Abstract
Metal and metal oxide nanoparticles are widely used to remove organic dyes from wastewater due to their higher surface area, high adsorption capacity, chemical stability, and photocatalytic activity. In present work, Azadirachta indica (neem) leaf extract was used as a reducing and capping agent for the green synthesis of copper nanoparticles (CuNPs) with homogeneous precipitation process. The developed green method of Azadirachta indica leaves extract was found to be simple, environment-friendly, and cost-effective as compared to existing toxic reducing agents. Synthesized CuNPs using green route were characterized by UV–visible spectroscopy, Fourier Transform Infrared spectroscopy (FTIR), and Scanning Electron Microscopy (SEM) analysis. UV–visible spectroscopy analysis showed characteristics absorption peaks at 800 nm confirming the formation of copper nanoparticles. FTIR spectra exhibited the presence of bioactive molecules in leaf extract which are responsible for the reduction of copper ions to CuNPs. Moreover, Scanning Electron Microscopic (SEM) analysis also revealed that synthesized CuNPs were in spherical shape with average size of particles ranging between 50 and 70 nm. Further, this study reports utilization of biosynthesized CuNPs for degradation of organic dye, namely, methylene blue dye from wastewater samples under sunlight. Result analysis revealed that approximately 60% of degradation of Methylene blue dye was observed within 120 min using green synthesized copper nanoparticles that exhibit good photocatalytic activity. This study demonstrates that green synthesized copper nanoparticles can be effectively used for removal of hazardous dye from industrial wastewater which contributes to cost-effective, environmental, and sustainable processes.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Water pollution has become a great concern worldwide due to accidental or arbitrarily dumping of polluted wastewater from industries into aquatic systems [1]. Organic dyes are extensively used in various industries such as paper, cosmetics, textile, plastics, and leather tanning. These synthetic dyes are highly toxic, carcinogenic, and non-biodegradable due to their complex chemical structure [2]. However, the release of such harmful dyes into the water bodies without appropriate treatment can cause environmental problems along with health problems in human beings such as vomiting, diarrhea, skin diseases, cancer, allergic reactions, and mutation for people [3]. According to “The United Nations World Water Development Report 2017,” over 800,000 deaths worldwide were caused by contaminated drinking water [4]. Hence, it is imperative to remove methylene blue dye from industrial wastewater before discharge into aquatic systems.
Different physical, chemical, and biological methods for the removal of dye from wastewater have been reported in the literature but most of them have certain demerits, such as requiring high temperature, harmful chemicals, high operating costs, and time-consuming [5]. In contrast, in recent years, green synthesized metal nanoparticles (NPs) have been employed in wastewater treatment along with medicine, drug delivery, and optoelectronics applications [6]. Moreover, antifungal, antibacterial, photocatalytic, properties are the important features of various metal nanoparticles [7]. Photocatalysts have been gaining more attention for the treatment of organic pollutants from past several years [1, 8]. Photocatalysis is a green method for the degradation of hazardous dyes in wastewater via sunlight or visible light-induced reaction with catalyst (i.e. nanoparticles) that produce free radical such as hydroxyl radical (OH–). These radicals can break down the organic dyes molecules and degrade them efficiently. It eliminates the need for hazardous chemicals and thus resulting in more sustainable, efficient, stable, and eco-friendly approach as compared to conventional treatment methods [3, 9].
In the last few years, different approaches for synthesis of metal NPs have been invented and reported, for instance sol gel, hydrothermal, chemical reduction, chemical vapor deposition, etc. [10]. These methods are energy intensive and employ the use of toxic, hazardous, and expensive chemicals which limits their use for wide implementation [11]. Nowadays, green method utilizing plant extract for NPs synthesis is more widely used as compared to conventional methods as these sources are inexpensive, easily and abundantly available, stable and nontoxic. In addition, active components of leaf extract namely polyphenols, flavonoids, alkaloids, and proteins play a vital role in the reduction of metal ions to nanoparticles and also act as capping agent to prevent agglomeration of nanoparticles [10].
In past few years, metal NPs synthesized via green route have been successfully applied in organic dye removal because of its less toxicity, easy separation, and its photocatalytic activity [5, 12, 13]. Among various metal NPs, copper nanoparticles (CuNPs) have been receiving considerable attention due to their attractive features including low cost, easy availability, catalytic properties, high electrical conductivity, optical and antibacterial properties [14]. In literature, copper-based nanoparticles have been synthesized from several plant and fruit extracts such as Jatropha curcas leaves, Celastrus paniculatus Willd. Leaves, Piper retrofractum Vahl fruit extract, Ficus carica extract [3, 6, 9, 14]. However, green synthesis of CuNPs using Azadirachta indica leaf (more commonly known as neem leaf) extract is reported in very few previous research studies [10, 11]. Figure 1 shows the image of dried neem leaf. It belongs to Meliaceae family and available abundantly in India and in nearby subcontinents. Products from neem have good antibacterial, antifungal, antiviral properties and also help in controlling the blood sugar level [12]. Therefore, the present work focused on green synthesis of CuNPs utilizing neem leaves extract as a reducing and capping agent.
The objective of this work is to synthesize copper nanoparticles (CuNPs) by using leaf extract of Azadirachta indica and then evaluate their photocatalytic activity for the decomposition of organic dye from wastewater. The green synthetized CuNPs were then characterized using UV–Visible Spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FESEM), and X-ray Diffractometer (XRD). Further, tests were carried out in a batch system to evaluate the effect of initial dye concentration and contact time on methylene blue (MB) dye removal efficiency. The green synthesized CuNPs have effective photocatalytic activity against methylene blue in sunlight. The present research work of green synthesis of CuNPs using aqueous leaf extract and its further application for removal of hazardous dye from industrial wastewater provides promising approach because of its low-costs, low energy requirements and thus contributes to protect the environment as well as health by avoiding the use of toxic chemicals.
2 Materials and Methods
2.1 Chemicals and Materials
Copper Sulfate (CuSO4·5H2O) and Methylene Blue dye (MB chemical formula C16H18ClN3S, molecular weight = 319.85 g/mol, λmax = 650 nm) of AR Grade was procured from SRL Chemicals and used without any further purification. Distilled water was employed for preparation of leaf extract, solutions of metal salt, and MB solutions.
Fresh Azadirachta indica leaves were collected from the campus of MIT-WPU, Pune, India in the month of September. In order to remove the dust and other debris, the leaves of Azadirachta indica were washed thoroughly using distilled water. Washed leaves were then dried in a hot air oven at 60 °C temperature for 120–180 min. The dried Azadirachta indica leaves were then grounded into fine powder for utilization in further experiments.
2.2 Preparation of Aqueous Extract of Azadirachta Indica Leaf
An aqueous extract of Azadirachta indica leaf was used for green synthesis of Cu-NPs. To prepare the aqueous extract, 20 g of dried Azadirachta indica leaves powder were added to 200 ml of distilled water in 250 ml 3 neck round bottom flask. The mixture was continuously stirred using a magnetic stirrer and heated at 60 °C for 60–80 min in oil bath.
The prepared extract of Azadirachta indica leaves was cooled down to room temperature and was filtered using Whatman filter Paper No. 42 to separate the leaf debris. The filtrate called dark pale-yellow color aqueous extract was used immediately or stored in refrigerator at 4 °C for further use. The prepared extract was used within one week of preparation for nanoparticles synthesis.
2.3 Synthesis of Copper Nanoparticles
In this study, copper nanoparticles were synthesized through the green reduction approach utilizing Azadirachta indica leaf extract which acts as primary reducing and capping agent. For the synthesis of copper nanoparticles, 200 ml of 0.05 M aqueous solution of CuSO4·5H2O was prepared which was then heated at 80 °C with magnetic stirring at 500 rpm. Afterwards, 50 ml of aqueous Azadirachta indica leaf extract was added to the 200 ml of copper sulfate solution in a dropwise manner with constant stirring and heating for one hour.
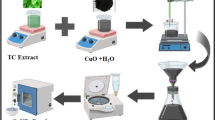
After complete addition of leaf extract, the resulting mixture was cooled down from 80 °C to room temperature. During the reaction, the color of the copper salt solution changed from bluish to yellowish to dark green, indicated the formation of CuNPs. The resulting dark color solution was centrifuged for 20 min at 7,000 rpm. Finally, pellets (dark brown color powder) were collected and was calcined in muffle furnace overnight at 40 °C. It was then stored at room temperature for further use. The schematic representation of green synthesis of CuNPs using neem leaf extract is as shown in Fig. 2.
2.4 Characterization of Synthesized Nanoparticles
The bioreduction of copper ions to CuNPs was initially confirmed by visual color change along with UV–Visible analysis (using UV–Visible Spectrophotometer Model UV-1800 by Shimadzu). The UV–Visible spectra for CuNPs confirmation and photocatalytic reduction of MB dye were recorded at a range of 200–800 nm. For FTIR analysis (Bruker, Model ALPHA II available at Central Instrumentation Facility, Savitribai Phule Pune University, Pune), CuNPs suspension was examined in a range of 500–4000 cm−1. The functional groups of active components of neem leaves extract responsible for reduction and capping of synthesized copper nanoparticles were determined from this FTIR spectrum. For determining the structure and size of synthesized CuNPs were examined under Field Emission Scanning Electron Microscopy (FESEM, Model FEI Nova NanoSEM 450 available at Central Instrumentation Facility, Savitribai Phule Pune University, Pune). The crystal structure of synthesized CuNPs was analyzed using X-ray diffractometer (Bruker-D8 Venture).
2.5 Photocatalytic Degradation of Organic Dye
Batch experiments were carried out to evaluate the photocatalytic activity of CuNPs for degradation of Methylene blue dye in presence of sunlight. Initially, stock solution of 10 mg/L of MB was prepared. In the first experimental run, 10 mg of the synthesized CuNPs was dispersed in 100 ml of 10 mg/L solution of methylene blue dye solution at pH value of 8.0 at ambient temperature.
Afterwards, the mixture was exposed to sunlight. At regular intervals of 10–15 min, 3 ml of sample was taken from the mixture. The progress of dye degradation reaction was examined using UV–Vis spectrophotometer with maximum absorbance around 650–665 nm and contact time of 120 min. Furthermore, initial concentration of MB was varied to 5 mg/L and 2.5 mg/L with the same dosage of photocatalyst (10 mg) to evaluate the effect of initial concentration of MB on dye removal efficiency of CuNPs.
3 Results and Discussion
3.1 UV–Visible Analysis of CuNPs
The formation of CuNPs was initially confirmed by the change in color from bluish to yellow to green upon the addition of aqueous extract of Azadirachta indica into aqueous copper sulfate solution and is presented in Fig. 3.
Further, UV–visible spectroscopy analysis was used to confirm the formation of CuNPs. Figure 4 shows the UV–visible spectrum of synthesized Cu-NPs using Azadirachta indica leaf extract. Spectrum clearly showed the characteristics absorption peak at 800 nm confirming the formation of copper nanoparticles. This absorption peak is also called as surface plasmon resonance (SPR) of CuNPs which is due to electron excitation in the conduction band of NPs surface. In addition, the absorption peaks between 200 and 400 nm is related to double bonds present in phenolic compound in extract. As per the literature study, when the wavelength of incident light exceeds the particle size diameter causes the transition of electrons from valence band to the conduction band and gives the absorption maxima at ~500–600 nm indicating the formation of CuNPs. This SPR band varies based on the size and shape of the NPs. Similar results of previous studies related to size variations of green synthesized CuNPs and SPR band support the findings of the present study [3, 11, 14, 15].
3.2 FTIR Analysis
FTIR analysis was carried out to identify the biomolecules present in neem leaf extract which are responsible for reduction and capping of CuNPs. The FTIR spectrum of green synthesized CuNPs suspension exhibited in Fig. 5 with absorptions peaks at 3263.51, 1635.77, 3000, 1098.97, 1260.84 cm−1, 941.91–506.33 cm−1 correspond to the functional groups of –OH, C═C, C–H, C–N, and C–O. These peaks indicate the presence of flavonoid and other phenolic compounds in the neem leaf extract which are responsible for the bio-reduction of Cu+ ions to CuNPs [3]. Thus, FTIR result analysis indicated that the surface of synthesized CuNPs was capped and stabilized by flavonoid and other phenolic compounds present in the Azadirachta indica leaf extract.
3.3 Morphology Analysis (SEM)
The morphology (shape and size) of synthesized CuNPs were characterized by FE-SEM. As shown SEM image in Fig. 6, synthesized CuNPs from leaf extract have spherical-shaped morphology which was consistent with previous findings [13]. Slight agglomeration was observed in the synthesized copper nanoparticles. The size of the particles was found to be in the range of 50–70 nm.
3.4 X-ray Diffraction Analysis
To analyze the crystal structure and phase identification of synthesized copper nanoparticles sample, XRD analysis was performed and displayed in Fig. 7. Three major peaks appeared at 2 θ values of 43.48°, 50.40°, and 74.54° which belongs to (111), (200), and (220) planes of face centered cubic crystal structure of copper. These XRD results are closely matched with the literature values of metallic copper [5]. In addition, the XRD pattern consists of small peaks (e.g. 36.50°, 47.5°) which indicates presence of small impurities.
3.5 Photocatalytic Degradation of Methylene Blue Dye
The photocatalytic activity of synthesized CuNPs for degradation of MB was evaluated under direct sunlight at different experimental conditions as initial concentration of MB (10, 5, and 2.5 ppm) and contact time (10–120 min). The photocatalytic performance of CuNPs was analyzed using the UV–Visible spectra of MB samples within the wavelength range of 400–800 nm with maximum absorption around 665 nm.
Figure 8 shows the UV–Visible spectra for the first case of 10 ppm initial concentration of MB at different irradiation time. The recorded spectrum exhibits the characteristic band of MB at λmax of ∼650–664 nm (Fig. 8). It was observed that as the exposure time increased, the absorption peak corresponding to MB decreased gradually. Results clearly indicating the presence of CuNPs reduces the intensity of the MB and thus, its photocatalytic activity. As a result, color of MB gradually lightened (from dark blue to bright blue). In the absence of CuNPs, MB aqueous solution was kept in sunlight for more than two hours, resulted in negligible change in color and dye degradation. These experimental findings exhibited that dye degradation was driven by a photocatalytic activity of CuNPs. The efficiency of the CuNPs for the degradation of MB can be calculated using the following equation [8].
where At and A0 are the absorbances at the time “t” and at the initial time, respectively. Figure 9 represents the degradation efficiency of the synthesized CuNPs for initial MB concentration of 10 ppm with time. Figure 9 clearly shows that the MB degradation efficiency of the CuNPs was approximately 42% after 120 min. Hence, the data elucidate that synthesized CuNPs through green route have good photodegradation activity for MB dye.
In general, steps in the degradation of methylene blue in presence of CuNPs and sunlight are as follows [3, 5, 8]. In first step, when the solar irradiation is absorbed by CuNPs, it gets photo excited due to SPR effect (Eq. 1). Secondly, the electron and holes generated in Eq. 1 can react with O2 (Eq. 2) and H2O (Eq. 3) molecules to provide active species, namely, hydroxyl radical (OH–), and anionic superoxide radical (O2–), respectively (Eq. 4). Finally, both oxidation and reduction proceed on the surface of the photocatalyst Eqs. 5 and 6.
Later, similar experimental runs were conducted with initial concentration of MB of 5 and 2.5 ppm with same dose of CuNPs and results of degradation process are exhibited in Figs. 10 and 11. It is clearly observed in Figs. 10 and 11 that with decrease in initial MB concentration leads to an increase in the rate of dye degradation when concentration of nanoparticles remained the same. Obtained percentage degradation efficiency of MB at 5 and 2.5 ppm is approximately 50% and 60% respectively. As a representative case, Fig. 12 depicts degradation efficiency for initial MB concentration of 2.5 ppm with time. This result indicated the approximately 19% increase in MB removal efficiency in 120 min of test was observed than that of 10 ppm. Comparison of these results indicated that the lower initial concentration of methylene blue or increasing the catalyst dosage leads to increment in the dye removal efficiency. The presence of a greater number of active sites on the Cu-NPs surface is related to this increased efficiency of dye removal and results were confirmed with the similar study carried out by Alhalili [13].
Furthermore, the obtained results of the present study were compared with previous studies of methylene blue degradation via green synthesized copper or copper oxide nanoparticles using different plant extracts [5, 9, 11, 15] and degradation results are presented in Table 1. It should be noted that the dye degradation efficiency is dependent on various process parameters such as active elements of plant extract, concentration of dye, dosage of nanoparticles, sunlight or UV light exposure and irradiation time. Although there are different possible factors that affects the photodegradation process, a comparative study clearly illustrates that our results exhibited the similar trend of dye degradation (refer Figs. 8, 9, 10, 11 and 12]. However, similar type of study using aqueous extract of Azadirachta indica leaves has been carried out by Ref. [11] and our findings are in close agreement with their results.
The kinetics of the photocatalyzed degradation of dye was described by a pseudo-first-order reaction for the concentration of MB [3].
where Co is the initial MB concentration and Ct is the MB concentration at the irradiation time (t) and k is the rate constant (min−1). The relationship between \(\mathrm{ln}{C}_{t}/{C}_{o}\) versus irradiation time (t) presented in Fig. 13 showed good linear correlation with the correlation coefficient of 0.94 (R2 > 0.94). The slope of the linear fitting line as shown in Fig. 13 depicts the rate constant (k) of the reaction was found 0.0084 min−1. From this kinetic study, it is concluded that the degradation of MB dye process resulted in pseudo-first-order kinetics with obtained value of rate constant 0.0084 min−1.
4 Conclusions
The copper nanoparticles (CuNPs) was successfully synthesized using leaf extract of Azadirachta indica (neem) which is simpler, green, environment-friendly, economical method and free of the use of any organic/toxic solvents, and specialized instruments. The UV–Visible Spectroscopy and FESEM analysis results confirmed the formation of spherical CuNPs with average size in the range of (50–70 nm). FTIR analysis confirmed the presence of various functional groups responsible for the reduction and stabilization of CuNPs. The results revealed that 60% of hazardous organic methylene blue dye was completely degraded using green synthesized CuNPs under sunlight and thus, showed good photocatalytic activity. The developed method provides an environmental and sustainable process for synthesis of CuNPs and its utilization for removal of industrial hazardous dye. This experimental study can be extended for green synthesis of other metal nanoparticles using suitable leaf extracts and evaluation of their applicability in dye removal.
References
Lin, J., Luo, Z., Liu, J., Li, P.: Photocatalytic degradation of methylene blue in aqueous solution by using ZnO–SnO2 nanocomposites. Mater. Sci. Semicond. Process. 87, 24–31 (2018)
Hou, Y., Yan, S., Huang, G., Yang, Q., Huang, S., Cai, J.: Fabrication of N-doped carbons from waste bamboo shoot shell with high removal efficiency of organic dyes from water. Biores. Technol. 303, 22939 (2020)
Mali, S.C., Dhaka, A., Githala, C.K., Trivedi, R.: Green synthesis of copper nanoparticles using celastrus paniculatus willd leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 27, 1–9 (2020)
UN WWDR: WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report 2017: Wastewater, The Untapped Resource. Paris, UNESCO (2017)
Sinha, T., Ahmaruzzaman, M.: Green synthesis of copper nanoparticles for the efficient removal (degradation) of dye from aqueous phase. Environ. Sci. Pollut. Res. 22, 20092–20100 (2015)
Usman, M., Ahmed, A., Yu, B., Peng, Q., Shen, Y., Cong, H.: Photocatalytic pote ntial of bio-engineered copper nanoparticles synthesized from Ficus carica extract for the degradation of toxic organic dye from waste water: growth mechanism and study of parameter affecting the degradation performance. Mater. Res. Bull. 120, 110583 (2019)
Khalafi, T., Buazar, F., Ghanemi, K.: Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 9, 6866 (2019)
Mehrabian, M., Esteki, Z.: Degradation of methylene blue by photocatalysis of copper assisted ZnS nanoparticle thin films. Optik 130, 1168–1172 (2017)
Ghosh, M.K., Sahu, S., Gupta, I., Ghorai, T.K.: Green synthesis of copper nanoparticles from an extract of jatropha curcas leaves: characterization, optical properties, CT-DNA binding and photocatalytic activity. RSC Adv. 10, 22027–22035 (2020)
Nagar, N., Devra, V.: Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 213, 44–51 (2018)
Rafique, M., Shaikh, A.J., Rasheed, R., Tahir, M.B., Gillani, S.S., Usman, A., Imran, M., Zakir, A., Khan, Z.U., Rabbani, F.: Aquatic biodegradation of methylene blue by copper oxide nanoparticles synthesized from azadirachta indica leaves extract. J. Inorg. Organomet. Polym Mater. 28, 2455–2462 (2018)
Ansilin, S., Nair, J.K., Aswathy, C., Rama, V., Peter, J., Persis, J.J.: Green Synthesis and characterisation of copper oxide nanoparticles using azadirachta indica (Neem) leaf aqueous extract. J. Nanosci. Technol. 2(5), 221–223 (2016)
Alhalili, Z.: Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: adsorption and design of experiments. Arab. J. Chem. 15, 103739 (2022)
Amaliyah, S., Pangesti, D.P., Masruri, M., Sabarudin, A., Sumitro, S.B.: Green synthesis and characterization of copper nanoparticles using Piper retrofractum Vahl extract as bioredutor and capping agent. Heliyon 6, e04636 (2020)
Manasa, D.J., Chandrashekar, K.R., Madhu Kumar, D.J., Niranjana, M., Navada, K.M.: Mussaenda frondosa L. mediated facile green synthesis of copper oxide nanoparticles–characterization, photocatalytic and their biological investigations. Arab. J. Chem. 14, 103184 (2021)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Datar, S., Kadu, S., Khurpade, P., Nandi, S. (2024). Experimental Study on Green Synthesis of Copper Nanoparticles and Its Utilization for Degradation of Organic Dye. In: Saxena, S., Shukla, S., Mural, P.K.S. (eds) Emerging Materials and Technologies in Water Remediation and Sensing. ICWT 2022. Lecture Notes in Civil Engineering, vol 439. Springer, Singapore. https://doi.org/10.1007/978-981-99-6762-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-6762-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6761-2
Online ISBN: 978-981-99-6762-9
eBook Packages: EngineeringEngineering (R0)