Abstract
We report the pregnancy and live birth achieved after in vitro maturation (IVM) of oocytes and PGT-A in a 23-year-old patient suffering from ovarian gonadotropin resistance. A woman with resistant ovary syndrome (ROS) had secondary amenorrhea, high FSH levels (25.34 mIU/mL) and LH (29.6 mIU/mL), low estradiol levels (15.2 pg/mL), and high serum AMH levels (38.0 ng/mL), associated with an increased antral follicle count (AFC) of 45. Without gonadotropin priming and HCG trigger, ultrasound-guided transvaginal oocyte retrieval was performed. Aspiration of antral-stage follicles allowed the retrieval of 15 immature oocytes. After oocyte collection, immature oocytes were cultured in the IVM medium. Following IVM, six of them reached metaphase II stage. Resultant matured oocytes were fertilized by intracytoplasmic sperm injection (ICSI). Embryos obtained were cultured to the blastocyst stage. On day 5, three embryos reached blastocyst stage. Trophectoderm biopsy and PGT-A were performed on two better quality embryos on day 5 after fertilization. Two biopsied embryos were reported to be euploid. PGT-A was performed utilizing next-generation sequencing (NGS\MPS). One embryo was transferred in an artificial thaw cycle and resulted in a viable intrauterine pregnancy and live birth. Our experience indicates that there is no requirement for gonadotropin stimulation and use of b-hCG trigger prior to IVM in patients with ROS. The results suggest that oocytes obtained with IVM in patients with ROS are capable of meiotic and mitotic division, fertilization, and generation of euploid embryos. IVM appears to be a valuable approach in patients with ROS, allowing them to have genetically connected offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first description of resistant ovarian syndrome (ROS) was made in 1969 by the pioneer of ovarian stimulation, Dr. Georgiana Jones. This condition was previously referred to as savage syndrome, named after the first patient in whom it was described [1].
The main characteristic of ROS is the resistance of antral follicles to both endogenous and exogenous gonadotropins in patients with typical female phenotype and karyotype. Additionally, ROS is characterized by elevated serum levels of FSH and LH, often in the post-menopausal range, normal level of AMH, normal antral follicle count, primary or secondary amenorrhea or progressively prolonged menstrual cycles, and normal secondary sexual characteristics [1, 2]. According to A Galvão et al., ROS is a heterogeneous condition with highly variable manifestations, both clinical and endocrine. According to their data, patients with ROS can have not only primary amenorrhea, but also a normal menstrual cycle, irregular cycles as well as secondary amenorrhea. These patients may also have secondary infertility, varying FSH levels, and normal or high serum AMH levels [3].
The etiology of this condition is still unclear and appears to be highly variable [4]. This can potentially be due to genetic and immunological causes. Hormone resistance may be caused by inactivating mutations in gonadotropin receptors, as well as post-receptor defects [5,6,7,8,9]. The presence of antibodies to endogenous and cross-reactivity to exogenous gonadotropins may also be the primary cause of ROS [10,11,12].
ROS can be classified as WHO Group III anovulation, which is characterized by hypergonadotropic hypogonadism, similar to patients with primary ovarian insufficiency (POI). Anovulatory infertility is a common complaint of women with ROS during reproductive age. In contrast to a true primary POI, in ROS, the number of antral follicles (AFC) and serum levels of anti-Müllerian hormone (AMH) are most often within the normal range, while few or no follicles are found in POI and AMH level being drastically reduced [13].
In vitro maturation (IVM) of oocytes is an alternative approach to conventional methods of artificial reproductive technologies. IVM is currently defined as the maturation in vitro of immature cumulus–oocyte complexes collected from antral follicles [14]. Before 2010, the treatment of infertility in patients with ROS was confined to IVF with donor oocytes due to the fact that native antral follicles were unresponsive to exogenous FSH stimulation. IVM has emerged as a viable alternative to egg donation for women with ROS. The first pregnancy and live birth after IVM in a woman with ROS were reported in 2013 [15]. This paved the way for patients diagnosed with ROS to have genetically related children, since donor oocytes to achieve pregnancy were no longer required.
ROS is a rare condition with variable etiology. Treatment of infertility in patients with ROS is a great challenge for both patients and clinicians. We report the first pregnancy and live birth achieved using IVM and preimplantation genetic testing for aneuploidy (PGT-A) in a woman with ROS.
Materials and methods
Informed consent
Written informed consent for publication of patent’s clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Transvaginal oocyte pick up
Aspiration of antral follicles, between 2 and 3 mm in diameter, was performed with a 19-gauge OPU Needle (Kitazato, Jap), using negative pressure of 200 mmHg.
In vitro maturation, ICSI, embryo culture, biopsy, and vitrification
Cumulus–oocyte complexes were collected into LAG medium (IVM system, Medicult, Origio). Oocytes were incubated in IVM medium (IVM system, Medicult, Origio), supplemented with 75 mIU/mL FSH (Gonal-F, Merck), 100mIU/mL hCG (Pregnyl, MSD), and 20% patient’s serum for 24 h in a four-well dish with oil overlay (Irvine) in an benchtop incubator containing 6% CO2 in air at 37°C, until progression to the MII stage was achieved. After denuding, mature oocytes were fertilized by intracytoplasmic sperm injection (ICSI) with fresh sperm. Fertilization was confirmed by the presence of two pronuclei 16–18 h post-ICSI. Embryos were then cultured in sequential media (CSM, IRVINE) under overoil (Irvine) until day 5. We performed zona pellucida laser-assisted hatching on day 4. Suitable blastocysts were biopsied and vitrified (Kitazato) 5 days after insemination.
Preimplantation genetic testing for aneuploidy
Preimplantation genetic testing for aneuploidy was performed with the use of NGS (VeriSeq, Illumina Inc, US/Singapore). Whole-genome amplification and sequencing were performed according to the manufacturer’s recommendations SurePlex DNA Amplification System (Illumina Inc, US/Singapore) (Part # 15053626 Rev. C), VeriSeq PGS Library preparation Guide (# RH-101-9001DOC), MiSeqDx® Reference Guide for Instruments with Dual Boot Configuration (# 15070067 Rev. A). Sequencing data analysis was performed using A Technical Guide to Aneuploidy Calling with VeriSeq PGS (# 15059470 Rev. A). We analyzed the data with Blue Fuse Multi software (Illumina Inc, US/Singapore).
Endometrium preparation and FET
Endometrial preparation was initiated with oral estradiol valerate (Progynova, DELPHARM Lille S.A.S., France) at 2 mg three times a day (TDS). When the endometrial thickness reached 7 mm, vaginal tablets of micronized progesterone (Utrogestan, Besins, Belgium) 600 mg/day were started for luteal phase support. Vitrified-warmed embryo transfer was performed on day 6 after initiation of progesterone. Nine days after embryo transfer, serum ß-hCG was measured and luteal phase support continued until eight complete weeks of gestation.
Results
Case description
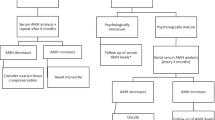
In December 2017, a 23-year-old woman with a 3-year history of primary infertility and secondary amenorrhea was referred to our IVF center. Her menarche occurred at 13 years of age. Up to 17 years old, the patient experienced progressively longer menstrual cycles of 50–60 days. From the age of 17, she had secondary amenorrhea and was prescribed a combined oral contraceptive pill. Her height and weight were 165 cm and 64 kg (body mass index: 23.5 kg/m2). The patient had mature secondary sexual characteristics and did not present any signs of androgen excess. She had no medical or surgical history of note and the physical examination was unremarkable. There was no personal or family history of inheritable conditions. She denied any autoimmune disorders. The patient’s karyotype was normal. Previously, the patient was diagnosed with heterozygous FSH receptor polymorphisms FSHR A919G, Thr307 Ala (rs6165), and FSHR A2039G, Asn680Ser (rs6166). She had elevated baseline FSH levels ranging from 21 to 25 IU/L and resistant to ovarian stimulation. Prior to presenting to our center, there were numerous attempts at ovulation induction and ovarian stimulation (Table 1). In two instances, clomiphene citrate was used, 50 mg and 100 mg. Three stimulated cycles of IVF were commenced, with recombinant FSH (rFSH) and dose ranging between 50 and 250 IU for 9 to 17 days with the addition of menotropins and aromatase inhibitors. None of these cycles produced any measurable ovarian response. Following her last cycle of ovarian stimulation, she was advised of a possible ROS diagnosis and encouraged to seek further treatment with donor oocytes.
Fertility investigation
Hormonal and ovarian reserve testing was repeated in the absence of hormone replacement therapy. The patient’s FSH was found to be elevated at 25.34 IU/L (normal range 3.5–12.5 IU/L) as was her luteinizing hormone (29.6 IU/L; normal range 2.4–12.6 IU/L). She had decreased estradiol levels (15.2 pg/mL; normal range 12.5–166 pg/mL). Despite the confirmed elevation of FSH, the patient’s AMH level was also elevated (38.0 ng/mL; normal range 1.58–13.5 ng/mL). Her thyroid-stimulating hormone (1.94 mIU/L; normal range 0.27–4.2 mIU/L) and prolactin (30.1 ng/mL; normal range 6–29.9 ng/mL) levels were normal. Transvaginal ultrasound showed the presence of more than 20 antral follicles up to 5 mm in each ovary. The uterus and ovaries were otherwise normal, and the endometrial stripe was 3 mm. These investigations were indicative of ROS and the previously diagnosed FSH receptor polymorphisms were consistent with the exhibited phenotype. After extensive discussion with the patient and her partner, aspiration of antral follicles with subsequent in vitro oocyte maturation was offered as a treatment option.
OPU, IVM, and PGT-a
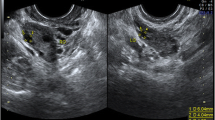
The patient was prescribed hormone replacement therapy (HRT) to induce regular withdrawal bleeding for 3 months. On the eighth day of the fourth month, while continuing to take hormone replacement therapy, without gonadotropin priming or trigger injection, 15 cumulus–oocyte complexes (COC) were retrieved under ultrasound guidance with a 19G single lumen aspiration needle, with aspiration pressure of 200 mmHg. Ten of them showed germinal vesicles and five had oocyte degeneration. The COCs were placed in the IVM medium. After denuding the oocytes, six had already reached the metaphase II stage (MII) (Fig. 1). Mature oocytes were fertilized by ICSI. At the 18-h point after ICSI, successful fertilization was confirmed for four oocytes by visualization of the expulsion of the second polar body and the presence of two pronuclei. On day 5 of embryo culture, three blastocysts had reached the full blastocyst stage 2BB, 5AA, and 5AA in accordance with the Gardner scoring system [16] (Table 2). Trophectoderm biopsy was performed on two better quality embryos (5AA and 5AA). All three blastocysts were cryopreserved. Based on the results of PGT-A, two euploid blastocysts were obtained.
Embryo transfer and follow-up
In the following month, after cessation of HRT and a withdrawal bleed, the patient was started on 2 mg of estradiol valerate (Progynova, DELPHARM Lille S.A.S., France) TDS. After reaching an endometrial thickness of 7 mm, micronized progesterone (Utrogestan, Besins, Belgium) 600 mg/day was added to the treatment regime. On day 6 after commencing progesterone, a 5AA euploid blastocyst was transferred under ultrasound guidance. Serum beta-human chorionic gonadotropin (B-hCG) was positive 9 days after embryo transfer. A transvaginal ultrasound confirmed viable intra-uterine pregnancy at 7-week gestation. Both estradiol and progesterone were continued up to eight completed weeks of pregnancy. The pregnancy progressed uneventfully and resulted in a normal vaginal delivery of a healthy male infant, weighing 4070 g in 2018. One euploid blastocyst remains in storage.
rFSH recombinant FSH
IVM in vitro maturation, MII mature oocytes
Discussion
There are isolated case reports in the literature describing live birth following IVM in patients with ROS. In 2013, Grynberg M et al. report the first case of pregnancy and live birth using IVM in a 29-year-old woman with multiple antral follicles that were unresponsive to exogenous gonadotropin stimulation [15]. In that case, the authors used IVM with a trial of stimulation using recombinant FSH and administration of 10,000 IU of human chorionic gonadotropin (hCG) for final oocyte maturation. Two days after ICSI, three embryos were transferred into the uterus. Fifteen days after embryo transfer, serum b-hCG was positive; the pregnancy progressed uneventfully and resulted in a live birth of a healthy infant.
Li Y et al. reported another case of live birth of a healthy child using IVM in a 33-year-old woman with secondary infertility of 3 years duration [17]. The authors also performed gonadotropin priming and hCG triggering prior to IVM. Five cumulus–oocyte complexes were obtained. On day 3 after ICSI, two good quality embryos were transferred into the uterus. The pregnancy progressed to an uneventfully delivered healthy boy.
In 2018, Galvão A et al. published the results of using IVM in nine patients with ROS [3]. Two of them had a regular menstrual cycle, three had oligomenorrhea, and only four patients presented with amenorrhea. The authors argued that all patients, including the women with a regular 28-day menstrual cycle, had the diagnosis of ROS. In this study, IVM resulted in a live birth in two patients with ROS. One case was related to oocyte donation, where the oocyte donor had FSH resistance. The first patient had secondary amenorrhea and ovarian resistance to FSH. Seven cycles of immature oocyte retrievals followed by IVM were conducted in this patient, resulting in three fresh embryo transfers. The last embryo transfer resulted in a live birth. In the final successful cycle, transvaginal OPU was performed after ovarian stimulation with gonadotropins (150 IU/day for 5 days) and administration of an ovulation trigger (hCG 10,000 IU). There were seven COCs collected and four had reached the metaphase II stage. Double cleavage-stage fresh embryo transfer was performed. The authors tested the basal serum FSH level in seven IVM cycles in this patient with the following results: 6.5, 8.6, 9.0, 5.2, 12.0, 12.6, and 7.9 IU/L. She also had an AFC of 40 and a baseline serum AMH of 2.11 μg/L. Whether this patient can be classified as having hypergonadotropic hypogonadism is debatable. The second patient underwent six IVM cycles with successful treatment in two instances. In the first IVM attempt, 14 COCs were collected after administration of an ovulation trigger (hCG 10,000 IU). Single cleavage-stage fresh embryo transfer was performed, which resulted in a live birth. In the second instance, no stimulation or trigger was used, ultimately resulting in the live birth of healthy twins. For the third patient in this group, COC collection was performed without hormonal pre-treatment and IVM was once again successful in producing MII oocytes with subsequent live birth.
In 2019, Flageole C. et al. [18] reported yet another pregnancy and live birth following IVM of oocytes in a 31-year-old patient suffering from ROS. Before transvaginal OPU was attempted, the authors reported using high daily doses of recombinant FSH (up to 600 IU) and a subcutaneous injection of hCG 10,000 IU. A single fresh cleavage-stage embryo transfer resulted in live birth.
In the majority of the described cases of successful treatment of infertility in patients with ROS, IVM was combined with short gonadotropin stimulation and trigger b-hCG injection before oocyte collection was undertaken. The utility of such a combined approach is unclear in the setting of ROS, since antral follicles do not appear to be responsive to either high or low dose of exogenous FSH stimulation. Increased endogenous FSH level typically observed in “true” ROS also makes such stimulation likely to be of limited benefit and probably futile.
FSH priming in humans was adopted based on the early techniques used in animal models. FSH priming generates immature, germinal vesicle stage oocytes that are capable of undergoing meiosis in vitro. An IVM cycle may exclude any and all forms of hormonal manipulation that are intended to trigger oocyte maturation in vivo [14]. It is the authors’ contention that IVM is the maturation in vitro of immature COCs collected from antral follicles, which may include priming with FSH, but excludes cycles primed with hCG or GnRH agonists to trigger oocyte maturation in vivo. It is also the definition used by the American Society for Reproductive Medicine (ASRM): “The maturation in culture of immature oocytes after their recovery from follicles that may or may not have been exposed to exogenous FSH but were not exposed to either exogenous LH or hCG prior to retrieval to induce meiotic resumption.” The use of exogenous LH or hCG prior to OPU is not IVM in the strictest sense [19]. It is recognized that during an oocyte collection, even from small follicles, an occasional MII oocyte can be found if b-hCG was administered. These oocytes clearly do not require IVM to be fertilized successfully [20,21,22].
We feel that the recovery and use of in vivo matured oocytes in IVM cycles, which include a b-hCG trigger, challenge the notion that such an approach can be defined as IVM. The use of gonadotropin priming in conjunction with IVM in polycystic ovarian syndrome patients may be beneficial. However, the use of gonadotropin priming and triggering in ROS patients is not consistent with the current understanding of pathophysiology of this condition.
In our case, we used the IVM technique, without the use of gonadotropin priming and b-hCG trigger. A distinctive feature of this case is the cultivation of the resulting embryos to the blastocyst stage, in contrast to the transfer of embryos at the cleavage stage described in previously published cases, and in line with current IVM protocols. Incubation to the blastocyst stage made it possible to utilize PGT-A, via trophectoderm biopsy, in order to select a euploid embryo and perform elective single-embryo transfer with reduced risk of multiple pregnancies [23]. We have decided to perform PGT-A on this patient because there are no data on the frequency of detection of euploidy, aneuploidy, and mosaicism during PGT-A in patients with ROS after IVM. Also, most IVF cycles in our clinic are performed with PGT-A.
Limited information is available about the chromosomal constitution of IVM embryos. According to Spits C et al., the rate and type of chromosomal abnormalities in embryos obtained from oocytes matured in vitro are not different from those observed in embryos obtained from oocytes matured in vivo [24]. However, data pertaining to aneuploidy rates in embryos after IVM in patients with ROS are absent.
It is imperative to monitor the health status of children born after IVM. Mostinckx L et al. showed comparable rates of congenital malformations and preterm birth in IVM offspring compared with their counterparts conceived after controlled ovarian stimulation (COS) in women with polycystic ovary syndrome [25]. Belva F et al. did not observe IVM’s adverse effects on growth parameters in offspring ∼2 years of age compared with COS [26]. Few publications have reported on the health of IVM offspring, specifically from women with ROS. Therefore, we still need to investigate these children after IVM in detail. Further research is required to ensure the safety of IVM, reliability of outcomes, and most importantly, to ensure that there are no long-term adverse health consequences for the children born with the help of this promising technology.
Conclusion
We report the first pregnancy and live birth obtained after IVM of oocytes and PGT-A in a patient with genetic variation of FSH receptor and phenotype consistent with ROS. The results indicate that oocytes obtained with IVM in patients with ROS are capable of meiotic and mitotic division, fertilization, generation of euploid embryos, and live birth of a healthy child. IVM can be considered to be an effective treatment for infertility in patients with hypergonadotropic hypogonadism, normal ovarian reserve, and gonadotropin resistance. IVM could be attempted in the setting of ROS where the only other viable alternative is donor oocyte utilization. Our experience and data from previous publications indicate that there is no requirement for gonadotropin stimulation and use of b-hCG trigger prior to IVM in patients with ROS.
References
Jones GS, De Moraes-Ruehsen M. A new syndrome of amenorrhea in association with hypergonadotropism and apparently normal ovarian follicular apparatus. Am J Obstet Gynecol. 1969;104(4):597–600.
Jequiez AM. Conception in the resistant ovary syndrome occurring during hormone replacement therapy: a report of two cases. Aust N Z J Obstet Gynecol. 1990;30:176–8.
Galvão A, Segers I, Smitz J, Tournaye H, De Vos M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J Assist Reprod Genet. 2018;35(12):2161–71. https://doi.org/10.1007/s10815-018-1317-z.
Huhtaniemi I, Alevizaki M. Gonadotropin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:561–76. https://doi.org/10.1016/j.beem.2006.09.003.
Khor S, Lyu Q, Kuang Y, Lu X. Novel FSHR variants causing female resistant ovary syndrome. Mol Genet Genomic Med. 2019;8(2):e1082. https://doi.org/10.1002/mgg3.1082.
Huhtaniemi IT, Themmen AP. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine. 2005;26:207–17. https://doi.org/10.1385/ENDO:26:3:207.
Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–83. https://doi.org/10.1210/edrv.21.5.0409.
Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol. 1999;51:97–9. https://doi.org/10.1046/j.1365-2265.1999.00745.x.
Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–68. https://doi.org/10.1016/0092-8674(95)90275-9.
Haller-Kikkatalo K, Salumets A, Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clin Dev Immunol. 2012;2012:762541. https://doi.org/10.1155/2012/762541.
Rogenhofer N, Pavlik R, Jeschke U, Wypior G, Ochsenkühn R, Thaler CJ. Effective ovarian stimulation in a patient with resistant ovary syndrome and antigonadotropin antibodies. Am J Reprod Immunol. 2015;73:185–91. https://doi.org/10.1111/aji.12306.
Meyer WR, Lavy G, DeCherney AH, Visintin I, Economy K, Luborsky JL. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response to exogenous gonadotropin. Obstet Gynecol. 1990;75:795–9.
Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev. 2016;37(6):609–35. https://doi.org/10.1210/er.2016-1047.
De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear - variations need defining. Hum Reprod. 2016;31:2411–5. https://doi.org/10.1093/humrep/dew208.
Grynberg M, Peltoketo H, Christin-Maître S, Poulain M, Bouchard P, Fanchin R. First birth achieved after in vitro maturation of oocytes from a woman endowed with multiple antral follicles unresponsive to follicle-stimulating hormone. J Clin Endocrinol Metab. 2013;98:4493–8. https://doi.org/10.1210/jc.2013-1967.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999. p. 378–88.
Li Y, Pan P, Yuan P, Qiu Q, Yang D. Successful live birth in a woman with resistant ovary syndrome following in vitro maturation of oocytes. J Ovarian Res. 2016;9:1–6. https://doi.org/10.1186/s13048-016-0263-6.
Flageole C, Toufaily C, Bernard DJ, Ates S, Blais V, Chénier S, et al. Successful in vitro maturation of oocytes in a woman with gonadotropin-resistant ovary syndrome associated with a novel combination of FSH receptor gene variants: a case report. J Assist Reprod Genet. 2019;36(3):425–32. https://doi.org/10.1007/s10815-018-1394-z.
Practice Committees of the American Society for Reproductive. M, the Society for Assisted Reproductive T. In vitro maturation: a committee opinion. Fertil Steril. 2013;99:663–6.
Hyman JH, Sokal-Arnon T, Son WY, Tan SL, Dahan MH. Live birth of twins after performing early hCG administration as a modification of natural cycle in vitro fertilization, in a women with decreased ovarian reserve. Arch Gynecol Obstet. 2015;291:219–22. https://doi.org/10.1007/s00404-014-3371-9.
Son WY, Chung JT, Dahan M, Reinblatt S, Tan SL, Holzer H. Comparison of fertilization and embryonic development in sibling in vivo matured oocytes retrieved from different sizes follicles from in vitro maturation cycles. J Assist Reprod Genet. 2011;28:539–44. https://doi.org/10.1007/s10815-010-9527-z.
Son WY, Chung JT, Herrero B, Dean N, Demirtas E, Holzer H, et al. Selection of the optimal day for oocyte retrieval based on the diameter of the dominant follicle in hCG-primed in vitro maturation cycles. Hum Reprod. 2008;23:2680–5. https://doi.org/10.1093/humrep/den332.
Klenov VE, Boulet SL, Mejia RB, et al. Live birth and multiple birth rates in US in vitro fertilization treatment using donor oocytes: a comparison of single-embryo transfer and double-embryo transfer. J Assist Reprod Genet. 2018;35(9):1657–64. https://doi.org/10.1007/s10815-018-1243-0.
Spits C, Guzman L, Mertzanidou A, Jacobs K, Ortega-Hrepich C, Gilchrist RB, et al. Chromosome constitution of human embryos generated after in vitro maturation including 3-isobutyl-1-methylxanthine in the oocyte collection medium. Hum Reprod. 2015;30(3):653–63. https://doi.org/10.1093/humrep/deu329.
Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, et al. Obstetric and neonatal outcome of assisted reproductive technology (ART) in patients with polycystic ovaries (PCO): in vitro maturation (IVM) of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34:1595–607. https://doi.org/10.1093/humrep/dez086.
Belva F, Roelants M, Vermaning S, Desmyttere S, De Schepper J, Bonduelle M, et al. Growth and other health outcomes of 2-year-old singletons born after IVM versus controlled ovarian stimulation in mothers with polycystic ovary syndrome. Hum Reprod Open. 2020;10(1):hoz043. https://doi.org/10.1093/hropen/hoz043.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kornilov Nikolai, Pavlova Marina, and Yakovlev Pavel. The first draft of the manuscript was written by Yakovlev Pavel and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval is required. Informed consent was obtained from participant included in the study.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kornilov, N.V., Pavlova, M.N. & Yakovlev, P.P. The live birth in a woman with resistant ovary syndrome after in vitro oocyte maturation and preimplantation genetic testing for aneuploidy. J Assist Reprod Genet 38, 1303–1309 (2021). https://doi.org/10.1007/s10815-021-02085-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02085-5