Abstract
Although IVM previously encompassed a very narrow scope given its experimental designation, its utility as a first-line treatment choice has received renewed attention. Indeed, IVM has traditionally been reserved for women with polycystic ovarian syndrome (PCOS) and prepubertal fertility preservation prior to gonadotoxic treatment, yet its benefits have the capacity to extend to all women undergoing ART, and indications have since broadened to include poor responders, egg donors, those who decline ovarian stimulation, and those who had failed egg maturation in regular IVF. However, clinical outcomes from IVM vary widely likely owing to differing techniques and protocols, including use of hCG triggering and gonadotropin treatment to support follicle growth. Although further research and refinements to the procedure are still ongoing, recent consensus guidelines recognize IVM as no longer an experimental technique and support its therapeutic role if the expertise exists in a particular centre.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Notwithstanding the burgeoning success of in vitro fertilization (IVF) [1], it is important to recognize the seminal role of in vitro maturation (IVM) in the development of IVF as well as its current widespread application amongst the arsenal of assisted reproductive techniques for over 25 years [2, 3]. While traditional IVF involves recovery of in vivo maturated oocytes, IVM refers to the recovery of immature oocytes from small antral follicles at the germinal vesicle (GV) or metaphase I (MI) stage and subsequent meiotic resumption under specifically controlled culture conditions. Cha et al. reported the first birth using IVM of immature oocytes collected at caesarean section within an oocyte donation programme in 1991 [4], but it was only after Trounson and colleagues reported the first pregnancy using a woman’s own immature oocytes collected by transvaginal ultrasound-guided follicle aspiration that IVM emerged as a viable alternative to IVF for patients with polycystic ovaries [5]. The popularization of IVM first emerged from work from McGill showing that a single injection of hCG with no other stimulation was able to achieve a live birth rate/cycle started of 40% [6]. Most recently, a consensus has emerged to promote more widespread expertise and training for IVM given its overall safety and reduced patient burden compared to hyperstimulation protocols with IVF [3].
Oocyte Physiology
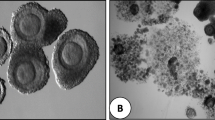
Oocyte maturation is the physiological event that precedes, and is required for, successful fertilization and embryo development. Oocytes initially mature during the foetal period and become arrested at the diplotene stage of prophase I (GV stage) until they are committed to ovulation or atresia. Resumption of meiosis and progression through maturation result in arrest at the metaphase II stage, with extrusion of the first polar body (Fig. 16.1). In vivo, the trigger for resumption of meiosis is the preovulatory surge of LH. However, removal of the oocyte from the inhibitory influence of the follicle also allows spontaneous resumption of meiosis. Importantly, the success of IVM relies on techniques that promote both nuclear and cytoplasmic maturation of the oocyte. Nuclear maturation consists of germinal vesicle breakdown (induced by the LH surge in vivo) followed by resumption of meiosis and extrusion of the first polar body (MII). Cytoplasmic maturation is more difficult to assess microscopically as it involves the redistribution of various organelles, including cortical granules, and accumulation of factors that prepare the oocyte for fertilization and embryonic development [7].
Different stages of oocyte development; (a) Oocyte at germinal vesicle stage (GV), the nuclear membrane is intact and the nucleolus is visible; (b) Metaphase I oocyte, the nuclear membrane dissolves and the nucleolus disappears; (c) Metaphase II oocyte, the first polar body extrudes. Note: these oocytes are after the removal of cumulus cells
Potential Indications for IVM
IVM offers several key advantages over other assisted reproduction techniques, including a lower risk of adverse events and reduced financial and emotional burden owing to the short duration of monitoring and relatively low medication requirements. Historically, IVM has primarily been proposed as an alternative to IVF particularly for PCOS patients at risk for ovarian hyperstimulation syndrome (OHSS) given that they generally have the highest number of antral follicles for potential retrieval and simultaneously at highest risk of developing OHSS under traditional stimulation protocols. Other indications include patients with limited time for ovarian stimulation as well as those with contraindications to sustained elevation of oestradiol (E2). However, studies have shown that IVM can be used in almost all areas where IVF and other assisted reproductive techniques are used [2, 3, 8].
IVM in the Era of Antagonist Protocol with Agonist Trigger
Despite increasingly widespread use of GnRH agonist trigger in GnRH antagonist cycles, OHSS remains an ever-present risk with gonadotropin stimulation [9]. Indeed, early severe OHSS can occur if there is agonist trigger with low-dose hCG (1500 IU) rescue [9], if there is agonist trigger with high-dose oestrogen and progesterone supplementation for fresh ET [10, 11], or even if there is agonist trigger and a freeze all embryos policy. Only IVM can avoid OHSS completely. Another recent development of pseudo double-lumen needle (Steiner-Tan needle) allows even better results for IVM egg collection [12].
IVM Instead of Natural Cycle IVF
Classical natural cycle IVF involves no ovarian stimulation, and triggering is generally performed once the leading follicle reaches 18-mm diameter; however, it is associated with up to 30% risk of premature LH surge and ovulation. Alternatively, modified natural cycle IVF requires daily GnRH antagonist and FSH injections once a follicle reaches 14 mm and continued until hCG triggering, which equates to at least three FSH and GnRH antagonist injections with a subsequent 15–20% clinical pregnancy rate per cycle started given that only one MII oocyte is retrieved. In stimulated IVM, three injections of FSH are given on days 4, 6, and 8, with subsequent hCG administration when the leading follicle is 12–14-mm, thereby forgoing the need of GnRH antagonist. Furthermore, several MII and GV oocytes can be obtained to generate multiple blastocysts, thereby increasing clinical pregnancy rates per cycle started of up to 45–50% in women up to 37 years of age. In some cases, over 100 oocytes can be obtained at a single collection [13].
IVM Instead of FSH/IUI
The first line of treatment in many fertility programs is FSH stimulation combined with IUI. This treatment requires approximately eight to ten daily injections of FSH and has a pregnancy rate of 15–20% and multiple pregnancy rate of 30% and may cost upwards of $2000 in North America for medications and IUI. In comparison, IVM costs ~$4000 but requires fewer injections and generally yields higher pregnancy rates, lower odds of multiple pregnancy, and no risk of OHSS. In fact, Hatirnaz et al. have achieved a 35% live birth rate per IVM cycle with elective single ET. This group has even achieved good results using letrozole 5 mg a day for 5 days in early to mid-follicular phase and hCG trigger at 12–14 mm [14] and eSET [14].
In fact, the group from Perth has even shown that although fresh ET from IVM cycles are lower than from IVF cycle, if the embryos are frozen, the FERC pregnancy rates for both IVM and IVF are comparable [15].
IVM and Fertility Preservation for Cancer
When presented with an oncology patient wanting fertility preservation, it is important to first determine whether ovarian stimulation can be safely performed and how long the patient can wait before the start of chemotherapy [16]. If hormone stimulation is not contraindicated and chemotherapy can wait, ovarian stimulation followed by mature egg collection should be performed. However, IVM with or without ovarian tissue freezing are the only viable options if hormone stimulation is contraindicated or there is no time. Importantly, IVM allows multiple egg collections to be performed at any phase of the menstrual cycle including the luteal phase [17]. In the past few years, there has been a lot of work in this field extending the area of IVM and cancer fertility preservation. For example, it has been shown to allow fertility preservation for breast cancer [18], and there is even greater promise to combine in vitro growth and in vitro maturation [19]. Other advances in this area involve the addition of L-carnitine and B-glutathione to the IVM media to increase survival of vitrified GV oocytes and fertilization to become blastocysts, at least in the animal model [20, 21].
IVM and Resistant Ovary Syndrome
A few small studies and case reports have investigated the utility of IVM amongst women with repeated ART failure owing to resistant ovary syndrome with promising results [22, 23]. For instance, Galvao et al. reported a case series of 9 women with repeated IVF failure who underwent 24 IVM cycles and achieved a live birth rate of 16.7% per started cycle and 33.3% per patient [22].
Safety Concerns
Although the long-term developmental outcomes of children conceived with IVM have only been studied in small numbers, current evidence suggests that foetal outcomes and incidence of congenital malformations are similar to pregnancies derived from IVF and spontaneous pregnancies in healthy women [24,25,26,27,28,29,30,31]. This reflects related cellular and molecular studies which found normal ultrastructural morphology by transmission electron microscopy (TEM) and no increase in imprinting errors rates at maternally or paternally methylated gene loci in IVM-derived oocytes [32, 33].
Conclusion
IVM is a well-studied and safe procedure that has been practiced for several decades. It is a low intervention, mild approach to ART that offers improved safety and a simplified clinical approach compared to IVF [34, 35]. However, adoption has been mixed as incentives leading to enhanced uptake of IVM in the ART clinic vary widely around the world. As further innovations improve the recovery rate of immature oocytes and embryo yield from IVM, REI physicians may soon have an obligation to offer this technology more widely given its potential advantages to offer new approaches to infertility management and social fertility preservation and towards a new clinical paradigm of minimal or zero stimulation ART. When the senior author of this chapter started his career with Robert Edwards, Edwards emphasized that he had started his career in IVM and always held the dream that one day, women would have the same success rates with IVM as with IVF. He used to say, drugs only benefit big pharma. No woman would willingly give herself daily injections of hormones if she believed she could have the same success rates without having to use drugs [2].
References
Tan SL, Betts J, Mason B, Edwards RG, Tan SL, Campbell S, et al. Cumulative conception and live birth rates after in-vitro fertilisation. Lancet. 1992;339(8806):1390–4.
Niederberger C, Pellicer A, Cohen J, et al. Forty years of IVF. Fertil Steril. 2018;110(2):185–324.e5.
Hillson K, Clemens SC, Madhi SA, Voysey M, Pollard AJ, Minassian AM. In vitro maturation: a committee opinion. Fertil Steril. 2020;115(2):298–304. https://doi.org/10.1093/humrep/dew109.
Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program**Prize paper, presented at the 45th Annual Meeting of The American Fertility Society, San Francisco, California, November 11 to 16, 1989. Fertil Steril. 1991;55(1):109–13.
Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients*. Fertil Steril. 1994;62(2):353–62.
Chian RC, Gülekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. New Engl J Med. 1999;341(21):1624–6. https://doi.org/10.1056/NEJM199911183412118.
Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod Biomed Online. 2004;8(2):148–66.
Plancha CE, Rodrigues P, Marques M, Almeida JM, Navarro-Costa P. The time is ripe for oocyte in vitro maturation. J Assist Reprod Gen. 2021;38(6):1281–3.
Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod. 2013;28(9):2522–8. https://doi.org/10.1093/humrep/det124.
Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101(4):1008–11. https://doi.org/10.1016/j.fertnstert.2014.01.019.
Gurbuz AS, Gode F, Ozcimen N, Isik AZ. Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online. 2014;29(5):541–4. https://doi.org/10.1016/j.rbmo.2014.07.022.
Rose BI, Laky D. A comparison of the Cook single lumen immature ovum IVM needle to the Steiner-Tan pseudo double lumen flushing needle for oocyte retrieval for IVM. J Assist Reprod Genet. 2013;30(6):855–60. https://doi.org/10.1007/s10815-013-0006-1.
Dahan MH, Ata B, Rosenberg R, Chunh JT, Son WY, Tan SL. Collection of 125 oocytes in an in vitro maturation cycle using a new oocyte collection technique: a case report. J Obstet Gynaecol Can. 2014;36(10):900–3. http://www.jogc.com/article/S1701216315304394/abstract
Hatırnaz S, Hatırnaz E, Dahan MH, Tan SL, Ozer A, Kanat-Pektas M, et al. Is elective single-embryo transfer a viable treatment policy in in vitro maturation cycles? Fertil Steril. 2016;106(7):1691–5.
Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30(1):88–96. https://doi.org/10.1093/humrep/deu248.
Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91(6):2391–8. https://doi.org/10.1016/j.fertnstert.2008.04.014.
Demirtas E, Elizur SE, Holzer H, Gidoni Y, Son WY, Chian RC, et al. Immature oocyte retrieval in the luteal phase to preserve fertility in cancer patients. Reprod Biomed Online. 2008;17(4):520–3.
Shalom-Paz E, Almog B, Shehata F, Huang J, Holzer H, Chian RC, et al. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod Biomed Online. 2010;21(4):566–71. https://doi.org/10.1016/j.rbmo.2010.05.003.
Telfer EE, Andersen CY. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil Steril. 2021;115(5):1116–25.
Moawad AR, Tan SL, Xu B, Chen HY, Taketo T. L-carnitine supplementation during vitrification of mouse oocytes at the germinal vesicle stage improves preimplantation development following maturation and fertilization in vitro. Biol Reprod. 2013;88(4):104. https://doi.org/10.1095/biolreprod.112.107433.
Moawad AR, Xu B, Tan SL, Taketo T. l-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum Reprod. 2014;29(10):2256–68. https://doi.org/10.1093/humrep/deu201.
Galvão A, Segers I, Smitz J, Tournaye H, Vos MD. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J Assist Reprod Gen. 2018;35(12):2161–71.
Kornilov NV, Pavlova MN, Yakovlev PP. The live birth in a woman with resistant ovary syndrome after in vitro oocyte maturation and preimplantation genetic testing for aneuploidy. J Assist Reprod Gen. 2021;38(6):1303–9.
Ho VNA, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod. 2019;34(6):1055–64.
Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization–embryo transfer. Fertil Steril. 2005;83(5):1461–5.
Fadini R, Renzini MM, Guarnieri T, Canto MD, Ponti ED, Sutcliffe A, et al. Comparison of the obstetric and perinatal outcomes of children conceived from in vitro or in vivo matured oocytes in in vitro maturation treatments with births from conventional ICSI cycles. Hum Reprod. 2012;27(12):3601–8.
Yu EJ, Yoon TK, Lee WS, Park EA, Heo JY, Ko YK, et al. Obstetrical, neonatal, and long-term outcomes of children conceived from in vitro matured oocytes. Fertil Steril. 2019;112(4):691–9.
Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, et al. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34(8):1595–607.
Roesner S, von Wolff M, Elsaesser M, Roesner K, Reuner G, Pietz J, et al. Two-year development of children conceived by IVM: a prospective controlled single-blinded study. Hum Reprod. 2017;32(6):1341–50.
Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110(4):885–91.
Söderström-Anttila V, Salokorpi T, Pihlaja M, Serenius-Sirve S, Suikkari AM. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod. 2006;21(6):1508–13.
Kuhtz J, Romero S, Vos MD, Smitz J, Haaf T, Anckaert E. Human in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2. Hum Reprod. 2014;29(9):1995–2005.
Coticchio G, Canto MD, Fadini R, Renzini MM, Guglielmo MC, Miglietta S, et al. Ultrastructure of human oocytes after in vitro maturation. Mhr Basic Sci Reprod Med. 2016;22(2):110–8.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome†‡. Hum Reprod Oxf Engl. 2018;33(9):1602–18.
Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open. 2019;2019(1):hoy021.
Acknowledgements
The authors acknowledge Dr. Mingju Cao for technical assistance in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tan, J., Tan, S.L. (2023). The Essential Role of In Vitro Maturation in Assisted Reproduction. In: Schenker, J.G., Birkhaeuser, M.H., Genazzani, A.R., Mettler, L., Sciarra, J.J. (eds) Hot Topics in Human Reproduction. Reproductive Medicine for Clinicians, vol 3. Springer, Cham. https://doi.org/10.1007/978-3-031-24903-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-24903-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24902-0
Online ISBN: 978-3-031-24903-7
eBook Packages: MedicineMedicine (R0)