition of nanoparticles, the orange color of the solution decreased significantly, become colorless with 74% degradation, and no changes were observed in control. The dye degradation efficiency of nanoparticles may be due to differences in size, shape and catalytic activity (Moon et al. 2015). Kumar et al. (2013), has also reported that metal nanoparticle synthesized by green approach was effectively degraded the dye with periodically increase in time. There are various parameters affecting the photocatalytic degradation, such as pH, dye concentration, catalytic dosage, irradiation time, reaction temperature, light intensity and the presence of electron acceptors. In particular, we investigated the effect of pH, dye concentration, catalytic dosage, and irradiation time through one factor at a time (OFAT) approach to study the impact of each factor ranges for the photocatalytic degradation of RO-M2R.
12.3.2 Evaluation of Process Parameters by OFAT
Effect of pH is one of the main factors for the degradation of industrial dye. To investigate the effect of pH, the experiments were carried out at different pH ranges from 3 to 12 at 2 gL−1 of CuO NP`s, 50 ppm of RO-M2R and irradiation time of 4 h. The result showed that by increasing the pH of the dye solution, the dye degradation was getting increased, as shown in Fig. 12.2. It was observed that degradation rate increases, with an increase in pH and the maximum photocatalytic degradation, was found to be at pH 10. The cause can be due to efficient formation of hydroxyl radicals in alkaline medium. Excess of hydroxyl anions increases the formation of hydroxyl radicals as the main oxidizing function responsible for dye degradation (Gopalapp et al. 2012). Similarly, Devadi et al. (2014) has observed the maximum degradation of methyl blue at pH 11 and at under acidic condition the degradation efficiency get decreased due to the columbic repulsion. Also, Daneshvar et al. (2003) reported that in the presence of ZnO NP`s, the photodegradation was considerably improved at high pH (pH = 11), While the lower at low pH is due to the photodecomposition of ZnO to Zn2+ takes place in acidic and neutral solutions and that efficient formation of hydroxyl radicals occurs in alkaline solution.
The effect of dye concentration for degradation of RO-M2R was varied between 10 to 100 ppm where pH was kept at 10, catalytic dosage at 2 gL−1 and irradiation time for 4 h. The degradation of RO-M2R at different concentration of dye is shown in Fig. 12.3. The result showed that up to 40 ppm the percentage of degradation was approximately the same and after 40 ppm the degradation rate was reduced. While maintaining a constant catalyst dose, it caused the fixed number of catalysis sites to be saturated rapidly, which is due to increasing dye concentration in the solution (Mohan and Pittman 2006). In addition, the light transmittance in the solution may be decreased due to the increased dye concentration; decreased light infiltration can diminish the activation rate of nanoparticles and delay the formation of OH radicals, results in decreased photocatalytic degradation.
Figure 12.4 shows the effects of different concentrations of nanoparticles as catalysts on degradation. The increase in concentration up to 3 gL−1 contains a high amount of active site and thus producing more free radicals which uninterruptedly increase the degradation of RO-M2R, and beyond 3 gL−1 there was a decrease in degradation rate. According to Akpan and Hameed (2009), this was due to the formation of turbid in the solution, which reduces the penetration of sunlight. Hence, it blocks the radiation to enter into the RO-M2R solution. Recently, Mortazavian et al. (2019) has reported in a study that the amounts of catalyst particles might aggregate leading to a reduced number of active sites because the increase of TiO2 particles in the solution might supply more active sites for the molecules of dye to be adsorbed and degraded. This phenomenon might be one of the reasons for decreasing the degradation rate beyond 3 gL−1 of CuO NP`s as a catalyst. Very recently, Hashemi et al. (2022) reported the optimization and assessment of photocatalytic degradation of methyl orange using silver nanoparticles synthesized through green route. As a result, observed that the methyl orange pollutant degradation was increased while increasing the concentration of nanocatalyst.
The effect of irradiation time on degradation was investigated by keeping catalyst amount at 3 gL−1, pH at 10, and dye concentration at 40 ppm and varying the irradiation time of the dye solution under sunlight. Figure 12.5 shows that degradation rate increased as time increased, and this is due to the increased number of photons. The maximum degradation efficiency was observed at 5th h, and after that the degradation remains the same.
12.3.3 Optimization Using Response Surface Methodology
Box Behnken Design (BBD) was employed to evaluate the effect of variables on the degradation of RO-M2R in the presence of sunlight. The influence of operating parameters on the photocatalytic degradation efficiency of RO-M2R were assessed, four main factors were chosen: pH, dye concentration (ppm), catalytic dosage (gL−1), irradiation time (hrs). A total of 29 experiments were used using Design expert software. The experimental outcomes of degradation of dye by nanoparticles were analyzed through RSM as tabulated in Table 12.1 to obtain an empirical model.
Based on the outcomes, an empirical relationship between the responses and independent variables were attained for the dye degradation expressed by the quadratic polynomial equation, as shown below.
Response surface methodology integrated the interaction effects of variables and helps us in simultaneously optimizing many process parameters within a minimum number of trial runs. Such statistically aided experimental designs can guide to considerably better performance of the nanoparticles. The P value acts as a tool for assessing the significance of each coefficient and is indicative of the interaction strength of all independent variable. Low values of P at (P < 0.05) indicate the high significance of the corresponding coefficients. In general, larger t, F and smaller P values indicate that the corresponding coefficient terms are significant.
From the ANOVA Table 12.2, the model F-value of the model (41.08) and values of P > F(<0.0500) implied that the model was significant statistically. The predicted R2 of the 0.8808 is in reasonable agreement with the adjusted R2 of 0.9525. Adequate precision measures signal-to-noise ratio (S/N ratio) and detect which experimental parameters generate signals that are large in comparison to the noise. The observed adequate precision ratio was 23.29, greater than 4, which indicated the selected model could be employed to precisely navigate the design space as reported by Chaibakhsh et al. (2016). The accuracy of the model is shown in Fig. 12.6, which evaluates the measured values against the predicted responses of the model for the degradation of RO-M2R. It can be observed that correlation between the experimental data and predicted values (R2 = 0.9762) showed the data fit well with the model in the range studied.
12.3.3.1 Analysis of Response Surface Plots
Three-dimensional surface can be used as a graphical representation of the regression equation to find the optimum levels of the variables studied and widely used to achieve a better understanding of the interactions between variables within the ranges.
Figure 12.7 represents the interaction effect of pH, nanoparticles concentration and dye concentration and irradiation time on the degradation of dye from aqueous solution. There was a significant degradation observed by increasing pH till 12. Similarly, nanoparticles concentrations also influencing the degradation rate of dye molecules up to 2.20 gL−1 then degradation rate declines. Whereas, dye concentration and irradiation time were not influencing the degradation rate as pH and nanoparticles concentration. Recently, Chaker et al. (2021), has studied a statistical modelling optimization approach for efficient photocatalytic degradation of azo dye using cerium-doped mesoporous ZnO by central composite design. Their results indicated that all the variable chosen had an impact on the photocatalytic process and pH was the least factor against the effect of catalyst concentration.
Three dimensional surface plot showing the effect of a nanoparticles concentration (gL−1) versus pH b dye concentration (mgL−1) versus pH c Irradiation time (h) versus pH d Dye concentration (mgL−1) versus nanoparticles concentration (gL−1) e Irradiation time (h) versus dye concentration (mgL−1) f Irradiation time (hrs) versus nanoparticles concentration (gL−1) on degradation of RO-M2R
The three-dimensional plots based on the interactions between the variables showed an increase in RO-M2R dye degradation as each variable increased to an optimum level, beyond the optimum level; decline rate could be observed (Fig. 12.7a–f). The optimal values obtained from the three-dimensional were also almost equal to the results obtained by the regression analysis (Eq. 12.1). From the model, a maximum dye degradation of 84% under the optimum condition of pH 11.8, nanoparticle concentration of 2.20 gL−1, dye concentration of 7.49 ppm and irradiation time of 4 h were predicted. The experiment was conducted in triplicate to validate the prediction of the model, and the maximum degradation of RO-M2R was found to be 83.41% which was close to the predicted maximum dye degradation of the model.
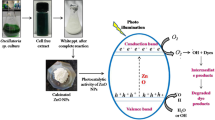
12.3.4 Mechanism of Photocatalytic Degradation by CuO NP`s
For the above observed results, a possible mechanism for the degradation of RO-M2R dye by CuO nanoparticles in aqueous solution can be proposed. A possible photocatalytic mechanism of CuO nanoparticles for the degradation of aqueous RO-M2R is shown in Fig. 12.8. Photocatalytic degradation of organic dyes through the utilization of CuO nanoparticles arises when the photocatalyst absorbs light of a suitable wavelength from sunlight. The light energy from the sun is exciting and promotes electrons in the valence band to conduction band of photocatalyst. As results, creation of positive charges (holes) and negative charges (electrons) on the valence and conduction bands respectively, leads to the formation of electron–hole pairs. This hole oxidizes the water molecule into hydrogen gas and hydroxyl radicals (OH•), whereas electron reduces the oxygen molecules to form superoxide radicals (O2•_). Thus radicals formed radicals, attack the dye molecule over the surface of the CuO NP’s and accomplish the dye degradation, and these formed radicals are also responsible for inactivation of bacteria through their cell walls degradation (Dalrymple et al. 2010).
12.4 Conclusions
We conclude that the photocatalytic degradation of RO-M2R by CuO NP`s as a catalyst in the aqueous medium was developed a quadratic model using Response Surface Methodology as a functional relationship between catalytic dosage, pH, dye concentration and irradiation time to determine the optimum condition for the degradation of RO-M2R. The optimized degradation of RO-M2R was achieved with 83.41% by Box-Behnken design. Therefore, CuO NP`s could be utilized as an efficient photocatalyst for the degradation of dye-containing waste water from textile industries.
References
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170(2–3):520–529
Chaibakhsh N, Ahmadi N, Zanjanchi MA (2016) Optimization of photocatalytic degradation of neutral red dye using TiO2 nanocatalyst via Box-Behnken design. Desalin Water Treat 57(20):9296–9306
Chaker H, Attar AE, Djennas M, Fourmentin S (2021) A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: a central composite design in response surface methodology. Chem Eng Res Des 171:198–212
Dalrymple OK, Stefanakos E, Trotz MA, Goswami DY (2010) A review of the mechanisms and modeling of photocatalytic disinfection. Appl Catal B 98(1):27–38
Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A Chem 157(1):111–116
Dawoud TM, Pavitra V, Ahmed P, Syed A, Nagaraju G (2020) Photocatalytic degradation of an organic dye using Ag doped ZrO2 nanoparticles: milk powder facilitated eco-friendly synthesis. J King Saud Univ Sci 32(3):1872
Devadi MAH, Krishna M, Murthy HN, Sathyanarayana BS (2014) Statistical optimization for photocatalytic degradation of methylene blue by Ag-TiO2 nanoparticles. Proc Mater Sci 5:612–621
Esplugas et al., 2002 Esplugas S, Giménez J, Contreras S, Pascual E, Rodríguez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36:1034–1042
Farooq S, Saeed A, Sharif M, Hussain J, Mabood F, Iftekhar M (2017) Process optimization studies of crystal violet dye adsorption onto novel, mixed metal Ni0. 5Co0. 5Fe2O4 ferrospinel nanoparticles using factorial design. J Water Proc Eng 16:132–141
Gopalapp H, Yogendra K, Mahadevan K (2012) Solar photocatalytic degradation of commercial azo dye acid orange 7 by synthesized CaZnO2 nanoparticle as an effective Catalyst. Int J Res Chem Environ 2:39–43
Hamami Z, Javanbakht V (2021) Biosynthesis of copper oxide nanoparticles using biomass, peel, and extract polysaccharides of Solanum Tuberosum for ultrasound-assisted adsorption of azo direct red 80 contaminants. Ceram Int 47(17):24170–24181
Hashemi Z, Mizwari ZM, Mohammadi-Aghdam S, Mortazavi-Derazkola S, Ebrahimzadeh MA (2022) Sustainable green synthesis of silver nanoparticles using Sambucus ebulus phenolic extract (AgNPs@ SEE): optimization and assessment of photocatalytic degradation of methyl orange and their in vitro antibacterial and anticancer activity. Arab J Chem 15(1):103525
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B 3:1145–1157
Indhira D, Krishnamoorthy M, Ameen F, Bhat SA, Arumugam K, Ramalingam S, Kumar GS (2022) Biomimetic facile synthesis of zinc oxide and copper oxide nanoparticles from Elaeagnus indica for enhanced photocatalytic activity. Environ Res 212:113323
Jantawasu P, Sreethawong T, Chavadej S (2009) Photocatalytic activity of nanocrystalline mesoporous-assembled TiO2 photocatalyst for degradation of methyl orange monoazo dye in aqueous wastewater. Chem Eng J 155:223–233
Kanmani P, Karthik S, Aravind J, Kumaresan K (2013) The use of response surface methodology as a statistical tool for media optimization in lipase production from the dairy effluent isolate Fusarium solani. Int Schol Res Notices 528708
Kaur H, Singh J, Rani P, Kaur N, Kumar S, Rawat M (2022) A novel and one-pot synthesis of Punica granatum mediated copper oxide having flower-like morphology as an efficient visible-light driven photocatalyst for degradation of textile dyes in waste water. J Mol Liq 355:118966
Kumar RV, Vinoth S, Baskar V, Arun M, Gurusaravanan P (2022) Synthesis of zinc oxide nanoparticles mediated by Dictyota dichotoma endophytic fungi and its photocatalytic degradation of fast green dye and antibacterial applications. S Afr J Bot. https://doi.org/10.1016/j.sajb.2022.03.016
Kumar P, Govindaraju M, Senthamil selvi S, Premkumar K (2013) Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Coll Surf B Biointerf 103:658–661
Ltaief S, Jabli M, Abdessalem SB (2021) Immobilization of copper oxide nanoparticles onto chitosan biopolymer: application to the oxidative degradation of Naphthol blue black. Carbohyd Polym 261:117908
Mathur N, Bhatnagar P, Sharma P (2012) Review of the mutagenicity of textile dye products. Univers J Environ Res Technol 2(2):1–18
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J Hazard Mater 137:762–811
Moon SA, Salunke BK, Alkotaini B, Sathiyamoorthi E, Kim BS (2015) Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract. IET Nanobiotechnol 9(4):220–225
Mortazavian S, Saber A, James DE (2019) Optimization of photocatalytic degradation of acid blue 113 and acid red 88 textile dyes in a UV-C/TiO2 suspension system: application of response surface methodology (RSM). Catalysts 9(4):360
Noor MHM, Azli MFZM, Ngadi N, Inuwa IM, Opotu LA, Mohamed M (2022) Optimization of sonication-assisted synthesis of magnetic Moringa oleifera as an efficient coagulant for palm oil wastewater treatment. Environ Technol Innov 25:102191
Ramya E, Thirumurugan A, Rapheal VS, Anand K (2019) CuO@SiO2 nanoparticles assisted photocatalytic degradation of 4-nitrophenol and their antimicrobial activity studies. Environ Nanotechnol Monit Manag 12:100240
Reza KM, Kurny ASW, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7(4):1569–1578
Selvam K, Albasher G, Alamri O, Sudhakar C, Selvankumar T, Vijayalakshmi S, Vennila L (2022) Enhanced photocatalytic activity of novel Canthium coromandelicum leaves based copper oxide nanoparticles for the degradation of textile dyes. Environ Res 211:113046
Thirumurugan A, Harshini E, Marakathanandhini BD, Kannan SR, Muthukumaran P (2017) Catalytic degradation of reactive red 120 by copper oxide nanoparticles synthesized by Azadirachta indica. In: Bioremediation and sustainable technologies for cleaner environment. Springer, Cham, pp 95–102
Uddin MK, Baig U (2019) Synthesis of Co3O4 nanoparticles and their performance towards methyl orange dye removal: characterisation, adsorption and response surface methodology. J Clean Prod 211:1141–1153
Acknowledgements
We authors sincerely thank the management for their support and providing all the facilities throughout the research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ramya, E., Thirumurugan, A., Govindan, N., Aravind, J., Gobikrishnan, S. (2023). Photocatalytic Degradation of Reactive Orange M2R Using Green Route Synthesized Copper Oxide Nanoparticles and Its Optimization Studies. In: Jeyaseelan, A., Murugasen, K., Sivashanmugam, K. (eds) Sustainable and Cleaner Technologies for Environmental Remediation. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-29597-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-29597-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-29596-6
Online ISBN: 978-3-031-29597-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)