Abstract
In vitro maturation (IVM) is a modified in vitro fertilisation (IVF) procedure, whereby immature oocytes are collected and complete their final stages of maturation in the laboratory or ‘in vitro’. Clinical pregnancy and live birth success rates following IVM differ considerably in the literature, as do the treatment regimens and culture conditions used to achieve them. Variations in hormonal priming and culture media/culture timing mean that there is no standard IVM protocol, and many clinics have adopted their own modifications, for their own systems, with varying degrees of success. This chapter will introduce IVM as a clinical treatment option for patients with polycystic ovary syndrome (PCOS) and outline the current trends in IVM protocols used around the world. As IVM is the only treatment option which can completely eliminate the risk of ovarian hyperstimulation syndrome for which PCOS patients are at a much greater risk, it is a useful addition to a clinics’ routine treatment options.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Human IVM was first reported more than 50 years ago [1]; however, with the introduction of gonadotrophins to stimulate multi-follicular growth [2], research into IVM treatment became less popular. This introduction of ovarian hyperstimulation also introduced the side effect of OHSS [3], which is a significant clinical consequence of gonadotrophin stimulation, resulting in patient discomfort in the mild stages and significant morbidity or mortality in the major forms of the condition [4]. Even though IVM provided a viable alternative to avoid ovarian hyperstimulation syndrome (OHSS), stimulated IVF treatment expanded worldwide, and it wasn’t until 1991 when the first live birth was recorded after IVM following collection by ovary biopsy [5]. Following this, in 1994 IVM success was achieved following a transvaginal oocyte aspiration (TVOA) [6], and its reported use in the literature has continued at a steady pace ever since. IVM treatment has now expanded to treat a range of conditions including gamete donation and follicle-stimulating hormone (FSH)-resistant ovaries and for fertility preservation. However, the predominant patient cohort who are suitable for IVM treatment are those diagnosed with polycystic ovarian morphology (PCOM) and/or polycystic ovary syndrome (PCOS). Additionally and most importantly, IVM is currently the only treatment option which completely eliminates the risk of OHSS [7].
This chapter will outline the use of IVM as an effective treatment method for patients with PCOS and PCOM, as well as summarise the different protocols for treatment regimes, hormonal priming and culture conditions with resulting reproductive outcomes. It will also address the limited current evidence available for the outcomes of children born following IVM and where IVM research is heading in the future.
2 IVM in the Treatment of Women with PCOM/PCOS
The defining feature for increased IVM success rates is an increased antral follicle count (AFC) [8]. Only PCOS patients with PCOM (more than 12 antral follicles) and PCOS patients with a high antral follicle (although not so high to be classified as PCOM) count could have best benefit from IVM. As the AFC is a determining factor for IVM treatment, patients with fewer than five antral follicles should not be considered for this treatment [9]. Considering women with PCOM/PCOS who typically have a very high AFC, they therefore respond better to IVM treatment and have more oocytes collected from IVM cycles than patients without the condition. While those patients with PCOM may still benefit from IVM treatment, a recent meta-analysis showed that implantation and clinical pregnancy rates are highest in patients with PCOS [10].
3 Treatment Regimes and Hormonal Priming
In theory, IVM requires no exogenous gonadotrophin administration as the immature oocytes complete their final stages of maturation under the influence of suitable culture conditions, tailored to mimic the intra-follicular environment. However, hormonal priming using follicle-stimulating hormone (FSH) or human menopausal gonadotrophin (hMG) and/or human chorionic gonadotrophin (hCG) is often used to ‘prime’ the follicles prior to oocyte aspiration. The results of these protocols are contradictory and difficult to evaluate, due to differences in priming and culture conditions. FSH priming plays an important role in increasing follicular growth and contributes to higher rates of oocytes collected, increased maturation [9], fertilisation, embryo development and implantation [9], and the highest rates of IVM success have been achieved with 3–5 days of FSH priming without hCG triggering when the dominant follicle is less than 12 mm [11]. Protocols for hCG priming achieve the best result with a 38 h interval from 10,000 IU hCG triggering to oocyte collection [12] when the dominant follicle is less than 14 mm [13].
The predominant controversial issue is the use of hCG or gonadotrophin-releasing hormone (GnRH) agonist triggers in IVM protocols, as they induce nuclear maturation in vivo [14] and, hence, are at odds with the core concept of IVM, where maturation by definition takes place in vitro. Additionally, as hCG can induce oocyte maturation in vivo in follicles greater than 9 mm [15], this methodology is logistically problematic, as oocytes are at varying stages of development when they are collected. This in turn leads to multiple insemination times for the same patient and subsequent variations in embryo culture stages. Recently, an effort to change the clinical definition of IVM has been suggested to categorise the different protocols currently employed around the world [16]. The authors suggested four definitions of IVM treatment protocols: (1) IVM without triggering, (2) natural cycle IVF with early triggering combined with IVM, (3) IVM with short gonadotrophin stimulation and (4) modified natural cycle IVF with early triggering combined with IVM. However, these definitions are confusing and still allow for a category of patients receiving both FSH priming and hCG triggering which others have suggested be termed ‘truncated IVF’ [17, 18].
More widely accepted definitions have been recommended to include three treatment groups, ‘truncated IVF’(where priming includes both FSH and hCG) and ‘hCG-primed IVM’ (where there is no FSH administered and patients receive an hCG or agonist trigger), and the definition of ‘IVM’ is suggested to be reserved for those cycles with or without FSH/FSH-analogue priming, without the use of ‘gonadotrophins that are intended to trigger oocyte maturation in vivo, such as hCG or GnRH agonists’ [18]. This is a necessary development for IVM both clinically and for research purposes, to avoid comparisons between outcomes of what some consider to be true IVM vs an abridged version of standard, stimulated IVF treatment. This will aid to avoid confusion for clinicians, patients and health professionals when IVM is discussed.
4 Immature Oocyte Collection
Similarly to priming protocols, procedures for immature oocyte collection in IVM cycles show considerable variation. They are mostly centred around a standard TVOA procedure with modifications that enable the collection of oocytes from small follicles. Clinicians may use a double or single lumen needle, depending on whether the protocol employs follicular flushing. Some clinics have reported flushing each follicle up to three times [19], and others do not employ follicular flushing [20]. Follicular flushing solutions include HEPES [21] or Hartmann’s supplemented with heparin [19]. Additionally, the aspiration pressure used in TVOA for IVM has been reported in varying ranges, from 7 kpa (52.5 mmHg) [22] to 200 mmHg [21].
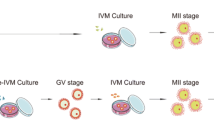
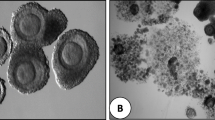
Such as in standard IVF, germinal vesicle (GV) stage oocytes can be identified by sight [11, 19], which involves additional training of the embryologist, or by filtering follicular aspirates through a mesh cell strainer [20, 23]. Figure 20.1 represents a GV stage oocyte located during an IVM oocyte collection. Once the cumulus-oocyte complex is removed from the follicle, there is a significant drop in the amount of gap junction communication between the cumulus cells and the oocyte and a dramatic decrease in cyclic adenosine monophosphate (cAMP) activity [24]. Following removal from the follicle, some oocytes may undergo spontaneous nuclear maturation, evidence by the progression to metaphase II (MII) status [25]. However, this does not necessarily correlate to cytoplasmic maturity, whereby the oocyte gains the capacity for activation and the resumption of meiosis, leading to successful fertilisation and ongoing embryo development [26]. This needs to be managed effectively in order to prevent spontaneous maturation and asynchrony between nuclear and cytoplasmic maturation, which can be detrimental to embryo growth, and this is predominantly managed through the development of specialised IVM culture systems.
5 Oocyte Maturation and Culture
IVM culture differs depending on the base media, culture timing and hormone and serum concentrations. A range of culture media have been formulated for use in IVM. The two mostly widely used commercially available IVM base media are Sage (CooperSurgical, USA) and MediCult (Origio, Denmark), which have shown similar success rates [27]. Media specifically formulated for blastocyst culture have been used successfully [11, 28] and have comparable success rates compared to Sage IVM media [29].
Unlike priming protocols, hormonal additives are common components for culture media in human IVM. Luteinising hormone (LH) and hCG are important mediators of oocyte maturation as they act on a common granulosa cell receptor to induce the intracellular rise in cAMP activity within the oocyte [30]. The cAMP cascade then promotes the resumption of meiosis and germinal vesicle breakdown [26]. Recombinant LH or hCG are added in concentrations from 0.1 IU/mL [23] to 0.75 IU/mL [31]. FSH is used to promote cumulus-oocyte complex (COC) expansion and a subsequent rise in cAMP activity leading to increased oocyte maturation [32, 33]. The concentration of FSH added is relatively consistent across IVM protocols at 0.075 IU/mL [23, 34] or 0.1 IU/mL [11, 19].
5.1 Other Culture Media Additives
Other additives have been suggested to be beneficial to IVM culture media by aiding in either maturation, fertilisation or embryo development. These include insulin-like growth factor (IGF-I) [35, 36], epidermal growth factor (EGF) [35, 37, 38], meiosis-activating sterols (MAS) [39, 40] and activin [41]; however, these are rarely used in routine culture.
5.2 Serum or Follicular Fluid in Culture
Human follicular fluid (HFF), inactivated autologous patient serum (maternal serum) or foetal bovine serum (FBS) are the three protein additives used most commonly in human IVM. HFF supplementation has been used in concentrations ranging from 30% [42] up to 70% [21]. Heat-inactivated FBS has also been used at either 10% [43] or 20% [14, 44]. Maternal serum is the most common protein supplement, used in concentrations of 10% [23, 45] or 20% [22, 46]. The use of human serum in IVM culture media may provide a number of nutrients and growth factors that are involved in the maturation process. There are, however, a number of negative aspects associated with using serum in embryo culture including contributing to ammonia formation, which can be damaging to embryo development through mitochondrial disruption [47], and the interruption of the aromatisation process involved in the conversion of androgens to oestrogens in granulosa cells [48].
Additionally, concentrations of amino acids, lipids, hormones, antibodies and other immunological mediators vary between women according to diet, genetics and infection status, such that serum can be highly variable in its potency and toxicity. Additionally, there is the risk of contributing unknown contaminants (microbial and otherwise) to the cultured embryos when using serum or HFF preparations. However, it is not clear whether the negative effects of serum relevant to embryo culture also relate to in oocyte maturation culture. In most circumstances, oocytes are only exposed to maternal serum for the first 24–48 h of in vitro culture, depending on the protocol employed, and are then moved into commercial embryo culture media prior to insemination. Regardless, more research is needed into the effects of protein additives to IVM culture.
Recent research has investigated the benefits of supplementing IVM culture media with cAMP modulators. These include cilostamide and forskolin, which are designed to prevent the loss of gap cell junctions following removal of the oocyte from the follicle and the subsequent decrease in cAMP activity [49]. cAMP modulators may also prove beneficial during a short pre-IVM period, immediately after removal from the follicle, known as simulated physiological oocyte maturation (SPOM) [50], although in humans, the benefits of this pretreatment are inhibited by heparin, which is most often used during the collection procedure [51]. Initial testing of another cAMP modulator 3-isobutyl-1-methylxanthine (IBMX) in human participants was shown to be a safe additive, in terms of the incidence of embryo chromosomal aneuploidy rates [52]. Further large-scale trials into its effect on human IVM success rates are required.
5.3 Culture Timing
Similarly to priming protocols and culture media contents, there are considerable variations in oocyte maturation culture time reported in the literature. Following the oocyte collection procedure and transport to the embryology laboratory, oocytes are placed into maturation culture for at least 24 h [19]; however, culture periods of up to 40 h have also been reported [53]. Additionally, if a hCG trigger is used, there will be variations in culture timings such as at 24 and 30 h [54] for the same oocyte cohort. At the initiation of maturation culture in non-hCG-primed cycles, the GV stage oocytes will have a tightly compacted cumulus oophorus (Figs. 20.2, 20.3 and 20.4), and after maturation culture, expansion of the coronal cells should be visible (Figs. 20.5, 20.6 and 20.7).
6 Fertilisation, Embryo Culture
IVM fertilisation is predominantly performed using intracytoplasmic sperm injection (ICSI) as fertilisation and implantation rates were originally reported as significantly lower than IVF-inseminated oocytes following IVM [23]. However, following a small, sibling oocyte study, no significant differences were seen between those fertilised by ICSI and IVF in outcomes of fertilisation, blastocyst development or implantation rates [28]. When using IVF as a fertilisation technique, it is important to consider that there is a potential conflict in the timing at which maturation/fertilisation check is performed between ICSI and IVF. IVF oocytes are allowed an additional 16–20 h prior to denudation and, therefore, additional time to undergo spontaneous and late maturation which may skew fertilisation results if they are calculated per oocyte matured.
Following fertilisation, embryo culture practices generally do not differ from standard IVF, and culture timing should reflect what is normally performed in any treating clinic. The majority of reports of clinical IVM culture perform 3 days of culture and transfer or freezing of cleavage stage embryos [22, 23, 44]; however, as with standard IVF treatment, blastocyst culture in IVM has become more prevalent [55]. Blastocyst culture may also be preferable in IVM as embryo development may be impaired during the early cleavage stages, with higher rates embryo arrest reported during the second and third cell cycles; however, embryo arrest during compaction and blastulation as well as kinetic time points were no different from standard ICSI treatment [56]. Blastocyst culture would therefore enable the deselection of embryos which may appear to be of suitable quality at the cleavage stage but fail to progress further.
7 Transfer/Cryopreservation
Endometrial priming is required if IVM-derived embryos are intended to be transferred in fresh cycles, and this is achieved using oestrogen and progesterone supplementation. This methodology shown to be beneficial when administration begins at the mid-follicular timing of the cycle [57] with at least 6 days of oestrogen necessary for endometrial receptivity [58]. High rates of implantation and live birth can be achieved by oestrogen supplementation 2 days prior and progesterone supplementation commencing on the day of oocyte collection, extending to the day of pregnancy test [11]. However, significantly higher rates of miscarriage and early pregnancy loss were seen following transfers in fresh IVM cycles compared to fresh IVF cycles, and this is not evident following frozen transfers [19]. Therefore, a freeze-all approach may provide the best outcomes for patients following IVM treatment.
8 Reproductive Outcomes
A recent systematic review and meta-analysis of treatment strategies for PCOS was inconclusive in regard to IVM as no RCTs were identified [59]. Success rates from non-RCT publications vary considerably in reported outcomes, and the results are difficult to compare due to the differences in treatment protocols. Reports of implantation rates for IVM range from 0% [9] to 34.5% [23] for cycles with no hormonal priming, 21.6% [9] to 47.7% [11] for cycles with recombinant FSH priming only, 8.9% [22] to 26.8% [60] for cycles with hCG priming only and 9.7% [46] for cycles with both FSH and hCG administered.
One of the primary reasons for the limited use of IVM around the world is that traditionally it is significantly less successful than standard IVF. There are only three reports of clinical IVM outcomes which include an IVF control group, all of which report lower live birth rates in PCOM/PCOS patients from fresh cycles [28, 34, 61]. In addition, miscarriage rates following fresh IVM cycles are significantly higher than in IVF and ICSI cycles [62], although this may be influenced by PCOS status and not the IVM procedure itself and/or insufficient endometrial conditions following fresh cycles, as this is not seen following frozen embryo transfer cycles [28]. The small size of these studies as well as the large variation in results further highlights the need for a large-scale, randomised clinical trial for IVM treatment.
9 Birth Outcomes
It is estimated that more than 3000 births have been achieved worldwide following IVM treatment, and although there is very limited research on the outcomes of these children, that which is available demonstrates a very positive outlook. Only six publications to date have reported on neonatal outcomes from IVM births. The reported incidence rates of congenital birth defects include 0% [11, 65], 2.1% [64], 7.1% [63], 5.1% [66] and 3% [19] (Table 20.1). Additionally, Walls et al. and Fadini et al. included IVF controls and found no difference in congenital birth defects between the two treatments. However, the number of live births included within these data sets was small, with some including multiple births, and therefore, further large-scale studies are needed to determine the true impact of IVM treatment on congenital malformation.
Other measurements of neonatal health including Apgar scores have been reported in multiple studies and are within normal ranges [65] or show no significant difference to controls for singleton live births [66,67,68]. The incidence of adverse outcomes is often confounded by multiple births as many IVF centres worldwide still routinely transfer multiple embryos, though evidence suggests this is not best practice [69]. Regardless of multiple birth outcomes, the incidence of congenital birth defects, preterm birth and low birth weight, which are often associated with ART treatments (especially in PCOS patients), is low following IVM treatment.
Such as for birth outcomes, long-term outcomes from children born following IVM are unknown, and there are currently no long-term data on children born from IVM. However, the limited reports of the follow-up to children born from this technique do not demonstrate any adverse outcomes. The first study published on the development of children following IVM reported follow-up at 6, 12 and 24 months after birth and found that physical growth at all stages as well as neurological and neuropsychological outcomes were normal [65]. There were minor developmental delays in 8/43 children at 12 months (19%), but this decreased to 3% at 24 months. Their findings did not include IVF controls; however, they were within normal ranges for the general population.
Very few reports of early childhood outcomes are available employing standard IVF controls for IVM treatment. However, in one such study, it was reported that no differences in height or weight were found in 6 and 24 months of age in IVM infants compared to standard IVF controls [68]. Additionally, there was no difference in mental developmental index and psychomotor scores between the two groups according to the Bayley Scales of Infant Development. Following these reports, in a cohort of French children, female infants born from IVM treatment displayed increased mean weight, height, body mass index (BMI) and head circumference at birth compared to those born following standard IVF treatment with ICSI. These outcomes remained significantly higher than the control group after 2 years of follow-up [67]. It is yet to be determined whether these findings are related to underlying infertility and PCOS rather than the IVM procedure itself. It appears that growth and development of IVM children falls within normal limits; however, further research is crucial to determine outcomes into early childhood, adolescence and adulthood.
10 The Risks of Aneuploidy and Epigenetic Variation
There is very little evidence of the effects of IVM on the risk of embryo chromosomal aneuploidy. Two case-control studies utilising fluorescence in situ hybridisation (FISH) reported no difference in the incidence of chromosomal abnormality between IVF- and IVM-derived embryos [70, 71]. Only one study has reported on the use of array comparative genomic hybridisation (aCGH) with IVM-derived embryos. This study involved the addition of the phosphodiesterase inhibitor IBMX into the culture media, and rates of aneuploidy were found to be similar to the researchers’ previously published data from standard IVF treatments [72]. The limited data available in regard to aneuploidy and IVM highlights the need for more research in this area.
Similar to the risks of aneuploidy, concerns have been raised about the possible interference of IVM with epigenetic mechanisms and in particular with genomic imprinting. A systemic review of the risks of imprinting defects following oocyte culture in animal studies shows reassuring evidence of correct imprinted DNA methylation while highlighting the need for further research [73]. Additionally, research into the impacts of IVM on epigenetic variation in human oocytes is limited, and there is currently no information available on a genome-wide scale. Instead, researchers have focused on the analysis of selected imprinting genes and their error rates following IVM treatment. In one study of IVM, it was found that for the selected genes LIT1, SNRPN, PEG3 and GTL2, there were no significant increases in imprinting mutations [74]. In a more recent study, researchers compared six imprinting, five tumour suppressors, two pluripotency and two metabolic genes from cord blood and chorionic villus samples. Two repetitive elements were included to detect genome-wide DNA methylation changes in both, to detect allele methylation errors and found no difference in epigenetic changes between samples from 11 IVM and 19 IVF control neonates [75]. Therefore, while there is a clear need for further research, the limited data available so far is reassuring with respect to the continued use of IVM as a treatment for infertility.
Conclusions
With significant improvements to success rates in recent years, IVM may be considered a valuable treatment option for ART clinics. This is especially important for patients with PCOS who are at a significantly higher risk of developing OHSS. There have been significant milestones made in animal models from the investigation of oocyte-secreted factors growth-differentiation factor nine (GDF9) and bone morphogenic protein 15 (BMP15). These form part of the transforming growth factor β (TGFβ) superfamily and are necessary components for functional fertility [76, 77]. These factors produced by the oocyte act through paracrine signalling as regulators of granulosa/cumulus cell expansion and differentiation [78, 79]. The addition of recombinant forms of these factors to IVM culture media and/or other additives such as cAMP modulators may help improve success rates even further.
However, there still remains a clear need for large-scale randomised controlled studies to validate IVM success compared to standard IVF. Additionally, further research into the long-term outcomes of children born following IVM is necessary, even though initial assessments of children born from the technique show promising results. Finally, in order for IVM to become a more widely accepted treatment method worldwide, there needs to be a more standardised approach to protocols which will enable clinics to more easily implement this important treatment option.
References
Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–51.
Porter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet (London). 1984;2:1284–5.
Rizk B, Smitz J. Ovarian hyperstimulation syndrome after superovulation using GnRH agonists for IVF and related procedures. Hum Reprod. 1992;7:320–7.
Saul T, Sonson JM. Ovarian hyperstimulation syndrome. Am J Emerg Med. 2009;27:250.e3–4.
Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109.
Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353.
Lindenberg S. New approach in patients with polycystic ovaries, lessons for everyone. Fertil Steril. 2013;99:1170–2.
Tan SL, Child TJ, Gulekli B. In vitro maturation and fertilization of oocytes from unstimulated ovaries: predicting the number of immature oocytes retrieved by early follicular phase ultrasonography. Am J Obstet Gynecol. 2002;186:684–9.
Mikkelsen A, Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction. 2001;122:587–92.
Siristatidis C, Sergentanis TN, Vogiatzi P, Kanavidis P, Chrelias C, Papantoniou N, et al. In vitro maturation in women with vs. without polycystic ovarian syndrome: a systematic review and meta-analysis. PLoS One. 2015;10:e0134696.
Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2012;98:888–92.
Son W-Y, Chung J-T, Chian R-C, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23:2010–6.
Son W-Y, Chung J-T, Herrero B, Dean N, Demirtas E, Holzer H, et al. Selection of the optimal day for oocyte retrieval based on the diameter of the dominant follicle in hCG-primed in vitro maturation cycles. Hum Reprod. 2008;23:2680–5.
Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–70.
Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71:132–43.
Dahan MH, Tan SL, Chung J, Son W-Y. Clinical definition paper on in vitro maturation of human oocytes. Hum Reprod. 2016;31:1383–6.
Coticchio G. IVM in need of clear definitions. Hum Reprod. 2016;31:1387–9.
De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear – variations need defining. Hum Reprod. 2016;31:2411–5. Invited Commentary
Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30:s88–96.
Hreinsson J, Rosenlund B, Fridén B, Levkov L, Ek I, Suikkari AM, et al. Recombinant LH is equally effective as recombinant hCG in promoting oocyte maturation in a clinical in-vitro maturation programme: a randomized study. Hum Reprod. 2003;18:2131–6.
Yoon H-G, Yoon S-H, Son W-Y, Lee S-W, Park S-P, Im K-S, et al. Clinical assisted reproduction: pregnancies resulting from in vitro matured oocytes collected from women with regular menstrual cycle. J Assist Reprod Genet. 2001;18:325–9.
Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936–42.
Söderström-Anttila V, Mäkinen S, Tuuri T, Suikkari A-M. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534–40.
Sasseville M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard FJ. Regulation of gap junctions in porcine cumulus-oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol. 2009;23:700–10.
Escrich L, Grau N, Mercader A, Rubio C, Pellicer A, Escribá M-J. Spontaneous in vitro maturation and artificial activation of human germinal vesicle oocytes recovered from stimulated cycles. J Assist Reprod Genet. 2011;28:111–7.
Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–9.
Pongsuthirak P, Vutyavanich T. Comparison of medicult and sage media for in vitro maturation of immature oocytes obtained during cesarean deliveries. J Fertil In Vitro IVF-Worldw Reprod Med Genet Stem Cell Biol. 2015;3:136. doi:10.4172/2375-4508.1000136.
Walls M, Junk S, Ryan J, Hart R. IVF versus ICSI for the fertilization of in-vitro matured human oocytes. Reprod Biomed Online. 2012;25:603–7.
Pongsuthirak P, Songveeratham S, Vutyavanich T. Comparison of blastocyst and Sage media for in vitro maturation of human immature oocytes. Reprod Sci. 2015;22:343–6.
Conti M. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod. 2002;67:1653–61.
Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier M-C, Chevalier N, et al. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20:420–4.
Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell enclosed mouse oocytes by follicle stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96.
Guoliang X, Byskov AG, Andersen CY. Cumulus cells secrete a meiosi inducing substance by stimulation with forskolin and dibutyric cyclic adenosine monophosphate. Mol Reprod Dev. 1994;39:17–24.
Gremeau A-S, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case–control study of 194 treatment cycles. Fertil Steril. 2012;98:355–60.
Gomez E, Tarin J, Pellicer A. Oocyte maturation in humans: the role of gonadotropins and growth factors. Fertil Steril. 1993;60:40–6.
Pawshe C, Appa Rao K, Totey S. Effect of insulin like growth factor I and its interaction with gonadotropins on in vitro maturation and embryonic development, cell proliferation, and biosynthetic activity of cumulus oocyte complexes and granulosa cells in buffalo. Mol Reprod Dev. 1998;49:277–85.
Das K, Stout L, Hensleigh H, Tagatz G, Phipps W, Leung B. Direct positive effect of epidermal growth factor on the cytoplasmic maturation of mouse and human oocytes. Fertil Steril. 1991;55:1000.
Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–44.
Smitz J, Picton HM, Platteau P, Rutherford A, Cortvrindt R, Clyde J, et al. Principal findings from a multicenter trial investigating the safety of follicular-fluid meiosis-activating sterol for in vitro maturation of human cumulus-enclosed oocytes. Fertil Steril. 2007;87:949–64.
Grøndahl C, Hansen TH, Marky-Nielsen K, Ottesen JL, Hyttel P. Human oocyte maturation in vitro is stimulated by meiosis-activating sterol. Hum Reprod. 2000;15(Suppl 5):3–10.
Alak BM, Coskun S, Friedman CI, Kennard EA, Kim MH, Seifer DB. Activin A stimulates meiotic maturation of human oocytes and modulates granulosa cell steroidogenesis in vitro. Fertil Steril. 1998;70:1126–30.
Son W-Y, Lee S-Y, Lim J-H. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum Reprod. 2005;20:3204–7.
Suikkari A-M, Tulppala M, Tuuri T, Hovatta O, Barnes F. Luteal phase start of low-dose FSH priming of follicles results in an efficient recovery, maturation and fertilization of immature human oocytes. Hum Reprod. 2000;15:747–51.
Cha KY, Han SY, Chung HM, Choi DH, Lim JM, Lee WS, et al. Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertil Steril. 2000;73:978–83.
Mikkelsen AL, Smith SD, Lindenberg S. In-vitro maturation of human oocytes from regularly menstruating women may be successful without follicle stimulating hormone priming. Hum Reprod. 1999;14:1847–51.
Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, et al. Combination of FSH priming and hCG priming for in-vitro maturation of human oocytes. Hum Reprod. 2003;18:1632–6.
Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;48:377–85.
Salha O, Nugent D, Dada T, Kaufmann S, Levett S, Jenner L, et al. The relationship between follicular fluid aspirate volume and oocyte maturity in in-vitro fertilization cycles. Hum Reprod. 1998;13:1901–6.
Y-m S, H-t Z, Ren Z, G-l Z, Liang X-y, Shen H-w, et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reprod. 2008;23:504–13.
Albuz F, Sasseville M, Lane M, Armstrong D, Thompson J, Gilchrist R. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011.
Zeng H-T, Ren Z, Guzman L, Wang X, Sutton-McDowall ML, Ritter LJ, et al. Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Hum Reprod. 2013;28:1536–45.
Spits C, Guzman L, Mertzanidou A, Jacobs K, Ortega-Hrepich C, Gilchrist RB, et al. Chromosome constitution of human embryos generated after in vitro maturation including 3-isobutyl-1-methylxanthine in the oocyte collection medium. Hum Reprod. 2015;30:653–63.
Son W-Y, Chung J-T, Dahan M, Reinblatt S, Tan SL, Holzer H. Comparison of fertilization and embryonic development in sibling in vivo matured oocytes retrieved from different sizes follicles from in vitro maturation cycles. J Assist Reprod Genet. 2011;28:539–44.
Farsi MM, Kamali N, Pourghasem M. Embryological aspects of oocyte in vitro maturation. Int J Mol Cell Med. 2013;2:99–109.
Barnes FL. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995;10:3243–7.
Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30:1842–9.
Russell JB, Knezevich KM, Fabian KF, Dickson JA. Unstimulated immature oocyte retrieval: early versus midfollicular endometrial priming. Fertil Steril. 1997;67:616–20.
Navot D, Anderson TL, Droesch K, Scott RT, Kreiner D, Rosenwaks Z. Hormonal manipulation of endometrial maturation. J Clin Endocrinol Metab. 1989;68:801–7.
Kollmann M, Martins WP, Lima ML, Craciunas L, Nastri CO, Richardson A, et al. Strategies to improve the outcomes of assisted reproduction in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2016;48:709–18.
Son WY, Lee SY, Yoon SH, Lim JH. Pregnancies and deliveries after transfer of human blastocysts derived from in vitro matured oocytes in in vitro maturation cycles. Fertil Steril. 2007;87:1491–3.
Child TJ, Phillips SJ, Abdul-Jalil AK, Gulekli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization for women with polycystic ovaries. Obstet Gynecol. 2002;100:665–70.
Buckett WM, Chian R-C, Dean NL, Sylvestre C, Holzer HEG, Tan SL. Pregnancy loss in pregnancies conceived after in vitro oocyte maturation, conventional in vitro fertilization, and intracytoplasmic sperm injection. Fertil Steril. 2008;90:546–50.
Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization–embryo transfer. Fertil Steril. 2005;83:1461–5.
Mikkelsen AL. Strategies in human in-vitro maturation and their clinical outcome. Reprod Biomed Online. 2005;10:593–9.
Söderström-Anttila V, Salokorpi T, Pihlaja M, Serenius-Sirve S, Suikkari A-M. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod. 2006;21:1508–13.
Fadini R, Mignini Renzini M, Guarnieri T, Dal Canto M, De Ponti E, Sutcliffe A, et al. Comparison of the obstetric and perinatal outcomes of children conceived from in vitro or in vivo matured oocytes in in vitro maturation treatments with births from conventional ICSI cycles. Hum Reprod. 2012;27:3601–8.
Foix-L'Helias L, Grynberg M, Ducot B, Frydman N, Kerbrat V, Bouyer J, et al. Growth development of French children born after in vitro maturation. PLoS One. 2014;9:e89713.
Shu-Chi M, Jiann-Loung H, Yu-Hung L, Tseng-Chen S, Ming-I L, Tsu-Fuh Y. Growth and development of children conceived by in-vitro maturation of human oocytes. Early Hum Dev. 2006;82:677–82.
Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2013;7:CD003416.
Zhang XY, Ata B, Son W-Y, Buckett WM, Tan S-L, Ao A. Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod Biomed Online. 2010;21:552–9.
Requena A, Bronet F, Guillén A, Agudo D, Bou C, García-Velasco JA. The impact of in-vitro maturation of oocytes on aneuploidy rate. Reprod Biomed Online. 2009;18:777–83.
Mertzanidou A, Wilton L, Cheng J, Spits C, Vanneste E, Moreau Y, et al. Microarray analysis reveals abnormal chromosomal complements in over 70% of 14 normally developing human embryos. Hum Reprod. 2013;28:256–64.
Anckaert E, De Rycke M, Smitz J. Culture of oocytes and risk of imprinting defects. Hum Reprod Update. 2013;19:52–66.
Kuhtz J, Romero S, De Vos M, Smitz J, Haaf T, Anckaert E. Human in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2. Hum Reprod. 2014;29:1995–2005.
Pliushch G, Schneider E, Schneider T, El Hajj N, Rösner S, Strowitzki T, et al. In vitro maturation of oocytes is not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2015;103:720–7.e1.
Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–83.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5.
Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77.
Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78:9–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Walls, M.L. (2018). In Vitro Oocyte Maturation. In: Palomba, S. (eds) Infertility in Women with Polycystic Ovary Syndrome. Springer, Cham. https://doi.org/10.1007/978-3-319-45534-1_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-45534-1_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45533-4
Online ISBN: 978-3-319-45534-1
eBook Packages: MedicineMedicine (R0)