Abstract
The gap between improvements in lifespan and age-related health is widening. Globally, the demographic of ageing is increasing and there has emerged a ‘diseasome of ageing’, typified by a range of non-communicable diseases which share a common underlying component of a dysregulated ageing process. Within this, chronic kidney disease is an emerging global epidemic.
The extensive inter-individual variation displayed in how people age and how their diseasome manifests and progresses, has required a renewed focus on their life course exposures and the interplay between the environment and the (epi)genome. Termed the exposome, life course abiotic and biotic factors have a significant impact on renal health.
We explore how the exposome of renal ageing can predispose and affect CKD progression. We discuss how the kidney can be used as a model to understand the impact of the exposome in health and chronic kidney disease and how this might be manipulated to improve health span.
Notably, we discuss the manipulation of the foodome to mitigate acceleration of ageing processes by phosphate and to explore use of emerging senotherapies. A range of senotherapies, for removing senescent cells, diminishing inflammatory burden and either directly targeting Nrf2, or manipulating it indirectly via modification of the microbiome are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Improvement in human life expectancy over the preceding 150 years has not been matched by a similar improvement in health span (i.e., years of healthy living). Consequently, there has been a significant shift in the global demographic of ageing, with the >60s predicted to outnumber the <15 year olds by 2050 (United Nations 2018).
This situation is compounded by the sequiturs of (i) multi-morbidity among the aged as they develop a ‘diseasome of ageing’ (ii) the staggering cost of addressing this, imposed on global social and health care systems (estimated pre-Covid pandemic at ~$47 trillion between 2010–2030) (Chen et al. 2018), (iii) low quality of life and (iv) the amplification of its effects by social deprivation (Shiels et al. 2021). Understanding the extrinsic factors underlying these health disparities is critical if we are to develop and apply suitable mitigation strategies to improve health and wellbeing. It is thus crucial that we have a better understanding of how we age at both a mechanistic biological level and an environmental/planetary level.
Ageing Is a Process
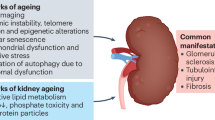
Ageing is a process starting pre-conception and ending in death. It is segmental, occurring at different rates in different organ systems and exhibiting significant inter-individual variation (Shiels et al. 2017; Stenvinkel and Shiels 2019). Its phenotype can be hallmarked with a series of features that are common across taxa (Stenvinkel and Shiels 2019). These comprise genomic instability, epigenetic dysregulation, telomere attrition, increasing cellular senescence, stem cell exhaustion, loss of proteostasis, dysregulated nutrient-sensing, mitochondrial dysfunction, and altered intercellular communication (López-Otín et al. 2013). In mammals, these are interfaced with diminished expression of the master cytoprotective regulator nuclear factor erythroid 2-related factor 2 (Nrf2), the chronic sterile inflammatory burden of ‘inflammageing’, alongside derepression of genomic retrotransposons and microbial dysbiosis (De Cecco et al. 2019; Simon et al. 2019; Mafra et al. 2022c, d). Whether these hallmarks act independently, cumulatively, or synergistically, remains to be determined (Shiels et al. 2019).
A Geroscience Approach to Age-Related Disease
Ageing is malleable and ill health in late life is not inevitable. Unequivocal evidence has shown that ageing can be forestalled by interventions that mitigate age-related deterioration of physiological function. The ‘diseasome of ageing’ comprises age-related non-communicable diseases where chronological age is a major risk factor. These include cancer, neurodegenerative diseases, osteoporosis, arthritis, non-alcoholic steatohepatitis, type 2 diabetes, and chronic kidney disease (CKD) (Shiels et al. 2021). They all, however, share a common underpinning feature of a dysregulated ageing process, in concert with inflammageing (chronic sterile inflammatory burden), phosphate toxicity, diminished systemic Nrf2 expression, and microbial dysbiosis (Ebert et al. 2020). The emergent phenotype of ageing reflects an interplay between the (epi)genome and the exposome (Fig. 5.1). The exposome constitutes the sum of all environmental exposures across the life course (Wild 2005). This entails interplay between biotic and abiotic environmental factors and the (epi)genome (Horton et al. 2014). Notably, only three basic exposome factors, comprising air pollution, tobacco smoke, and diet, are thought to be responsible for ~50% of annual global deaths (Lim et al. 2012).
The exposome of ageing. The totality of biotic and abiotic environmental exposures over the life course has a dramatic impact on health and is reflected in substantial inter-individual variation in health and both physical and physiological capability. Exposome effects are mediated by interaction with the (epi)genome
Conventionally, the individual disease modalities within the ‘diseasome of ageing’ are treated separately, typically resulting in a short improvement in health span. This is pertinent to the prediction that curing cancer or heart disease in a typical 50-year-old woman would only add 2–3 years of extra health span, while treating the underpinning component of ageing may add up to 25 years (Burch et al. 2014). Geroscientists have thus been characterizing basic mechanisms of ageing across a range of species to develop candidate anti-ageing therapies (e.g., nutritional, lifestyle, and pharmacological interventional strategies) that are now tested in human trials. This approach is not always as straightforward, or intuitive, as it first appears. Extrapolation of data from standard laboratory pre-clinical models to human interventions is often fraught. A consistent feature of this is the failure of drug therapies in late-stage clinical trials after successful pre-clinical testing. Additionally, many standard laboratory models are metabolically morbid (Stenvinkel et al. 2021a) and neglect to test for the effects of antagonistic pleiotropy (i.e., what is good for you when young, is not necessarily good for you when you are old and vice versa) (Williams 1957). In this instance, cellular senescence is a case in point, being onco-protective at older age, but diminishing physiological capability and increasing inflammatory burden at younger age. This is important when determining when and where in the life course any interventions need to be instigated, as these may engender substantially different effects. As such, age-related physical and physiological decline can be ameliorated differentially. To compensate for this, it may be better to consider ageing as a systemic burden of ‘wear and tear’. In this respect, we have modelled ageing in the kidney to explore how this might be used to mitigate decline in age-related health span (Kooman et al. 2014; Mafra et al. 2021).
Modelling Ageing Using the Kidney
Studies of patients with kidney failure have presented a unique opportunity to compare the ageing process in terms of both normative and dysregulated ageing. While analysis of renal transplants as a source of healthy tissue has been used to track normative age-related renal function (Shiels et al. 2017), CKD represents the flip side of the coin. CKD is a progressive and deteriorating health condition that poses a major social and economic challenge to the global population (Carney 2020). As the ageing population continues to grow around the globe, more people are presenting with CKD, either independently or together with other co-morbidities within the ‘diseasome of ageing’. Emerging data show that changes in the environment, such as air pollution and global warming, increase the risk of kidney disease (Stenvinkel et al. 2020). CKD is characterized by a gradual decline in the physiological functions of the kidney over time. As the disease progresses, the kidneys are unable to remove harmful substances and excess fluids from the bloodstream, leading to the build-up of toxins, water, and electrolytes in the body. This eventually causes kidney failure in patients and earlier age-related mortality. Compared with the general population, CKD patients have an approximately 20–70% reduction in overall life expectancy depending on their levels of kidney function and the age of onset (Bikbov et al. 2020).
The estimated global prevalence of CKD is around 10–12% with the all-cause mortality rate increasing by >40% within 27 years (1990–2017). The growing burden of CKD is directly proportional to the dominant demographic of ageing, regional socioeconomic disparities, as well as associated risk factors, including hypertension and diabetes. These contributing factors were estimated to be responsible for more than half of the CKD-associated mortality rate in 2017 (Bikbov et al. 2020). CKD-associated premature ageing has been linked with a range of biochemical features, including the excessive accumulation of uremic toxins, hyperphosphatemia, and mitochondrial dysfunction/oxidative stress (Kooman et al. 2014). The impact of accumulated levels of uremic toxins on the phenotype has been described in depth elsewhere (Ebert et al. 2020). Presently, >150 uremic solutes have been reported (Falconi et al. 2021). Many of these uremic toxins including indoxyl sulfate and p-cresyl sulfate cause extreme and irreversible damage to the kidney and surrounding organs (e.g., heart, liver, brain, or lung) (Vanholder et al. 2014).
Potential underlying mechanisms (Fig. 5.2) include accelerating cellular senescence, impairing Nrf2-mediated stress responses, increasing oxidative stress, and increasing inflammatory burden (Lisowska-Myjak 2014). A clear evidence base indicates that uremia shares underpinning molecular processes with the ageing process, including the hallmarks of cumulative cellular senescence, telomere attrition, post-translational protein modification, stem cell exhaustion, epigenetic dysregulation, and mitochondrial dysfunction (Kooman et al. 2014; Ebert et al. 2022).
Akin to chronic exposure to uremic toxins, CKD patients also develop immuno-senescence and vascular senescence, due to dialysis bio-incompatibility with complement activation (Losappio et al. 2020). Overactivation of both immune cells (particularly macrophages and neutrophils) and the renin-angiotensin system result from this (Losappio et al. 2020). As Nrf2 expression diminishes in the course of CKD, cytoprotection via antioxidant factors also systemically diminishes (Stenvinkel and Shiels 2021). Even early-stage CKD patients experience this systemic loss of redox homeostasis and display increased catabolic metabolism. These events trigger an increase in ATP consumption, thus forming a vicious cycle of mitochondrial dysfunction-oxidative stress-inflammation as CKD progresses (Peter 2021).
CKD also reflects a substantial relationship between the exposome and age-related renal health. Patients with CKD are predominantly reported in low- and middle-income nations (78% of the total patients), as well as less-developed regions in richer countries, where a significant health disparity exists (Mills et al. 2015). Exposome factors, such as socioeconomic position (SEP) and imbalanced diet are significant drivers of this health disparity (Craven et al. 2021). The mechanistic basis of this is not fully determined, but recent research from the National Longitudinal Study of Adolescent to Adult Health has indicated that the influence of SEP can be tracked from the late third decade of life using transcriptional signatures that can predict later life disease risk (Shanahan et al. 2022). These data have highlighted SEP-based inequalities in immune, inflammatory, and metabolic pathways which play key roles in the ageing process. Other exposome factors, such as microbial dysbiosis and hyperphosphataemia have already been linked directly to poorer renal function and increased disease risk among those at lower SEP, correlated with an imbalanced diet (McClelland et al. 2016; Craven et al. 2021). This pattern has also been confirmed by the mechanistic correlation between global DNA hypermethylation and renal dysfunction (Shiels et al. 2017). Further exposome effects on renal dysfunction can be gauged by the impact of the Covid pandemic (Stenvinkel et al. 2021b), global warming, and air pollution which have had a disproportionate effect on the renal health of the elderly (Stenvinkel et al. 2020; Avesani et al. 2022).
These environmental risk factors trigger an inflammatory response that accelerates the ageing process in CKD patients (Kooman et al. 2017). Consistent with this, global warming negatively affects the diversity of microbiota composition (Bestion et al. 2017). This condition might be worsened by long-term exposure to air pollution, which increases the risk of CKD and CVD. It has been shown that exposure to traffic-related air pollution for one year increases CKD incidence and risk of CKD development in older individuals (Kuźma et al. 2021). A more tractable exposome factor affecting renal health is the diet. In this respect, phosphate biology is highly pertinent.
Phosphate Biology
Serum phosphate (PO4, often denoted as Pi for ‘inorganic phosphate’) levels correlate strongly with lifespan in mammals (Kuro-o 2010a; Stenvinkel et al. 2018). In man, high serum Pi levels correlate with accelerated biological ageing and poorer renal function (McClelland et al. 2016), as well as all-cause and cardiovascular mortality risk (Chang et al. 2014). Additionally, in the general population higher Pi levels are associated with accelerated vascular ageing (Yoo et al. 2016). In CKD, phosphate is also considered a uremic toxin which provides a direct mechanistic link with the exposome of ageing. The source of phosphate, which mostly comes from protein-rich food consumption such as meat, fish, and eggs, is naturally regulated by vitamin D, parathyroid hormone and FGF-23/Klotho (Buchanan et al. 2020). Food additives contain phosphate (Ritz et al. 2012). Dietary-induced hyperphosphatemia contributes to poorer health in the general population while acting as a key driver of premature ageing in CKD patients (McClelland et al. 2016).
Phosphorus is one of the most abundant atoms in our bodies and an essential element for all known forms of life (Penido and Alon 2012). Phosphate has numerous vital biological functions: it is present in nucleic acids (DNA and RNA), energy-storing molecules such as ATP and GTP, the phospholipids that make up cellular and organellar bio-membranes, and mineralised tissues such as bone and teeth (Penido and Alon 2012). The addition or removal of Pi groups from proteins (phosphorylation and dephosphorylation), carried out by kinases, phosphatases, and phosphorylases, is a key mechanism of post-translational regulation of protein activity (Johnson 1997). Thus, it is of paramount importance for organisms to ensure adequate availability of this molecule. Indeed, one of the functions of mineralised bone is to serve as a reservoir of calcium and Pi and to buffer the levels of these ions in the blood (Copp and Shim 1963).
Serum phosphate levels are regulated by a bone-kidney endocrine axis that includes fibroblast growth factor-23 (FGF-23), Klotho, vitamin D, and parathyroid hormone (PTH) (Fig. 5.3) (Ebert et al. 2020). When the Pi level is high, FGF-23 secreted from the bone stimulates Pi excretion in the kidneys by binding to the FGF-23 receptor (and the transmembrane form of Klotho as obligate co-receptor) and reduces absorption in the intestine by lowering serum vitamin D levels (Kuro-o 2010b). Vitamin D, on the other hand, is transformed into the active 1,25-dihydroxyvitamin D3 in the kidneys and promotes Pi absorption in the intestine and release from the bone (Kuro-o 2010b). Parathyroid hormone stimulates vitamin D synthesis, but also has a parallel effect that induces Pi excretion, thus leading to an increase in blood calcium concentration without a parallel increase in blood Pi concentration (Kuro-o 2010b).
Interestingly, Klotho also has a soluble form, whose role in Pi homeostasis is still poorly understood (Tan et al. 2017; Batlahally et al. 2020). Soluble Klotho concentration decreases with age and negatively correlates with morbidity and mortality risk (Tan et al. 2017; Batlahally et al. 2020). The FGF23-Klotho pathway is dysregulated in CKD, leading to hyperphosphatemia, upregulation of the pro-inflammatory NF-κB signalling, systemic inflammation, and premature ageing (Ebert et al. 2020). Mice with a defective Klotho gene display a progeroid phenotype and have a greatly reduced lifespan (2–3 months) compared to wild-type (wt) mice (2–3 years), as well as increased serum Pi concentration (~50% higher than wt mice) (Kuro-o 2010a).
The normal serum calcium and Pi concentration ranges in humans are 2.2–2.5 mM (8.8–10.2 mg/dL) and 0.8–1.5 mM (2.5–4.5 mg/dL), respectively (Kuro-o 2010a). These physiological concentrations are high enough for the spontaneous formation of calcium phosphate (CaP) crystals (Brylka and Jahnen-Dechent 2013). CaP crystals are also toxic to cells, especially in the vasculature, and cause cellular damage, oxidative stress, and ectopic calcification (Ewence et al. 2008; Montezano et al. 2010). One of the main mechanisms of toxicity is thought to be the endocytosis of CaP particles by vascular smooth muscle cells (VSMCs), the dissolution of the crystals, and the release of calcium ions, which thus disrupt cytoplasmic calcium signalling (Ewence et al. 2008). This in turn can trigger cell death through apoptosis, osteogenic differentiation, deposition of extracellular CaP crystals (and thus ectopic vascular calcification), and inflammation (Ewence et al. 2008; Montezano et al. 2010). Magnesium deficiency has been shown to increase calcification and osteogenic differentiation of VSMCs, while magnesium supplementation has a protective effect (Montezano et al. 2010).
Several defence mechanisms have evolved to prevent the growth of CaP crystals. The plasma protein fetuin-A has a key role in preventing calcification by binding to and sequestering circulating CaP crystals into calciprotein particles (CPP) and preventing their further growth (Brylka and Jahnen-Dechent 2013). Indeed, fetuin-A deficiency has been associated with ectopic calcification (Brylka and Jahnen-Dechent 2013). Similarly, an increase in calcium and Pi concentration in the blood, commonly observed in CKD patients due to the diminished clearance capacity of the kidneys, can overwhelm the body’s protective capacity and lead to calcification (Kuro-o 2013; Ebert et al. 2020). For this reason, a diet low in Pi is recommended for CKD patients (Kuro-o 2013). Indeed, fetuin-A functions as a circulating inhibitor of vascular calcification and its levels have been associated with accelerated ageing in CKD (Carrero et al. 2008).
The fundamental importance of phosphate to the ageing process can also be gauged from the observation that its progeric phenotype in the Klotho mouse can be reversed by knock-out of the NaPi2a transporter (a Pi transporter) when the animal is fed a normal diet. However, when these animals are fed a high Pi diet, the phenotype reappears, indicating that elevated Pi is driving ageing in these animals (Ohnishi et al. 2009; Ohnishi and Razzaque 2010). The mechanistic basis of this is well understood. High concentrations of extracellular phosphate are toxic to cells. Calciprotein particles have the potential to induce extra-osteogenic transformation of vascular smooth muscle cells and cause cell death when endocytosed as a consequence of intra-cellular calcium release resulting in elevated oxidative stress and mitochondrial dysfunction (Buchanan et al. 2020). Conversely, high intake of dietary Pi can shorten life span in Klotho-deficient mice via activation of the AKT/mammalian target of rapamycin complex 1 (mTORC1) (Kawai et al. 2016).
Modulating the Exposome to Treat CKD
Diet has been considered the single easiest lever with which to leverage improved renal health. The Global Burden of Disease Study 2017 has indicated that 22% of global deaths were attributable to poor diet in 2017 (Willett et al. 2019; Afshin et al. 2019). Modulation of the diet to reduce intake of inorganic phosphate, maintain a salutogenic microbiome, and promote Nrf2-mediated cytoprotective responses is readily achievable. This firmly falls within the concept of Food as Medicine (Mafra et al. 2021). In this respect, increased intake of more plant-based protein, while still maintaining an omnivorous diet, offers renal health benefits. This approach has already shown some success, as it has been demonstrated to decrease CKD risk and progression and to improve on comorbid burden (Carrero et al. 2020; Avesani et al. 2022). Importantly, it has been applied successfully to the treatment of patients undergoing haemodialysis, where use of resistant starch cookies to support growth of saccharolytic salutobionts resulted in reduction of inflammatory burden and enhanced Nrf2 expression (Esgalhado et al. 2020; Kemp et al. 2021; Mafra et al. 2022b).
A positive sequitur from this strategy of eating more plant protein and reducing red meat consumption, is that it offers the possibility to alter food production systems to reduce factory farming, industrial mono-culture farming, and the impact of beef production (Stenvinkel et al. 2020), and thus improve Planetary health (Avesani et al. 2022). Additionally, a shift from a Western diet containing high levels of Ultra Processed Foods (UPFs) would have profound benefits, again mediated through supporting a salutogenic microbiome and better maintenance of Nrf2 agonism. The Western diet supports an industrialized human microbiome, that has outpaced its natural symbiotic evolution with humans and thus presents as a potentially dangerous unknown for human health. Its link to the prevalence of mild to moderate CKD (Craven et al. 2021; Chen et al. 2019) is already indicative of this.
Nutritional modulation of Pi intake can also radically affect age-related renal health, including through modulation of vitamin D metabolism and renal Klotho expression. Low Pi diets can be challenging and difficult to adhere to, as Pi and protein content in food tend to be positively correlated. While low protein intake can help slow down progression of CKD, it can also lead to malnutrition and adverse health outcomes (Buchanan et al. 2020). A high-fat diet (HFD) has been shown to alter renal Klotho expression in older mice and impair the balance between dietary Pi absorption and vitamin D (Yoshikawa et al. 2018).
Iron deficiency and anaemia upregulate FGF23 and are commonly observed in CKD patients. Thus, iron-based phosphate binders such as ferric citrate have been successfully used to decrease FGF23 expression, and ameliorate both hyperphosphatemia and iron deficiency, and slow CKD progression in mice (Courbon et al. 2020). At the same time, caution is warranted, as excess iron intake has been associated with increased oxidative stress and risk of accelerated ageing (Arruda et al. 2013; Tian et al. 2022). The role of iron in ageing is also supported by a recent multivariate genomic scan (Timmers et al. 2020). A diet high in magnesium can also reduce vascular calcification, but it can also lead to lower bone mineral density and carries a risk of osteomalacia (Buchanan et al. 2020).
Notably, poor quality diets, often observed in lower SEP strata, are typically characterized by high phosphate intake (including from additives), high fat, low vitamin D, and limited quantities of fruits and vegetables rich in polyphenolic and other bioactive compounds (Buchanan et al. 2020). This contributes to an increased risk of CKD and poor health outcomes in people of lower socioeconomic status or who experience food insecurity, at least in part mediated by overexpression of FGF23. Encouragingly, this demographic appears to be also more amenable to improvement upon dietary supplementation, e.g., with vitamin D (Buchanan et al. 2020).
Dietary fibre intake and supplementation with pre- and probiotics are also parameters of interest in the context of CKD, as they are powerful tools for promoting a healthy gut microbiome and avoiding CKD-associated gut dysbiosis (Buchanan et al. 2020; Kemp et al. 2021, p. 2021). Salutogenic intestinal microbes can produce Nrf2-activating compounds, modulate vitamin D/FGF23 homeostasis, produce short-chain fatty acids (SCFA) that promote the health of the intestinal epithelium, maintain integrity of the intestinal barrier, and reduce inflammation (Buchanan et al. 2020; Kemp et al. 2021, p. 2021).
Future Treatment Strategies
Changing medical interventions and tackling exposome factors concurrently may have lasting beneficial effects for health span. An emerging treatment category, termed senotherapy, tackles cumulative cellular senescence and its senescence-associated secretory phenotype (SASP); these often manifest in old age as inflammation and diminished cellular stress responses (Kooman et al. 2014). Senotherapeutics is a broad term which encompasses both pharmaceutical and bioactive agents which affect physiological senescence, often by directly targeting senescent cells (SCs) and their secretome. These agents can have specific effects. For example, senolytics induce SC apoptosis, geroprotectors inhibit or reverse senescence, whilst senomorphics (also known as senostatics) target products of the SASP and its by-products (Tchkonia et al. 2013; Mafra et al. 2021). These treatments can be applied to improve the dysregulation of ageing in CKD, thus pre-empting the development of the disease or mitigating its effects.
Examples of senotherapeutic interventions include use of Rapamycin and metformin, two repurposed clinical agents targeting the mTOR pathway. These attempt to switch cellular metabolism from catabolic to anabolic, and thus reduce the SASP phenotype (Nayeri Rad et al. 2022). Senolytic drugs, including dasatinib, in combination with quercetin, fisetin, navitoclax, or piperlongumine can induce apoptosis pathways in SCs, which are naturally resistant to apoptosis by nature of their senescent state of growth arrest, thus driving removal of SCs from tissues and organs (Nayeri Rad et al. 2022). However, dual approaches are also beneficial; the first in-human CKD trial using senotherapy, produced encouraging results, demonstrating improvement in renal function with the combination of dasatinib (a repurposed clinical chemotherapeutic agent) and quercetin (a bioactive agent), which complement each other in creating a senolytic effect (Nayeri Rad et al. 2022).
A further strategic development has been the adoption of a biomimetic approach to understand and treat diseases of ageing (Stenvinkel et al. 2018). The application of biomimetics to human health takes advantage of evolution by natural selection to produce, from within the natural world, solutions to human health problems (Stenvinkel et al. 2021a). One example of the insight such an approach can provide has been the identification and subsequent therapeutic targeting of Nrf2. Nrf2 responds to cellular stressors by upregulating over 350 cytoprotective genes which act in concert to reduce oxidative stress and damage, inflammatory burden, as well as modulating energy metabolism (Shiels et al. 2017, 2021; Stenvinkel et al. 2018). Additionally, it has provided a nexus for the re-envisionment of the Hippocratic concept of ‘Food as Medicine’. Foods, particularly fruits and vegetables, are rich in phenolic acids broken down by the gut microbiota to generate alkyl catechols, which are potent agonists for Nrf2, thus improving cytoprotection in response to stress (Shiels et al. 2021; Mafra et al. 2022a). A direct sequitur of this approach is a reduction in the number of SCs and thus the effects of the SASP. Notably, within the animal kingdom, Nrf2 forms the molecular basis of stress responses, particularly in long-lived animals, such as the naked mole rat and animals living under extremes of environmental stress, such as the ocean Quahog (Stenvinkel and Shiels 2019). A further benefit of this approach is the reduction in the use of inorganic phosphate preservatives within food stuffs and a return to fermentation of food stuffs to preserve them. This enhances the maintenance of a salutogenic microbiome and promotes renal health (Esgalhado et al. 2020; Kemp et al. 2021; Mafra et al. 2022b).
Similarly, this approach has also identified several bioactive molecules with promise as senotherapeutic agents. One of these, sulforaphane, found naturally in cruciferous vegetables, is a candidate senotherapeutic of promise, exhibiting both geroprotective and senolytic properties and already in a range of human trials (Cardozo et al. 2021). Another natural compound, fisetin, has also been shown to have a senolytic effect, improving both health and life span in a range of pre-clinical testing (Shiels et al. 2021). Whilst both bioactive and pharmaceutical agents have been identified as improving organ function, it is also important to consider that the promotion of cytoprotective effects and impairment of senescence may have adverse consequences, possibly increasing the risk of diseases such as B cell lymphoma (Franzin et al. 2021; Chaib et al. 2022).
As senolytics remove senescent cells which must be replaced to maintain tissue and organ homeostasis, it may lead to replicative exhaustion and dysfunction. Clearance of senescent pancreatic β-cells using senolytics has already been shown to lead to diabetes in mice (Helman et al. 2016). Additionally, senomorphic or senostatic drugs cannot eliminate SASP sources permanently, requiring repeated administration to ensure efficacy. This may result in suppression of other essential pathways and disturbance in tissue homeostasis, due to blocking of the SASP. Another concern is the lack of information regarding the optimal time points of administration of senolytic or senomorphic agents within the life course, pertinent considering antagonistic pleiotropy. Therapeutics may exert either beneficial, neutral, or negative impacts on different organs at a specific point over the life course. It remains unclear whether they provide protection and improve future health span when administered early in life or should only be administered at middle age or later. Indeed, it remains to be seen if, used in combination, they work synergistically, independently, cumulatively, or competitively. Furthermore, cryptic side effects need to be considered. Already, such effects have been identified while using senotherapeutic drugs. For instance, sulforaphane significantly reduces the water intake in young mice (Bose et al. 2020), which resonates with fluid retention issues observed clinically with the original use of Bardoxolone (Bose et al. 2020). To minimize the risks, further research is needed to determine the safety of these approaches.
Despite these concerns, it would be churlish not to recognize the genuine benefits and promise that senotherapies offer to the treatment of renal disease and the ‘diseasome of ageing’ in general. Their regular clinical use is now much anticipated.
References
Afshin A, Sur PJ, Fay KA et al (2019) Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393:1958–1972. https://doi.org/10.1016/S0140-6736(19)30041-8
Arruda LF, Arruda SF, Campos NA et al (2013) Dietary iron concentration may influence aging process by altering oxidative stress in tissues of adult rats. PLoS ONE 8:e61058. https://doi.org/10.1371/journal.pone.0061058
Avesani CM, Cardozo LFMF, Yee-Moon Wang A et al (2022) Planetary health, nutrition and chronic kidney disease: connecting the dots for a sustainable future. J Ren Nutr. S1051227622001613. https://doi.org/10.1053/j.jrn.2022.09.003
Batlahally S, Franklin A, Damianos A et al (2020) Soluble Klotho, a biomarker and therapeutic strategy to reduce bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Sci Rep 10:12368. https://doi.org/10.1038/s41598-020-69296-1
Bestion E, Jacob S, Zinger L et al (2017) Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat Ecol Evol 1:0161. https://doi.org/10.1038/s41559-017-0161
Bikbov B, Purcell CA, Levey AS et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395:709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Bose C, Alves I, Singh P et al (2020) Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 19. https://doi.org/10.1111/acel.13261
Brylka L, Jahnen-Dechent W (2013) The role of Fetuin-A in physiological and pathological mineralization. Calcif Tissue Int 93:355–364. https://doi.org/10.1007/s00223-012-9690-6
Buchanan S, Combet E, Stenvinkel P, Shiels PG (2020) Klotho, aging, and the failing kidney. Front Endocrinol 11:560. https://doi.org/10.3389/fendo.2020.00560
Burch JB, Augustine AD, Frieden LA et al (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69:S1–S3. https://doi.org/10.1093/gerona/glu041
Cardozo LFMF, Alvarenga LA, Ribeiro M et al (2021) Cruciferous vegetables: rationale for exploring potential salutary effects of sulforaphane-rich foods in patients with chronic kidney disease. Nutr Rev 79:1204–1224. https://doi.org/10.1093/nutrit/nuaa129
Carney EF (2020) The impact of chronic kidney disease on global health. Nat Rev Nephrol 16:251–251. https://doi.org/10.1038/s41581-020-0268-7
Carrero JJ, Stenvinkel P, Fellström B et al (2008) Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med 263:302–312. https://doi.org/10.1111/j.1365-2796.2007.01890.x
Carrero JJ, González-Ortiz A, Avesani CM et al (2020) Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol 16:525–542. https://doi.org/10.1038/s41581-020-0297-2
Chaib S, Tchkonia T, Kirkland JL (2022) Cellular senescence and senolytics: the path to the clinic. Nat Med 28:1556–1568. https://doi.org/10.1038/s41591-022-01923-y
Chang AR, Lazo M, Appel LJ et al (2014) High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr 99:320–327. https://doi.org/10.3945/ajcn.113.073148
Chen S, Kuhn M, Prettner K, Bloom DE (2018) The macroeconomic burden of noncommunicable diseases in the United States: estimates and projections. PLoS ONE 13:e0206702. https://doi.org/10.1371/journal.pone.0206702
Chen Y-Y, Chen D-Q, Chen L et al (2019) Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J Transl Med 17:5. https://doi.org/10.1186/s12967-018-1756-4
Copp DH, Shim SS (1963) The homeostatic function of bone as a mineral reservoir. Oral Surg Oral Med Oral Pathol 16:738–744. https://doi.org/10.1016/0030-4220(63)90081-1
Courbon G, Martinez-Calle M, David V (2020) Simultaneous management of disordered phosphate and iron homeostasis to correct fibroblast growth factor 23 and associated outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 29:359–366. https://doi.org/10.1097/MNH.0000000000000614
Craven H, McGuinness D, Buchanan S et al (2021) Socioeconomic position links circulatory microbiota differences with biological age. Sci Rep 11:12629. https://doi.org/10.1038/s41598-021-92042-0
De Cecco M, Ito T, Petrashen AP et al (2019) L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566:73–78. https://doi.org/10.1038/s41586-018-0784-9
Ebert T, Pawelzik S-C, Witasp A et al (2020) Inflammation and premature ageing in chronic kidney disease. Toxins 12:227. https://doi.org/10.3390/toxins12040227
Ebert T, Tran N, Schurgers L et al (2022) Ageing – oxidative stress, PTMs and disease. Mol Aspects Med 86:101099. https://doi.org/10.1016/j.mam.2022.101099
Esgalhado M, Kemp JA, de Paiva BR et al (2020) Resistant starch type-2 enriched cookies modulate uremic toxins and inflammation in hemodialysis patients: a randomized, double-blind, crossover and placebo-controlled trial. Food Funct 11:2617–2625. https://doi.org/10.1039/C9FO02939G
Ewence AE, Bootman M, Roderick HL et al (2008) Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 103. https://doi.org/10.1161/CIRCRESAHA.108.181305
Falconi CA, da Cruz Junho CV, Fogaça-Ruiz F et al (2021) Uremic toxins: an alarming danger concerning the cardiovascular system. Front Physiol 12:686249. https://doi.org/10.3389/fphys.2021.686249
Franzin R, Stasi A, Ranieri E et al (2021) Targeting premature renal aging: from molecular mechanisms of cellular senescence to senolytic trials. Front Pharmacol 12:630419. https://doi.org/10.3389/fphar.2021.630419
Helman A, Klochendler A, Azazmeh N et al (2016) p16Ink4a-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med 22:412–420. https://doi.org/10.1038/nm.4054
Horton R, Beaglehole R, Bonita R et al (2014) From public to planetary health: a manifesto. Lancet 383:847. https://doi.org/10.1016/S0140-6736(14)60409-8
Johnson LN (1997) From phosphorylase to phosphorylase kinase. In: Heilmeyer L (ed) Interacting protein domains. Springer, Berlin, Heidelberg, pp 9–14
Kawai M, Kinoshita S, Ozono K, Michigami T (2016) Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an α -Klotho–deficient model. J Am Soc Nephrol 27:2810–2824. https://doi.org/10.1681/ASN.2015040446
Kemp JA, Regis B, Fragoso dos Santos H, Emiliano de Jesus H, Craven H, Ijaz UZ, Shiels PG, Mafra D (2021) The impact of enriched resistant starch type-2 cookies on the gut microbiome in hemodialysis patients. Mol Nutr Food Res 65:e2100374. https://doi.org/10.1002/mnfr.202100374
Kooman JP, Kotanko P, Schols AMWJ et al (2014) Chronic kidney disease and premature ageing. Nat Rev Nephrol 10:732–742. https://doi.org/10.1038/nrneph.2014.185
Kooman JP, Dekker MJ, Usvyat LA et al (2017) Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol-Ren Physiol 313:F938–F950. https://doi.org/10.1152/ajprenal.00256.2017
Kuro-o M (2010a) A potential link between phosphate and aging—lessons from Klotho-deficient mice. Mech Ageing Dev 131:270–275. https://doi.org/10.1016/j.mad.2010.02.008
Kuro-o M (2010b) Overview of the FGF23-Klotho axis. Pediatr Nephrol 25:583–590. https://doi.org/10.1007/s00467-009-1260-4
Kuro-o M (2013) A phosphate-centric paradigm for pathophysiology and therapy of chronic kidney disease. Kidney Int Suppl 3:420–426. https://doi.org/10.1038/kisup.2013.88
Kuźma Ł, Małyszko J, Bachórzewska-Gajewska H et al (2021) Exposure to air pollution and renal function. Sci Rep 11:11419. https://doi.org/10.1038/s41598-021-91000-0
Lim SS, Vos T, Flaxman AD et al (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. https://doi.org/10.1016/S0140-6736(12)61766-8
Lisowska-Myjak B (2014) Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract 128:303–311. https://doi.org/10.1159/000369817
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Losappio V, Franzin R, Infante B et al (2020) Molecular mechanisms of premature aging in hemodialysis: the complex interplay between innate and adaptive immune dysfunction. Int J Mol Sci 21:3422. https://doi.org/10.3390/ijms21103422
Mafra D, Borges NA, Lindholm B et al (2021) Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol 17:153–171. https://doi.org/10.1038/s41581-020-00345-8
Mafra D, Borges NA, Alvarenga L et al (2022a) Fermented food: should patients with cardiometabolic diseases go back to an early neolithic diet? Crit Rev Food Sci Nutr:1–24. https://doi.org/10.1080/10408398.2022.2077300
Mafra D, Cardozo L, Ribeiro-Alves M et al (2022b) Short report: choline plasma levels are related to Nrf2 transcriptional expression in chronic kidney disease? Clin Nutr ESPEN 50:318–321. https://doi.org/10.1016/j.clnesp.2022.06.008
Mafra D, Ribeiro M, Fonseca L et al (2022c) Archaea from the gut microbiota of humans: could be linked to chronic diseases? Anaerobe 77:102629. https://doi.org/10.1016/j.anaerobe.2022.102629
Mafra D, Ugochukwu SA, Borges NA et al (2022d) Food for healthier aging: power on your plate. Crit Rev Food Sci Nutr:1–14. https://doi.org/10.1080/10408398.2022.2107611
McClelland R, Christensen K, Mohammed S et al (2016) Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging 8:1135–1149. https://doi.org/10.18632/aging.100948
Mills KT, Xu Y, Zhang W et al (2015) A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88:950–957. https://doi.org/10.1038/ki.2015.230
Montezano AC, Zimmerman D, Yusuf H et al (2010) Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56:453–462. https://doi.org/10.1161/HYPERTENSIONAHA.110.152058
Nayeri Rad A, Shams G, Avelar RA et al (2022) Potential senotherapeutic candidates and their combinations derived from transcriptional connectivity and network measures. Inform Med Unlocked 30:100920. https://doi.org/10.1016/j.imu.2022.100920
Ohnishi M, Razzaque MS (2010) Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24:3562–3571. https://doi.org/10.1096/fj.09-152488
Ohnishi M, Nakatani T, Lanske B, Razzaque MS (2009) In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin D levels. Circ Cardiovasc Genet 2:583–590. https://doi.org/10.1161/CIRCGENETICS.108.847814
Penido MGMG, Alon US (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27:2039–2048. https://doi.org/10.1007/s00467-012-2175-z
Peter S (2021) Mitochondrial dysfunction as part of an inflammatory intermediate phenotype that drives premature ageing. J Intern Med 290:231–234. https://doi.org/10.1111/joim.13243
Ritz E, Hahn K, Ketteler M et al (2012) Phosphate additives in food. Dtsch Ärztebl Int. https://doi.org/10.3238/arztebl.2012.0049
Shanahan MJ, Cole SW, Ravi S et al (2022) Socioeconomic inequalities in molecular risk for chronic diseases observed in young adulthood. Proc Natl Acad Sci 119:e2103088119. https://doi.org/10.1073/pnas.2103088119
Shiels PG, McGuinness D, Eriksson M et al (2017) The role of epigenetics in renal ageing. Nat Rev Nephrol 13:471–482. https://doi.org/10.1038/nrneph.2017.78
Shiels PG, Buchanan S, Selman C, Stenvinkel P (2019) Allostatic load and ageing: linking the microbiome and nutrition with age-related health. Biochem Soc Trans 47:1165–1172. https://doi.org/10.1042/BST20190110
Shiels PG, Painer J, Natterson-Horowitz B et al (2021) Manipulating the exposome to enable better ageing. Biochem J 478:2889–2898. https://doi.org/10.1042/BCJ20200958
Simon M, Van Meter M, Ablaeva J et al (2019) LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab 29:871–885.e5. https://doi.org/10.1016/j.cmet.2019.02.014
Stenvinkel P, Shiels PG (2019) Long-lived animals with negligible senescence: clues for ageing research. Biochem Soc Trans 47:1157–1164. https://doi.org/10.1042/BST20190105
Stenvinkel P, Shiels PG (2021) Metabolic syndrome in combination with chronic kidney disease—it’s a gut feeling. J Intern Med 290:1108–1111. https://doi.org/10.1111/joim.13363
Stenvinkel P, Painer J, Kuro-o M et al (2018) Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol 14:265–284. https://doi.org/10.1038/nrneph.2017.169
Stenvinkel P, Shiels PG, Painer J et al (2020) A planetary health perspective for kidney disease. Kidney Int 98:261–265. https://doi.org/10.1016/j.kint.2020.03.024
Stenvinkel P, Avesani CM, Gordon LJ et al (2021a) Biomimetics provides lessons from nature for contemporary ways to improve human health. J Clin Transl Sci 5:e128. https://doi.org/10.1017/cts.2021.790
Stenvinkel P, Painer J, Shiels PG et al (2021b) SARS-COV-2 and biomimetics: what saves the planet will save our health. J Intern Med 289:244–246. https://doi.org/10.1111/joim.13128
Tan S-J, Smith ER, Holt SG et al (2017) Soluble klotho may be a marker of phosphate reabsorption. Clin Kidney J 10:397–404. https://doi.org/10.1093/ckj/sfw146
Tchkonia T, Zhu Y, van Deursen J et al (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123:966–972. https://doi.org/10.1172/JCI64098
Tian Y, Tian Y, Yuan Z et al (2022) Iron metabolism in aging and age-related diseases. Int J Mol Sci 23:3612. https://doi.org/10.3390/ijms23073612
Timmers PRHJ, Wilson JF, Joshi PK, Deelen J (2020) Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat Commun 11:3570. https://doi.org/10.1038/s41467-020-17312-3
United Nations (2018) World Population Ageing 2017: Highlights. United Nations
Vanholder R, Schepers E, Pletinck A et al (2014) The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 25:1897–1907. https://doi.org/10.1681/ASN.2013101062
Wild CP (2005) Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 14:1847–1850. https://doi.org/10.1158/1055-9965.EPI-05-0456
Willett W, Rockström J, Loken B et al (2019) Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393:447–492. https://doi.org/10.1016/S0140-6736(18)31788-4
Williams GC (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11:398. https://doi.org/10.2307/2406060
Yoo KD, Kang S, Choi Y et al (2016) Sex, age, and the association of serum phosphorus with all-cause mortality in adults with normal kidney function. Am J Kidney Dis 67:79–88. https://doi.org/10.1053/j.ajkd.2015.06.027
Yoshikawa R, Yamamoto H, Nakahashi O et al (2018) The age-related changes of dietary phosphate responsiveness in plasma 1,25-dihydroxyvitamin D levels and renal Cyp27b1 and Cyp24a1 gene expression is associated with renal α-Klotho gene expression in mice. J Clin Biochem Nutr 62:68–74. https://doi.org/10.3164/jcbn.17-20
Acknowledgements
PSh is a Scientific reviewer for Mars UK Ltd and funded by an IPP award between Constant Pharma and UoG.
PSt is funded by Swedish Medical Research Council, CIMED, ALF and The Heart and Lung Foundation.
ON is funded by an IPP award to PS between Constant Pharma and UoG.
NT is supported by MINDSHIFT—H2020-MSCA-ITN-2020, Grant Agreement number: 954798.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shiels, P., Tran, N., McCavitt, J., Neytchev, O., Stenvinkel, P. (2023). Chronic Kidney Disease and the Exposome of Ageing. In: Harris, J.R., Korolchuk, V.I. (eds) Biochemistry and Cell Biology of Ageing: Part IV, Clinical Science. Subcellular Biochemistry, vol 103. Springer, Cham. https://doi.org/10.1007/978-3-031-26576-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-26576-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26575-4
Online ISBN: 978-3-031-26576-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)