Abstract
Disruptions in normal circadian rhythms and sleep cycles are effects of aging and profoundly affect health. The circadian clock that regulates these rhythms is dynamic throughout the lifespan of mammals. For instance, rhythmic activities, such as sleep/wake patterns, change markedly with age, and in many cases, they become increasingly fragmented. Although several factors contribute to these changes, emerging research suggests that age-related changes in the mammalian central circadian clock within the suprachiasmatic nucleus (SCN) of the hypothalamus may be a key factor. The circadian output from the SCN may decline with age due to the disorganization of the neural circuit within the SCN. This chapter addresses the regulatory mechanisms underlying circadian rhythms in mammals and summarizes the recent literature describing the effects of aging on the circadian system, with a focus on the SCN.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Circadian rhythm
- Circadian output
- Clock gene
- Neural firing activity
- PER2::LUCIFERASE
- Suprachiasmatic nucleus
1 Introduction
Circadian systems do not escape aging as well as other physiological functions. In humans, the sleep/wake rhythm of a newborn baby appears indistinguishable and then becomes entrained to external cues such as maternal behavior and light (Brooks and Canal 2013). In adults, the sleep/wake rhythm becomes stable, and humoral secretion rhythms also exhibit a robust circadian rhythm. However, older adults tend to have sleep disorders, such as early morning awakening and nocturnal awakening, and the amplitudes of rhythms in physiological functions also decline (Hood and Amir 2017). Age-related declines in circadian rhythms are mainly due to functional declines in the suprachiasmatic nucleus (SCN) of the hypothalamus, which is known to be the central circadian clock that regulates circadian rhythms for the whole body. This chapter addresses the regulatory mechanisms underlying the circadian rhythms in mammals and summarizes the recent literature describing the effects of aging on the circadian system, with a focus on the SCN.
2 Effects of Aging on Circadian Rhythms
The effects of aging on circadian rhythms have been reported in numerous species. In aplysia, a mollusk that is commonly used as in neuroscience research, long-term recordings of neural firing activities in the retina, which has the function as a biological clock, revealed that circadian amplitudes drastically decreased in the 12-month-old organisms compared with the 3-month-old organisms (Sloan et al. 1999). Behavioral rhythms in rodents, represented by rats and mice, are also altered with age (Pittendrigh and Daan 1974). In aged mice, the levels of locomotor activity were decreased, fragmented locomotor activity appeared, and free-running periods were lengthened during constant darkness (Fig. 4.1). In particular, the balance of the activity/rest phase in aged animals is “ambiguous” (Valentinuzzi et al. 1997). Although mice used in the laboratory are nocturnal and their results cannot be applied to humans, the cause of early morning awakening in humans is interpreted as a change in the endogenous period, and a shortened/lengthened period has been verified in aged rodents. Thus, rodents can be useful models for studying neural circuits associated with aging.

Adopted from Nakamura et al. (2016)
Effects of aging on the circadian locomotor activity rhythms in mice. Double-plotted actograms showing wheel-running activity in young (left) and aged (right) C57BL/6J mice. The vertical axis indicates the day and the horizontal axis indicates the time course (48 h). The mice were maintained under light/dark (LD = 12 h: 12 h) cycles for 2 weeks and then transferred to constant darkness (DD). In aged mice, the levels of locomotor activity are decreased, fragmented locomotor activity appears, and free-running periods are lengthened in DD.
3 The Central Circadian Clock in the SCN
The central circadian clock, which regulates circadian behavioral/physiological rhythms, is located in the SCN. The SCN is located above the optic chiasm where each optic nerve crosses, and the one-paired nuclei are proximal to either side of the third ventricle. The SCN is a distinctive structure in which each nucleus, containing approximately 10,000 neurons, is densely packed with small cell bodies. Thus, we can easily identify the SCN even though it is the size of a “poppy seed” in mice (Fig. 4.2). The SCN is directly connected to the retina and is entrained to an environmental light/dark cycle. Since the 1990s, clock genes have been identified in mammals, and more recent research has revealed that the system called “cellular clock” was in each SCN cell. Several clock genes form a cellular clock that oscillates for approximately 24 h driven by a transcriptional-translational negative feedback loop. In brief, the heterodimers of clock gene products BMAL1 and CLOCK drive transcription of circadian responsive genes, including Period (Per) and Cryptochrome (Cry) via E-box elements found in their promoters. The gene products of Per and Cry, in turn, suppress the transactivation of BMAL1/CLOCK (Reviewed in Takahashi 2017). Although an individual SCN cell generates rhythmicity, the SCN generates a more robust rhythm as a nucleus by interacting with individual SCN cells (Nakamura et al. 2012). In addition, cellular clocks exist in almost all organs of the entire body (Honma 2018), and each organ can autonomously oscillate (Yoo et al. 2004). If this whole-body clock system is compared to an orchestra, the SCN plays the role of the conductor, and the clocks existing in each tissue/organ (peripheral clock) play the role of each musical instrument. Similar to a conductor, the SCN conducts and integrates each peripheral clock to adjust the time for the physiological function of the organ. Just as an incompetent conductor who misleads the harmony of music, functional decline and dysfunction of the SCN disrupt the cellular clocks in the whole body.
4 Age-Related Decline in Circadian Rhythms Caused by SCN Disorganization
Many studies have reported that the decline in behavioral/physiological functions is in line with dysfunction of the SCN due to aging (Reviewed in Nakamura et al. 2016). A fetal SCN transplant into the third ventricle in an aged rat with reduced circadian rhythms improved circadian rhythms in locomotor activity, body temperature, and drinking behavior (Li and Satinoff 1998). In addition, there are many reports on circadian rhythms in the SCN of aged rodents. For instance, multi-unit neural activity (MUA) recordings of extracellular potentials in SCN slices revealed that the aged SCN showed significantly smaller amplitudes than the young SCN in hamsters (Watanabe et al. 1995). In dispersal cell cultures, individual cells of the aged SCN showed neural activity rhythms with decreased amplitudes and fluctuating peak phases (Aujard et al. 2001). These results suggest that the amplitudes of the neural activity rhythm in the whole SCN are decreased due to the desynchronization of individual cells in aged animals. However, quantitative examination of Per2 rhythms in the SCN revealed that these rhythms were not significantly influenced by aging (Asai et al. 2001). Per1 rhythms in SCN slice cultures with a luciferase reporter also revealed that the rhythms were not significantly influenced, even though the period was slightly shortened (Yamazaki et al. 2002). These results suggest that aging does not have a large impact on the cellular clocks composed of clock genes in the SCN. Thus, there is a discrepancy in the effects of aging between the results of MUA rhythms reflecting neural outputs and clock gene expressions reflecting cellular clock generation.
5 Age-Related Dysfunction of SCN Outputs
Gene expression analyses and MUA recordings in aged mice were performed to clarify the discrepancies described in the previous section (Nakamura et al. 2011). First, experimental mice were dissected at certain intervals and examined the PER2 expressions in the SCN by immunohistochemistry. There was no difference in the rhythms of PER2 expressions between young and aged mice. Second, PER2 rhythms were recorded in SCN slice cultures using the PER2::LUCIFERASE (PER2::LUC) reporting system. The amplitude of PER2::LUC in aged SCN declined with each cycle, whereas the free-running period was indistinguishable from that of young SCN. Finally, an in vivo MUA recording system that can record neural firing subpopulations in the SCN of freely moving mice using bipolar electrodes was constructed (Nakamura et al. 2008). The method revealed that the MUA rhythms in aged wild-type SCN were essentially maintained and the counts of MUA were high during the day and low during the night, whereas the variance per recording unit (1 min) significantly increased, and the robustness of day/night activities was lost relative to the young wild-type SCN. The amplitudes of the MUA rhythms in aged mice were significantly lower than those in young mice (Fig. 4.3).

Adopted from Nakamura et al. (2011)
Effects of aging on multi-unit neural activity (MUA) rhythms in the suprachiasmatic nucleus (SCN). In vivo MUA rhythms in the SCN of mice: the MUA rhythms in the SCN were recorded by chronically inserting electrodes into the SCN of the freely moving mice. The vertical axis indicates neural activity counts per min and the horizontal axis indicates the time course. The gray square indicates the dark phase. The MUA in the SCN is high during the day and low during the night. The MUA in aged SCN shows ambiguous rhythms, even though the difference in day/night activities was maintained.
It is considered that the SCN projects to the dorsomedial nucleus of the hypothalamus (DMH) via the subparaventricular zone (SPZ) located just above the SCN, and that the timing signals for behavioral/physiological functions are transmitted from the DMH to some functional centers (Saper 2013). Thus, the pathway of SCN-SPZ reflects the SCN outputs. The amplitudes of the MUA rhythms in aged mice were found to decline, even in the SPZ (Nakamura et al. 2011). These results indicated that the decline in circadian rhythms at the individual level in aged mice was due to the dysfunction of SCN outputs. The age-related decline of behavioral rhythms was not observed in mice with artificial degeneration of dopaminergic neurons in the substantia nigra of the midbrain, mimicking an aged brain (Tanaka et al. 2012). This result also supports the relationship between age-related decline in behavioral rhythms and dysfunction of SCN outputs. If these results were likened to an orchestra, the conductor (SCN) became hazy and lazy.

Adopted from Nakamura et al. (2015)
Constant darkness uncovers effects of aging on the cellular clock. The luminescence rhythms in the suprachiasmatic nucleus (SCN) of PER2::luciferase (PER2::LUC) mice, a luciferase is linked to PER2, were recorded in SCN slice cultures using a photomultiplier tube (PMT). PER2::LUC rhythms of mice housed in a normal light/dark (LD) cycle (upper) and constant darkness (DD) for 10 days (lower) are shown. The vertical axis indicates luminescent counts and the horizontal axis indicates the time course (days). There were no differences between the young and aged SCN during the periods and amplitudes in the LD cycle. In DD conditions, however, the aged SCN showed decreased amplitudes and fluctuated peak phases compared with the young SCN.
6 Mechanisms Underlying SCN Output Dysfunction
Because several reports using rodents revealed that the cellular clock was almost normal even in aged SCN, and the discrepancy in the effects of aging between the neural rhythm and the cellular clock in the SCN was unsolved. In our own work, we hypothesized that rhythms in the SCN were also influenced by aging and examined the rhythms of clock gene expressions in the SCN in a non-external cues environment (constant darkness condition) (Nakamura et al. 2015). Although PER2::LUC mice have been exposed to the light/dark cycle in many experiments, in the study we cultured SCN slices from PER2::LUC mice housed in constant darkness conditions for 10 days. There were no differences in circadian periods and amplitudes between the young and aged SCN of mice housed in the light/dark environment. In contrast, the period was lengthened and the amplitude was decreased in the aged SCN of mice housed in constant darkness conditions. In brief, the effects of aging on the cellular clock in the SCN were remarkable under the constant darkness condition (Fig. 4.4). These results suggest that a light/dark environment masks the dysfunction of the cellular clock in aged SCN. Moreover, we performed PER2::LUC imaging of aged SCN with a high-sensitivity charge-coupled device camera under the same experimental conditions. Fifty cells were picked from each SCN, and the luminescence rhythms of each cell were recorded for 5 days. The rhythms in aged SCN were gradually desynchronized under culture conditions. Statistical analysis revealed that the variance of the peak phases significantly increased, although the amplitudes of individual cells were maintained (Fig. 4.5). These results suggest that aging disturbs the synchronization of individual SCN cells, rather than influencing individual cellular clocks.

Adopted from Nakamura et al. (2015)
Effects of aging on individual suprachiasmatic nucleus (SCN) cellular clock. (Upper panel): PER2::LUC imaging of the SCN with a high-sensitivity camera. OC: optic chiasm, 3 V: the third ventricle. (Lower panel): serial plots of each PER2 rhythm picked up from individual 50 cells in the SCN for 5 days. The rhythms in the aged SCN are gradually desynchronized in culture conditions.
7 Conclusion
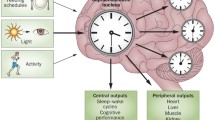
Taken together, many experiments using rodents reveal that aging: (1) disrupts SCN internal-synchronization and (2) induces SCN output dysfunction. Therefore, the SCN cannot transmit correct timing signals to each behavioral/physiological function (Fig. 4.6). Aging does not influence the scale or number of SCN neurons. However, there are more reports of a decrease in neurotransmitters in the SCN, such as gamma aminobutyric acid, with age (Hood and Amir 2017). Thus, it is considered that a functional decline in circadian rhythm due to aging is mainly due to a decline in SCN synchronization (a dysfunction of the SCN neural circuit), which may be supported by the reduction in SCN outputs caused by age-dependent alterations in specific neurotransmitter signaling (Farajnia et al. 2014). In addition, circadian rhythm disorders in neurodegenerative diseases, such as Alzheimer’s disease, are considered to be a result of SCN dysfunction (Musiek and Holtzman 2016). Therapy and medicine to improve SCN neural circuits in these diseases, including aging, are expected in the future.
Summary of aging effects on the clock system. Circadian rhythm disorders of sleep arousal and physiological functions appear with age. The main cause is dysfunction of the timing signal outputs from the SCN, which is the central circadian clock. It is considered that the decline in synchronization of individual SCN cells results in the decline of functional rhythm outputs from the whole SCN in aged animals
References
Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133–1139
Aujard F, Herzog ED, Block GD (2001) Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106:255–261
Brooks E, Canal MM (2013) Development of circadian rhythms: role of postnatal light environment. Neurosci Biobehav Rev 37:551–560
Farajnia S, Deboer T, Rohling JH, Meijer JH, Michel S (2014) Aging of the suprachiasmatic clock. Neuroscientist 20:44–55
Honma S (2018) The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J Physiol Sci 68:207–219
Hood S, Amir S (2017) The aging clock: circadian rhythms and later life. J Clin Invest 127:437–446
Li H, Satinoff E (1998) Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am J Physiol 275:R1735–R1744
Musiek ES, Holtzman DM (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354:1004–1008
Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T (2008) In vivo monitoring of circadian timing in freely moving mice. Curr Biol 18:381–385
Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD (2011) Age-related decline in circadian output. J Neurosci 31:10201–10205
Nakamura TJ, Michel S, Block GD, Colwell CS (2012) Neural circuits underlying circadian oscillations in mammals: clocks in a dish. In: Isolated central nervous system circuits. pp 183–210
Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD (2015) Age-related changes in the circadian system unmasked by constant conditions. eNeuro 2:ENEURO.0064-15.2015
Nakamura TJ, Takasu NN, Nakamura W (2016) The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci 66:367–374
Pittendrigh CS, Daan S (1974) Circadian oscillations in rodents: a systematic increase of their frequency with age. Science 186:548–550
Saper CB (2013) The central circadian timing system. Curr Opin Neurobiol 23:747–751
Sloan MA, Levenson J, Tran Q, Kerbeshian M, Block GD, Eskin A (1999) Aging affects the ocular circadian pacemaker of Aplysia californica. J Biol Rhythms 14:151–159
Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18(3):164–179
Tanaka M, Yamaguchi E, Takahashi M, Hashimura K, Shibata T, Nakamura W, Nakamura TJ (2012) Effects of age-related dopaminergic neuron loss in the substantia nigra on the circadian rhythms of locomotor activity in mice. Neurosci Res 74:210–215
Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW (1997) Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol 273:R1957–R1964
Watanabe A, Shibata S, Watanabe S (1995) Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res 695:237–239
Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A 99:10801–10806
Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 101:5339–5346
Acknowledgments
This study was supported by JSPS KAKENHI (19K06360 & 21K06363).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Miyazaki, S., Nakamura, W., Nakamura, T.J. (2023). Age-Related Decline in the Central Circadian Clock. In: Jagota, A. (eds) Sleep and Clocks in Aging and Longevity. Healthy Ageing and Longevity, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-031-22468-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-22468-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22467-6
Online ISBN: 978-3-031-22468-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)