Abstract
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has now plagued the world for almost 3 years. Although vaccines are now available, the severity of the pandemic and the current dearth of approved effective medications have prompted the need for novel treatment approaches. Curcumin, as a food nutraceutical with anti-inflammatory and antioxidant effects, is now under consideration for the prevention and treatment of COVID-19. Curcumin has been demonstrated to retard the entrance of SARS-CoV-2 into cells, interfere with its proliferation inside cells, and curb the hyperinflammatory state caused by the virus by modulating immune system regulators, minimizing the cytokine storm effect, and modulating the renin-angiotensin system. This chapter discusses the role of curcumin and its derivatives in the prevention and treatment of COVID-19 infection, considering the molecular mechanisms involved. It will also focus on the molecular and cellular profiling techniques as essential tools in this research, as these can be used in the identification and development of new biomarkers, drug targets, and therapeutic approaches for improved patient care.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coronaviruses are single-stranded ribonucleic acid (RNA) viruses belonging to the family of Coronaviridae. They were first recognized as enzootic infection factors and also as human-contaminating agents [1]. Coronavirus disease 2019 (COVID-19) is a newly emerged disease with a rapid rise in mortality cases after its first detection in December 2019 [2]. The international virus classification committee proposed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as the name of the virus which causes COVID-19 disease [3]. The symptoms of COVID-19 infection can resemble those of the common cold and acute respiratory diseases, with infection of the respiration system (e.g., pneumonia or bronchitis) [4]. Compared to the other members of the coronavirus family, such as SARS and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), SARS-CoV-2 has higher transmissibility. The significance of this fact is that this virus can engage a higher number of people through contact with an infected patient [5].
COVID-19 infection results in acute upper respiration symptoms, such as sneezing, sore throat, fever, dry coughs, fatigue, sputum, dyspnea, and headache [6, 7]. Severe cases of this disease are marked by pneumonia, metabolic acidosis, septic shock, and hemorrhage [8]. The laboratory results of most cases indicate a reduction in the number of white blood cells and lymphocytes [6, 9]. In acute cases, neutrophil counts, urea, and creatinine levels also show a significant rise while the number of lymphocytes is reduced. Inflammatory factors, such as interleukin 6 and 17, and necrosis factors, such as tumor necrosis factor (TNF-α), often increase [10]. The current anti-virus treatments target human cells or the virus itself. Currently, there are eight approved treatments for use in the European Union, and this field is rapidly evolving [11]. In addition to investigating the effect of the chemical-pharmaceutical agents on the pathogenicity of the virus, several studies have addressed the influence of phytochemicals on coronavirus due to evidence of the antiviral efficacy of some plant-based compounds. Some phytochemicals have also been found to boost the immune system against various diseases through diverse cellular mechanisms [12, 13].
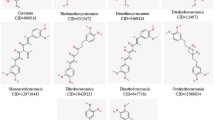
Curcumin is an important phytochemical compound that is extracted from the rhizome of Curcuma longa, also known as the turmeric plant. Turmeric includes an extensive spectrum of phytochemicals, such as curcumin, demethoxycurcumin, zingiberene, curcumenol, eugenol, triethyl curcumin, and turmerones. However, most of the therapeutic features of turmeric have been ascribed to curcumin [14]. Numerous studies have confirmed curcumin‘s antioxidant and anti-inflammatory effects, making it a viable candidate for treating diseases marked by disturbances in these pathways [15,16,17,18,19,20,21,22,23,24,25,26,27,28]. The anti-inflammatory effects of curcumin are comparable with those of anti-inflammatory steroids and non-steroid drugs [29] and appear to be mediated by inhibition and suppression of the prostaglandins synthesis and inhibition of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), as well as suppression of the production of cytokines, such as gamma interferon (INFγ) and tumor necrosis factor-alpha (TNFα) and activation of transcription factors such as nuclear factor-κB (NF-κB) [30]. Some studies have confirmed the role of curcumin in the inhibition of the proliferation of some viruses, such as human papillomavirus (HPV) and human immunodeficiency virus (HIV) [31]. Antiviral effects have also been proven against COVID-19 disease [32, 33]. Hence, curcumin appears to be a potential phytochemical for preventing and treating COVID-19 infection.
Recently, phytochemical investigations have been conducted to unveil the various molecular effects and pharmacological properties involved in their mechanisms of action [34]. This is important as some patients do not respond to particular drug therapy or suffer from adverse effects limiting the drug development process [35, 36]. Therefore, validated biomarkers that predict the effects of drugs and establish optimal therapeutic dosages are urgently needed [37]. In addition, the emergence of systems biology techniques has brought dawn to researchers in COVID-19 medication, and there are also many new technologies and strategies for drug design that can promote research in this field [38]. Accordingly, this study was conducted to review the molecular mechanisms and methods used in the study of curcumin and its derivatives in the prevention and treatment of COVID-19.
2 Methods
This review was carried out in a narrative manner by searching the databases PubMed, Web of Science, and Science Direct using coronavirus-19, COVID-19, SARS-CoV-2, curcumin, nanocurcumin, turmeric, nutraceutical, and phytochemical keywords without any limiting search items. The main objective was to report on the clinical trials which have investigated the effects of various forms of curcumin in the treatment of COVID-19 patients (Table 21.1). A secondary objective was to report on the molecular techniques used in these studies as biomarkers of efficacy or toxicities.
3 Results
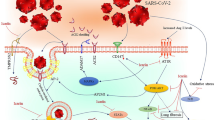
Results of the studies on the mechanisms of action showed the effect of curcumin on the prevention and treatment of COVID-19 at four stages of the virus life cycle, including entry into the cell, viral replication, cytokine storm effects, and involvement of the renin-angiotensin system. It should be noted that all the hypotheses mentioned in this study are based on the assumption that the immune response against COVID-19 is similar to that caused by other coronaviruses, which should be confirmed with further studies. This is important to aid preparedness for future coronavirus pandemics. These four stages are discussed in the following sections.
3.1 Cellular Entry of SARS-CoV-2 and Curcumin
Angiotensin-converting enzyme 2 (ACE2) receptor on host cell surface acts as an attachment and entry port for the SARS-CoV-2 virus. The SARS-COV-2 spike glycoprotein has two structural subunits (S1 and S2), which play separate roles in identifying and binding to the receptor and promoting fusion with the host cell membrane [39]. The attachment stage occurs through the binding of the receptor-binding domain (RBD) in the S1 subunit to ACE2 on the target cells [40]. Studies have shown that curcumin interacts with and can block this stage of SARS-COV-2 infection [41]. Various amino acid residues of ACE2 (e.g., alanine 348, asparagine 394, glutamate 402, histidine 378, and Tyrosine 385) have been identified as being in the active binding site in the interaction with curcumin [41]. Also, curcumin reduces the expression of TMPRSS-2 [42], which is one of the main activating proteases of host cells, permitting entry of the SARS-Cov-2 virus [39]. This enzyme cleaves the spike glycoprotein between the S1 and S2 domains to allow the fusion of the S2 subunit with the host cell membrane. Moreover, curcumin has a high binding affinity to the SARS-CoV-2 nucleocapsid proteins, which regulates replication of the viral RNA, inhibits protein translation, alters the cell cycle, and promotes apoptosis in host cells [43]. Thus, some of the antiviral properties of curcumin might arise by blocking the actions of this protein.
3.2 SARS-CoV-2 Proliferation and Curcumin
A large number of studies have investigated the key factors and enzymes involved in SARS-CoV-2 replication. Most of these have been carried out on the RNA-dependent RNA polymerase (RdRp) and the main protease (MPro; a 3CL-like enzyme). The MPro enzyme is responsible for the proteolytic cleavage of the viral polyprotein into distinct active peptides and the RdRp, which is also known as non-structural protein 12 (NSP12), is responsible for catalyzing the synthesis of the new viral RNA as part of the replication and transcription process [44]. In silico molecular docking studies have suggested that curcumin can bind directly to the MPro enzyme and the RdRp [45,46,47]. Other molecular docking studies have shown the effects of curcumin in blocking SARS-CoV-2 reproduction by targeting NSP9 of the viral replicase. NSP9 binds to single-stranded RNA and works in concert with the RdRp complex in the replication process [48]. Although a number of drugs have now been identified which can inhibit the activity of the RdRp complex, as well as the MPro enzyme and NSP9 [49], these will require further in vitro and in vivo validation. However, as curcumin has shown inhibitory properties against the viral replication cycle, it is possible that it achieves these effects by targeting some or all of the above SARS-CoV-2 proteins [50, 51]. Thus, curcumin has the potential to interfere with the process of replication of SARS-CoV-2 RNA in the generation of new virus particles.
3.3 COVID-19, the Cytokine Storm, and Curcumin
In any type of viral infection, inflammatory cytokines such as IL-1, IL-6, and TNF-α are actively released by immune cells into the bloodstream [51]. The release of large amounts of cytokines into the systemic circulation is often referred to as a cytokine storm [52]. Such an increase in cytokine levels in COVID-19 cases is associated with conditions related to acute respiratory distress syndrome (ARDS) and multiple organ damage, which can lead to a poorer prognosis [53, 54]. Curcumin also shows promise as a novel and effective treatment of the cytokine storm effects as the immunomodulatory activities of this molecule have been well established in different studies [32, 55, 56]. The ability of curcumin to suppress the cytokine storm and its potential in treating viral disorders, including those caused by coronaviruses, supports the case that it may be an effective treatment for COVID-19 [32]. The inflammatory factors released in the cytokine storm include IL-1, IL-2, IL-6, IL-10, transforming growth factor-β (TGF-β), interferons (IFNs), and TNF-α. The expression of IL-6 and TNF-α is mainly associated with COVID-19-related ARDS, which may contribute to organ damage in severe cases [53, 54]. Curcumin has a suppressive effect on IFN-α, and its lowering effect on inflammatory cytokines has been attributed to the inhibition of NF-κB signaling [55]. In a study conducted on the effects of nanocurcumin on the modulation of the inflammatory cytokines in COVID-19 patients, curcumin was found to temper the virus-related increase in inflammatory cytokines at both the mRNA and protein levels [57]. In another study of mild and severe nanocurcumin-treated COVID-19 patients, a significant decrease was observed in the number of inflammatory markers, including pro-inflammatory Th17-related cytokines [58]. Hassaniazad et al. also investigated the effect of curcumin nanomicelles in a clinical study on the cellular immune response in COVID-19 patients [59]. This revealed a rise in the levels of the anti-inflammatory cytokines IL-4 and TGF-β in the group receiving nanocurcumin, compared to the placebo group on the day 14.
The effect of curcumin to stimulate the production of anti-inflammatory factors may aid in at least a partial restoration of the cytokine balance by modulating key regulatory elements of immune and inflammatory pathways, thereby reducing the cytokine storm response to viral infection and minimizing the potentially damaging oxidizing effects of excessive reactive oxygen species (ROS) production [32].
3.4 Renin-Angiotensin System, SARS-CoV-2, and Curcumin
ACE2 present on the surface of host cells provides a target for the spike glycoprotein of SARS-CoV-2 as an entry point for viral infection via endocytosis [40]. Simultaneous internalization of ACE2 has been reported during cellular entry of SARS coronaviruses, including SARS-CoV-2 [39, 60]. As ACE2 normally acts to inactivate angiotensin II (AngII), a decrease in cell surface ACE2 levels can lead to the accumulation of this peptide hormone and high levels of AngII have been associated with acute lung injury in COVID-19 patients [54]. The mechanism of this is likely due to the fact that AngII is a peptide hormone that acts as a vasoconstrictor, which can lead to high blood pressure and trigger an inflammatory response. High AngII levels can stimulate the AT1 angiotensin receptor, which can have multiple adverse effects on physiology, including those mentioned above, as well as fibrosis and ARDS [61].
Curcumin has been reported to modulate the level of AngII expression and prevent inflammation-associated fibrosis [62]. Furthermore, the modulation of ACE2 levels by curcumin has also been documented [62, 63]. Such modulation of ACE2 expression by curcumin could lead to reduced AngII cell signaling and the subsequent damage and pathological consequences. Furthermore, curcumin has been found to inhibit high blood pressure by lowering the expression of angiotensin 1 converting enzyme (ACE1) in a rat model of hypertension [64]. Curcumin has also been found to reduce the expression of the angiotensin receptor AT1 in an AngII infusion model of fibrosis in rats [62]. Consistent with this, curcumin treatment was found to reduce AngII-induced hypertension in a mouse model [65].
Taken together, these findings indicate that treatment with curcumin can be used in the treatment of COVID-19 disease effects via multiple complementary pathways (Fig. 21.1).
4 Cellular and Molecular Profiling Techniques Used in the Assessment of Curcumin Efficacy in the Treatment of COVID-19 Disease
Different cellular and molecular profiling techniques were used to evaluate the biomarkers linked with the mechanism of action of curcumin treatment in the above studies. Valizadeh et al. used both real-time PCR and an enzyme-linked immunosorbent assay (ELISA) approach for evaluating the effects of curcumin on the production of the IL-1β, IL-6, IL-18, and TNF-α in peripheral blood mononuclear cells (PBMCs) and serum from COVID-19 patients [57]. The real-time quantitative PCR method allowed simultaneous multiplex detection of the different cytokines through the use of nucleotide probes linked with different fluorescent tags, as described by Hawkins and Guest [66]. In a similar manner, Hassaniazad et al. used real-time PCR analysis to examine immune response gene expression changes in the transcription factors TBX21, GATA-3, FOXP3, and RAR-related orphan receptor γt (ROR-γT) and ELISA to measure the serum levels of IFN-γ, IL-4, IL-17, and TGF-β cytokines in investigating the effects of curcumin nanomicelles on clinical cellular immune responses in COVID-19 patients [59]. Tahmasbi et al. used a flow cytometry technique to measure the frequency of circulating Th17 cells in patients with COVID-19 and healthy subjects using monoclonal antibodies against surface and intracellular markers [67]. They also evaluated mRNA expression profiles of Th17 cell-related factors RORγt, IL-17, IL-21, IL-23, using real-time PCR and the secreted levels of serum IL-17, IL-21, IL-23, and granulocyte-macrophage colony-stimulating factor (GM-CSF) via ELISA. Similar approaches were employed by Asadirad et al. in the measurement of TNF-α, IL-1β, IL-6, and IFN-γ inflammatory cytokines by the real-time PCR method and analyses of serum levels of TNF-α, IL-1β, and IL-6 using cytokine ELISA kits [68].
Other multiple analyte probing techniques have also been described, which can also be applied in the study of risk factors for COVID-19 and for assessing the effects of this disease in cells and circulation, and also for monitoring the response to various pharmaceutical and phytochemical treatments such as curcumin. These techniques include multiplex immunoassay [69] and biochip arrays [70] for the simultaneous measurement of multiple analytes such as inflammation- and immune-related factors. Given the multi-faceted nature of COVID-19 disease, the multiplex biomarker technologies listed above enable the simultaneous analysis of numerous analytes for interrogation of disease and treatment effects on specific protein pathways such as inflammation and oxidative damage. There are also methods for measuring effects on entire protein pathways, such as kits developed for determining total antioxidant capacity and coagulation status [71] in blood samples. Figure 21.2 indicates some of the main multiplex molecular profiling technologies employed in studying the effects of curcumin on COVID-19 disease.
5 Conclusions and Future Perspectives
In this chapter, we have summarized the disrupted molecular pathways that are potentially targeted by the phytochemical curcumin in COVID-19 disease prevention and treatment. We have also described the main methods which have been used to investigate these effects, with a focus on multiplex molecular profiling techniques such as real-time PCR and multi-analyte immunoassay. Curcumin has protective effects in preventing viruses from entering the cells, reducing virus proliferation, decreasing the cytokine storm effects, and modulating the renin-angiotensin pathway. Further studies on the possible use of natural compounds such as curcumin and the application of the appropriate molecular profiling approaches to monitor disease and treatment effects can lead to improved management of coronavirus and other viral infections during the current pandemic and future outbreaks.
References
Moreno-Eutimio MA, Lopez-Macias C, Pastelin-Palacios R (2020) Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect 22(4–5):226–229
Lai CC, Shih TP, Ko WC, et al (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 55(3):105924. https://doi.org/10.1016/j.ijantimicag.2020.105924
Gorbalenya AE, Baker SC, Baric RS, et al (2020) Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group. BioRxiv. https://doi.org/10.1038/s41564-020-0695-z
Wang M, Cao R, Zhang L, et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30(3):269–271
Elena SF, Sanjuán R (2005) Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J Virol 79(18):11555–11558
Guan WJ, Ni ZY, Hu Y, et al (2020) Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med 382(18):1708–1720
Guo YR, Cao QD, Hong ZS, et al (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res 7(1):1–10
Li JY, You Z, Wang Q, et al (2020) The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect 22(2):80–85
Liu K, Fang YY, Deng Y, et al (2020) Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chinese Med J 133(09):1025–1031
Huang C, Wang Y, Li X, et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
European Medicines Agency; COVID-19 Treatments; Approved for use in the European Union (2022). https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments. Accessed October 24, 2022
Sahebkar A, Henrotin Y (2016) Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials. Pain Med 17(6):1192–1202
Praditya D, Kirchhoff L, Brüning J et al (2019) Anti-infective properties of the golden spice curcumin. Front Microbiol 10912. https://doi.org/10.3389/fmicb.2019.00912
Khurana A, Ho CT (1988) High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma longa L. J Liq Chromatogr 11(11):2295–2304
Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, et al (2018) Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev 17(2):125–135
Hasanzadeh S, Read MI, Bland AR, et al (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921
Iranshahi M, Sahebkar A, Takasaki M, et al (2009) Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev 18(5):412–415
Panahi Y, Ghanei M, Bashiri S, et al (2014) Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res 65(11):567–573
Parsamanesh N, Moossavi M, Bahrami A, et al (2018) Therapeutic potential of curcumin in diabetic complications. Pharmacol Res 136:181–193
Sahebkar A (2010) Molecular mechanisms for curcumin benefits against ischemic injury. Fertil Steril 94(5):e75–e76
Shakeri A, Cicero AFG, Panahi Y, et al (2019) Curcumin: A naturally occurring autophagy modulator. J Cell Physiol 234(5):5643–5654
Afshari AR, Jalili-Nik M, Abbasinezhad-Moud F, et al (2021) Anti-tumor effects of curcuminoids in glioblastoma multiforme: An updated literature review. Curr Med Chem 28(39):8116–8138
Alidadi M, Jamialahmadi T, Cicero AFG, et al (2020) The potential role of plant-derived natural products in improving arterial stiffness: A review of dietary intervention studies. Trends Food Sci Technol 99:426–440
Gorabi AM, Kiaie N, Hajighasemi S, et al (2019) The effect of curcumin on the differentiation of mesenchymal stem cells into mesodermal lineage. Molecules 24(22):4029. https://doi.org/10.3390/molecules24224029
Mohajeri M, Bianconi V, Ávila-Rodriguez MF, et al (2020) Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacological Research 156:104765. https://doi.org/10.1016/j.phrs.2020.104765
Heidari Z, Daei M, Boozari M, et al (2022) Curcumin supplementation in pediatric patients: A systematic review of current clinical evidence. Phytother Res 36(4):1442–1458
Panahi Y, Khalili N, Hosseini MS, et al (2014) Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement Ther Med 22(5):851–857
Kurien BT,Scofield RH (2009) Oral dministration of heat-solubilized curcumin for potentially increasing curcumin bioavailability in experimental animals. Int J Cancer 125(8):1992. https://doi.org/10.1002/ijc.24547
Masferrer JL, Zweifel BS, Manning PT, et al (1994) Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Nat Acad Sci USA 91(8):3228–3232
Surh YJ, Chun KS, Cha HH, et al (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res 480–481:243–268
Sui Z, Salto R, Li J, et al (1993) Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg Med Chem 1(6):415–422
Zahedipour F, Hosseini SA, Sathyapalan T, et al (2020) Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res 34(11):2911–2920
Vahedian-Azimi A, Abbasifard M, Rahimi-Bashar F, et al (2022) Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: A systematic review of clinical trials. Nutrients 14(2):256. https://doi.org/10.3390/nu14020256
Alam S, Sarker MMR, Afrin S, et al (2021) Traditional herbal medicines, bioactive metabolites, and plant products against COVID-19: update on clinical trials and mechanism of actions. Front Pharmacol 12:671498. https://doi.org/10.3389/fphar.2021.671498
Yang Y, Blomme EA, Waring JF (2004) Toxicogenomics in drug discovery: from preclinical studies to clinical trials. Chem Biol Interact 150(1):71–85
Rahmoune H, Guest PC (2017) Application of multiplex biomarker approaches to accelerate drug discovery and development. Methods Mol Biol 1546:3–17
Saigusa D, Matsukawa N, Hishinuma E, et al (2021) Identification of biomarkers to diagnose diseases and find adverse drug reactions by metabolomics. Drug Metab Pharmacokinet 37:100373. https://doi.org/10.1016/j.dmpk.2020.11.008
Guest PC (2022) Multiplex Biomarker Techniques: Methods and Applications for COVID-19 Disease Diagnosis and Risk Stratification; Methods in Molecular Biology, 251; Humana Press; Totowa, NJ, USA. ISBN-13: 978-1071623947
Hoffmann M, Kleine-Weber H, Schroeder S, et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e278
Shang J, Wan Y, Luo C, et al (2020) Cell entry mechanisms of SARS-CoV-2. Proc Nat Acad Sci USA 117(21):11727–11734
Maurya VK, Kumar S, Prasad AK, et al (2020) Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease 31(2):179–193
Katta S, Srivastava A, Thangapazham RL, et al (2019) Curcumin-gene expression response in hormone dependent and independent metastatic prostate cancer cells. Int J Mol Sci 20(19):4891. https://doi.org/10.3390/ijms20194891
Suravajhala R, Parashar A, Malik B, et al (2020) Comparative docking studies on curcumin with COVID-19 proteins. Preprints 2020050439. https://doi.org/10.20944/preprints202005.0439.v3
Molavi Z, Razi S, Mirmotalebisohi SA, et al (2021) Identification of FDA approved drugs against SARS-CoV-2 RNA dependent RNA polymerase (RdRp) and 3-chymotrypsin-like protease (3CLpro), drug repurposing approach. Biomed Pharmacother 138:111544. https://doi.org/10.1016/j.biopha.2021.111544
Horowitz RI, Freeman PR (2020) Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med Hypotheses 143:109851. https://doi.org/10.1016/j.mehy.2020.109851
Huynh T, Wang H, Luan B (2020) In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2’s main protease. J Phys Chem Lett 11(11):4413–4420
Enmozhi SK, Raja K, Sebastine I, et al (2021) Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn 39(9):3092–3098
Kumar M, Sodhi KK, Singh DK (2021) Addressing the potential role of curcumin in the prevention of COVID-19 by targeting the Nsp9 replicase protein through molecular docking. Arch Microbiol 203(4):1691–1696
Chandel V, Sharma PP, Raj S, et al (2022) Structure-based drug repurposing for targeting Nsp9 replicase and spike proteins of severe acute respiratory syndrome coronavirus 2 J Biomol Struct Dyn 40(1):249–262
Marín-Palma D, Tabares-Guevara JH, Zapata-Cardona MI, et al (2021) Curcumin inhibits in vitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms. Molecules 26(22):6900. https://doi.org/10.3390/molecules26226900
Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, et al (2019) The role of interleukin 6 during viral infections. Front Microbiol 101057. https://doi.org/10.3389/fmicb.2019.01057
Hu B, Huang S,Yin L (2021) The cytokine storm and COVID-19. J Med Virol 93(1):250–256
Vardhana SA, Wolchok JD (2020) The many faces of the anti-COVID immune response. J Exp Med 217(6):e20200678. https://doi.org/10.1084/jem.20200678
Hirano T, Murakami M (2020) COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 52(5):731–733
Xu Y, Liu L (2017) Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway. Influenza Other Respir Viruses 11(5):457–463
Kunnumakkara AB,Harsha C,Banik K et al (2019) Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin Drug Metab Toxicol 15(9):705–733
Valizadeh H,Abdolmohammadi-Vahid S,Danshina S et al (2020) Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol 89107088 https://doi.org/10.1016/j.intimp.2020.107088
Thimmulappa RK, Mudnakudu-Nagaraju KK, Shivamallu C, et al (2021) Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 7(2):e06350. https://doi.org/10.1016/j.heliyon.2021.e06350
Hassaniazad M, Eftekhar E, Inchehsablagh BR, et al (2021) A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res 35(11):6417–6427
Zhu J, Bultynck G, Luyten T, et al (2013) Curcumin affects proprotein convertase activity: Elucidation of the molecular and subcellular mechanism. Biochimica Biophys Acta (BBA)-Mole Cell Res 1833(8):1924–1935
D’ardes D, Boccatonda A, Rossi I, et al (2020) COVID-19 and RAS: unravelling an unclear relationship. Int J Mol Sci 21(8):3003. https://doi.org/10.3390/ijms21083003
Pang XF, Zhang LH, Bai F, et al (2015) Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des Devel Ther 9:6043–6054
Subhan F, Khalil AAK, Zeeshan M, et al (2020) Curcumin: from ancient spice to modern anti-viral drug in COVID-19 pandemic. Life and Science 1(supplement):69–73. https://doi.org/10.37185/LnS.1.1.137
Akinyemi AJ, Thome GR, Morsch VM, et al (2015) Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in L-NAME induced hypertensive rats. J Funct Foods 17:792–801
Yao Y, Wang W, Li M, et al (2016) Curcumin exerts its anti-hypertensive effect by down-regulating the AT1 receptor in vascular smooth muscle cells. Sci Rep 6(1):1–8
Hawkins SF, Guest PC (2022) Multiplex Quantitative Polymerase Chain Reaction Diagnostic Test for SARS-CoV-2 and Influenza A/B Viruses. Methods Mol Biol 2511:53–65
Tahmasebi S,El-Esawi MA,Mahmoud ZH et al (2021) Immunomodulatory effects of Nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J Cell Physiol 236(7):5325–5338
Asadirad A, Nashibi R, Khodadadi A, et al (2022) Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res 36(2):1023–1031
Guest PC, Abbasifard M, Jamialahmadi T, et al (2022) Multiplex Immunoassay for Prediction of Disease Severity Associated with the Cytokine Storm in COVID-19 Cases. Methods Mol Biol 2511:245–256
Zahedipour F, Guest PC, Majeed M, et al (2022) Evaluating the Effects of Curcumin on the Cytokine Storm in COVID-19 Using a Chip-Based Multiplex Analysis. Methods Mol Biol 2511:285–295
Guest PC, Rahmoune H (2022) Point-of-Care Device for Assessment of Blood Coagulation Status in COVID-19 Patients. Methods Mol Biol 2511:345–354
Pawar KS, Mastud RN, Pawar SK, et al (2021) Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol 1056. https://doi.org/10.3389/fphar.2021.669362
Askari G, Sahebkar A, Soleimani D, et al (2022) The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials 23(1):1–10
Competing Interests
MM is the founder of Sami-Sabinsa group of companies.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Golpour-Hamedani, S. et al. (2023). Antiviral Mechanisms of Curcumin and Its Derivatives in Prevention and Treatment of COVID-19: A Review. In: Guest , P.C. (eds) Application of Omic Techniques to Identify New Biomarkers and Drug Targets for COVID-19. Advances in Experimental Medicine and Biology(), vol 1412. Springer, Cham. https://doi.org/10.1007/978-3-031-28012-2_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-28012-2_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28011-5
Online ISBN: 978-3-031-28012-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)