Abstract
Atrial fibrillation is the most common cardiac arrhythmia in the world and is something that will be encountered in the primary care setting. While not a life-threatening arrhythmia, patients with atrial fibrillation are at significantly higher risk of cardiovascular complications including stroke and heart failure. In those individuals who have both atrial fibrillation and heart failure, their symptoms may be closely intertwined and difficult to attribute to only one diagnosis. Furthermore, the presence of one diagnosis will increase the likelihood of the other. This is the case in heart failure patients with both reduced and preserved ejection fraction. Treatment of patients with atrial fibrillation and heart failure should be aimed at rhythm management whenever possible. This includes, but is not limited to, atrioventricular nodal blocking agents, antiarrhythmic therapy, and catheter ablation. Patients are best served by a multidisciplinary approach involving primary care, cardiology, and other specialists as indicated. Engagement with appropriate lifestyle modification for the patient is imperative to the optimal control of both comorbid conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In clinical practice, atrial fibrillation (AF) is the most common cardiac arrhythmia. By 2030, it is anticipated that the incidence and prevalence of AF will more than double, to 2.6 million and 12.1 million respectively [1]. Atrial fibrillation is a cardiac dysrhythmia that is characterized by abnormal electrical signals originating in the atria that fire rapidly with uncoordinated atrial activation and consequently, ineffective atrial contraction as no one single signal can depolarize the atria completely. As a result of the uncoordinated atrial activation, the ensuing ventricular response is characteristically irregularly irregular.
Atrial fibrillation and heart failure (HF) often occur in conjunction. The presence of one increases the likelihood of the other and each can be caused by the existence of the other [2]. This is the case in both heart failure with preserved ejection fraction (HFpEF) as well as reduced ejection fraction (HFrEF). Data from the original Framingham Heart Study examined over 10,000 individuals with new onset AF or HF between 1980 and 2012, and among 1737 individuals with new AF, 37% had HF [3]. Patients face greater mortality risk in the presence of both AF and HF compared with neither condition, particularly among those with HFrEF [3].
Atrial fibrillation is classified as paroxysmal, persistent (including early and longstanding persistent), or permanent. Paroxysmal AF includes those episodes that terminate spontaneously within 7 days. If AF is present for more than 7 days, it is termed persistent. Early persistent AF encompasses those episodes that last for more than 7 days but less than 3 months in duration. Longstanding persistent AF is continuous AF of more than 12 months. Finally, permanent AF is AF for which a decision has been made by the patient and their provider not to pursue restoration of sinus rhythm by any means. “Chronic” AF is no longer used, as the disease state of AF itself is chronic.
The symptoms associated with atrial fibrillation can vary significantly from patient to patient. Many often report palpitations or the sensation of heart racing. Chest discomfort/pressure, dyspnea, edema, dizziness, lightheadedness, syncope, fatigue, and exertional intolerance are also common complaints. Many of these symptoms may also overlap with those related to their heart failure and it can often be difficult to determine if one or both of their comorbidities are responsible for their complaints.
2 Atrial Fibrillation-Induced Heart Failure
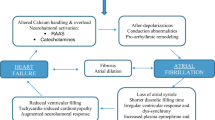
There are multiple ways in which AF can cause or worsen HF. Patients who have AF often have heart rates that are either too fast or too slow. Tachycardia and bradycardia, or other abrupt changes in the heart rate and rhythm, can potentially decrease cardiac output. Those who have persistent tachycardia related to their AF may develop tachycardia-induced cardiomyopathy [2]. Chronically elevated rates may produce significant structural changes in the heart including dilation of the left ventricle, marked reduction in left ventricular ejection fraction (LVEF), elevated filling pressures, and increased systemic vascular resistance [4, 5]. In most cases, LVEF returns to baseline once the tachycardia is controlled although in some cases LVEF may not return to baseline. In those who have preexisting cardiomyopathy, persistently elevated heart rates may cause further worsening of their cardiac function. The diagnosis of tachycardia-induced cardiomyopathy is typically made following the initiation of rate lowering therapy or restoration of normal sinus rhythm and then reevaluating the patient’s cardiac function [6]. It is also important to exclude other potential causes of cardiomyopathy such as ischemic heart disease.
As noted above, patients with AF have loss of atrial systole, also called atrial “kick.” Atrial systole promotes optimal ventricular filling. In the setting of diastolic heart failure, peak left ventricular filling occurs in late diastole and is more sensitive to the loss of effective atrial contraction. Finally, activation of neurohormonal vasoconstrictors, including angiotensin II and norepinephrine, can contribute to adverse hemodynamic changes. Some studies suggest angiotensin II is involved in the electrical and structural remodeling of the atrial myocardium [7, 8]. Structural remodeling of the atria includes fibrosis that perpetuates the development and maintenance of AF. Further, the presence of AF results in remodeling of the atrium over time, explaining the well-established concept that AF begets AF. The longer a patient has been in continuous AF, the less likely it is to terminate spontaneously and the harder it is to restore and maintain normal sinus rhythm [9].
3 Heart Failure Induced Atrial Fibrillation
The fibrillatory conduction throughout the atria is the result of various foci in the heart firing rapidly. The most common site of the rapid atrial firing that triggers AF is in the pulmonary veins (PV). When the atrium is stretched, as may be the case in those who present with volume overload and increased left atrial pressure, the likelihood of rapid firing from the PVs increases due to the stretch of sensitive ion channels [10]. Once AF has been induced, the patient will be more prone to have recurrent AF in the future, even in the absence of volume overload due to the electrical and structural remodeling of the atria as discussed above. Thus, the cyclical relationship between AF and HF begins [9].
4 Other Causes of Atrial Fibrillation
Aside from HF, there are other potential causes for AF. Non-modifiable risk factors include genetics, age, and sex. Several mutations have been identified that are responsible for familial AF, and those with a first degree relative with a history of AF have a 40% increased risk of developing it themselves [11].
A community-based cohort study in Olmstead County, Minnesota, found that the age-adjusted incidence of AF per 1000 person-years increased significantly between 1980 and 2000 from 4.4 to 5.4 in men and from 2.4 to 2.8 in women [12]. Screening for thyroid disease is also important in the patient with AF, particularly hyperthyroidism. Modifiable risk factors for AF include obesity, decreased physical activity, smoking, diabetes, sleep apnea, alcohol consumption, and hypertension [13]. Many of these are also independent risk factors for the development of HF [13].
Other cardiac abnormalities associated with AF include ischemic heart disease, mitral valve disease, hypertrophic cardiomyopathy, and dilated cardiomyopathy. Less often, you will find restrictive cardiomyopathies such as amyloidosis or constrictive pericarditis. Special attention should be paid to those with mitral valve disease. A stenotic or regurgitant mitral valve can cause left atrial enlargement and structural changes that perpetuate AF. The more severe the valvular insufficiency becomes, the more likely the patient will develop persistent and refractory AF [14].
5 Special Considerations
Patients with AF have a fivefold increase in stroke risk compared with those without AF, and special consideration must be given to these patients to prevent thromboembolic complications [15, 16]. Of those strokes that result from AF, 90% of them are due to a thrombus originating in the left atrial appendage. The use of the CHA2DS2-VASc score can assist in estimating thromboembolic risk in patients with AF to determine who would benefit from anticoagulant therapy [15, 16] (Fig. 10.1).
CHA2DS2-VASc scoring system (Reprinted from Chest, 137(2), Lip G et al., Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach, 263–272, 2010, with permission from Elsevier [16])
In adults with AF, thromboembolic risk is higher in females than in males, but female sex is associated with increased risk primarily in those with at least two non-sex risk factors [17, 18]:
-
For CHA2DS2-VASc ≥ 2 in males or ≥3 in females (two or more non-sex risk factors), the benefit of oral anticoagulation (OAC) outweighs bleeding risk [15, 16, 19].
-
For CHA2DS2-VASc 1 in males or 2 in females, the risk of thromboembolism varies depending on the non-sex risk factor. Age of 65–74 has the greatest effect on risk and use of OAC is recommended [15,16,17].
-
For CHA2DS2-VASc score of 0 in males or 1 in females, the thromboembolic risk is low and OAC is not recommended [15,16,17].
In selecting anticoagulation, novel oral anticoagulants (NOACs: dabigatran, rivaroxaban, apixaban, and edoxaban) are recommended over warfarin in NOAC-eligible patients with AF (except with moderate-to-severe mitral stenosis or a mechanical heart valve) [20].
It is also imperative to consider individual bleeding risk when making the decision to initiate anticoagulation. The HAS-BLED score was developed to estimate the 1-year risk for major bleeding (intracranial, hospitalization, hemoglobin decrease >2 g/L, and/or transfusion) [21, 22]. Patients with AF are divided into 3 risk stratifications. A score of 0 indicates low risk, 1–2 indicates moderate risk, and ≥3 indicates high risk [21, 22] (Fig. 10.2). Evaluating both the bleeding risk and the stroke risk is important to maximize appropriate anticoagulant therapy yet minimize adverse events resulting in net clinical benefit for the patient [21].
HAS-BLED bleeding risk score (Reprinted from Chest, 138(5), Pisters R et al., A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation, 1093–1100, 2010, with permission from Elsevier [21])
6 Treatment of Atrial Fibrillation and Heart Failure
For patients with AF, goals of therapy should include prevention of arterial thromboembolism (namely stroke), control of symptoms, and prevention of heart failure and/or hemodynamic compromise. Effective treatment and management of patients with atrial fibrillation and heart failure often require multidisciplinary collaboration between primary care and various subspecialties of cardiology, including but not limited to, electrophysiology, general cardiology, and advanced heart failure.
In a patient who presents with acute decompensation, initial strategy must focus on achieving euvolemia, preventing stroke/systemic embolism, and controlling the heart rate [23]. This may require admission for inpatient treatment depending on the severity of the decompensation. If the patient has HFpEF and they present with pulmonary congestion or hypotension, rate control can be attempted first with nondihydropyridine calcium channel antagonists, including diltiazem or beta blockers. In those with HFrEF, digoxin or intravenous amiodarone (in an inpatient setting) may be used. Avoid use of beta blockers and nondihydropyridine calcium channel blockers until stabilization of the decompensated HF as their negative inotropic properties may worsen the clinical condition. In most cases, slowing the ventricular response in AF will improve the clinical status of the patient [23]. Cardioversion in the setting of acutely decompensated HF is not likely to be successful and should only be considered after attempts to rate control and/or decrease pulmonary congestion have failed [24]. Careful attention must also be paid to those patients who have not been adequately anticoagulated since these patients have an increased risk of embolization following cardioversion [25]. In these situations, a transesophageal echocardiogram is used to exclude thrombus in the left atrium/left atrial appendage [26].
Once the patient has been stabilized, attention can be turned to overall treatment goals of AF, specifically, rate versus rhythm control. If rate control is selected, goals of therapy target adequate control of the heart rate while the patient remains in AF [27]. For patients who have HFpEF, calcium channel blockers may be more appropriate, while beta blockers and/or digoxin should be used in those with HFrEF [27]. Oral amiodarone can be used for rate control if other medications are not successful or other comorbidities prevent optimal titration as in hypotension [27]. Careful monitoring for long-term side effects of chronic amiodarone use is imperative and includes baseline, bi-annual, and annual monitoring for pulmonary, hepatic, thyroid, and ocular toxicity [28]. The guidelines for amiodarone surveillance include the following: (1) 12 lead electrocardiograms (ECG) at baseline and then only if symptoms and physical exam dictate, (2) Chest X-ray should be done at baseline and every 12 months (chest X-ray every 6 months is not needed if no pulmonary symptoms are present), (3) Pulmonary Function Testing with DLCO is needed at baseline and then only if abnormal findings are present on the annual chest X-ray or the patient is symptomatic, (4) Liver function testing (LFT) and Thyroid Stimulating Hormone (TSH) testing should be done at baseline, at 6 months, and every 12 months while patients are taking oral amiodarone. If the patient has any visual impairment an eye exam should be done at baseline and then again if visual changes arise [28]. In rhythm control, treatment strategies focus on maintenance of sinus rhythm often utilizing various medications, procedures (catheter and surgical based), and risk factor modification [29].
7 Heart Failure and Rhythm Control
Maintenance of sinus rhythm is preferred to AF for most patients with reduced EF. Rhythm control can be achieved with the use of antiarrhythmic drug therapy, catheter ablation, or surgical ablation [29]. Long-term maintenance of sinus rhythm is significantly influenced by how long the patient has been in AF, the size of the left atrium, and the patient’s engagement in risk factor modification. The initial approach to rhythm control includes electrical cardioversion and choosing an appropriate antiarrhythmic drug [29]. For those with HFrEF, amiodarone, sotalol, or dofetilide is recommended [29]. Other antiarrhythmic medications, including propafenone, dronedarone, and flecainide, have been associated with poor outcomes in HF patients [29].
As in those patients with HFrEF, rhythm control is also preferred to rate control for most patients with HFpEF [30]. Those strategies mimic those noted above. However, in patients that have preserved ejection fraction, consideration can be made to use propafenone or flecainide as antiarrhythmic therapy as long as the patient does not have evidence of any ischemic heart disease [29]. In addition, use of nondihydropyridine calcium channel blockers may also be used to assist with rate control [31]. Digoxin is used more cautiously in HFpEF [31].
7.1 Antiarrhythmic Medications
Dofetilide has a favorable side effect profile and efficacy, but its use is limited due to strict guidelines for administration and dose adjustments based on renal function. It is typically initiated in a hospital to monitor the QT interval at peak dosing [29]. Sotalol also has a favorable side effect profile and can be used in those with mild renal dysfunction, but should be avoided in those with EF <30% [29]. In those with HF or a structurally abnormal heart, sotalol should also be initiated in an inpatient setting [29]. Amiodarone can be started in an outpatient setting and is appropriate to use in those with renal failure. It should be noted that amiodarone takes several weeks to reach therapeutic benefit and has the potential for significant long-term side effects [29]. In patients with more persistent AF, the use of both antiarrhythmic therapy and cardioversion is recommended, as medical therapy alone is unlikely to restore sinus rhythm [29] (Table 10.1). Due to the potential for significant short- and long-term side effects, initiation of antiarrhythmic therapy should be done in close consultation with a cardiology provider.
7.2 Catheter Ablation
In patients who continue to have symptomatic recurrent atrial fibrillation, or are intolerant of antiarrhythmic therapy, referral to a cardiac electrophysiologist is recommended for evaluation for catheter ablation (CA) [31]. Patients with heart failure have a high recurrence of AF and more frequently require repeat ablation procedures [31]. The catheter ablation versus standard conventional therapy in patients with left ventricular dysfunction and atrial fibrillation (CASTLE-AF) trial randomized 363 patients to CA or medical therapy. The participants had symptomatic paroxysmal or persistent AF, NYHA Class II, III, or IV HF; an LVEF ≤35%; failure or unwillingness to take antiarrhythmic therapy; and prior placement of an implantable cardioverter defibrillator (ICD). After a median follow-up of nearly 38 months, the primary composite end point of death from any cause or hospitalization for worsening HF occurred in fewer patients in the CA group and fewer patients in the CA group died from any cause. The AF burden (time in AF) was monitored using their ICD and was significantly lower in those having had CA versus medical therapy [32].
It is important to note that catheter ablation may be used in conjunction with antiarrhythmic therapy. The goal of CA in this subset of patients should be to reduce AF burden and improve HF-related symptoms which may require multiple treatment modalities [32].
8 Heart Failure and Rate Control
If rhythm control cannot be achieved and treatment goals focus on rate management, those patients with HFrEF should receive beta blockers as first-line therapy [31]. Initial choices of beta blocker can include carvedilol, extended-release metoprolol succinate, or bisoprolol [33]. Generally, it is recommended to optimize the dose prior to considering a second agent for the treatment of atrial fibrillation. Digoxin may also be used but may be less efficacious than beta blockers. Avoid use of nondihydropyridine calcium channel blockers such as verapamil or diltiazem. The adequacy of rate control in AF should be assessed in a resting state as well as typical exertion for the patient [34]. A heart rate goal of ≤80–90 beats/minute at rest and ≤110–115 beats during moderate exercise is advised [34].
If rate control with beta blockers and digoxin cannot be achieved, then it may be reasonable to consider the use of amiodarone, either alone or in combination with other rate lowering medications. Amiodarone should be avoided for chronic rate control due to its potential for long-term side effects [34].
Finally, if the above noted strategies fail or are contraindicated for the patient, rate control can be achieved with ablation of the atrioventricular node and subsequent permanent pacemaker placement [34]. If the LVEF is <45%, strong consideration should be made for cardiac resynchronization therapy with a biventricular or His bundle pacing system as opposed to the standard right ventricular pacing system [34].
9 Putting It All Together
9.1 Case Study
Jean is a 73-year-old female who presents for a routine wellness exam. Her past medical history includes hypertension, diastolic heart failure, hypothyroid, obstructive sleep apnea, and hyperlipidemia. Her current medications include Lisinopril 10 mg daily, furosemide 20 mg daily as needed for swelling, levothyroxine 75 mcg daily, and atorvastatin 10 mg daily. She has a CPAP for treatment of her sleep apnea and reports approximately 70% compliance with use. Past surgical history includes cholecystectomy 5 years ago. She is married and lives with her husband. She worked as a church secretary for 20 years and is now retired. She remains very active in her church and with her five grandchildren. She routinely walks one to two miles per day in the early mornings, 3–4 times per week. She has never smoked and does not drink alcohol. She consumes 2–3 caffeinated beverages per day, mainly coffee. Pertinent family history includes her mother who died of a stroke and a brother who has had myocardial infarction with coronary artery bypass grafting at the age of 67.
In the office, she notes that she largely has been feeling well but in the last 2–3 months, she has felt more tired than usual and has trouble keeping up with her grandchildren. She finds that she short-winded after completing her morning walks and has attributed this to “getting older.” She has also noted that her ankles have a “sock ring” when she takes them off in the evenings. She has used her prn furosemide on a few occasions “if it gets really bad.” She has good urine output when she takes this. Her last dose of furosemide was about 1 week ago. She denies any chest discomfort, paroxysmal nocturnal dyspnea, or orthopnea. She sometimes notes her heart rate is increased during periods of emotional stress or if she’s “really pushing it” with activities. She has had no syncope or near syncope. She has been taking all of her medications as directed and without difficulties.
9.2 Objective
Height is 5′2″ and her weight is 160 lbs (BMI 29.26). Blood pressure is 128/72 mmHg and her pulse is 113. She is afebrile. Upon examination, she is noted to have an irregularly irregular rhythm and 1–2+ bilateral lower extremity edema. Other physical exam findings include: lungs = fine bibasilar rales; abdomen = no distention; neck = negative for thyromegaly or JVD; neurologic = normal coordination and gate. She had lab work done a week prior to her appointment which included a lipid panel, basic metabolic panel, TSH, free T4, and Vitamin D level. All were within acceptable range with the exception of TSH of 0.23 and free T4 of 2.5 An ECG is performed due to irregularly irregular rhythm noted and shows atrial fibrillation with rapid ventricular response. She had a previous echocardiogram 5 years prior as part of her clearance for cholecystectomy which showed left ventricular systolic function of 50–55%, grade I diastolic dysfunction, mild concentric left ventricular hypertrophy, mild left atrial enlargement, and trace to mild mitral regurgitation. She has never previously had an ischemic evaluation.
9.3 Assessment
Newly diagnosed atrial fibrillation with elevated rates and associated symptoms of fatigue, exertional dyspnea, and increased lower extremity edema for the last 2–3 months. She has evidence of fluid overload on exam concerning for exacerbation of diastolic heart failure versus declining left ventricular systolic function, but does not appear acutely decompensated. Contributing factors to atrial fibrillation include age, history of hypertension, history of diastolic heart failure, suboptimal treatment of obstructive sleep apnea, medication-induced hyperthyroid state, and possibly her family history. Her mother died due to complications from a stroke, which could have been caused by undiagnosed atrial fibrillation. She also has a first degree relative with ischemic heart disease.
9.4 Plan
Initial plans should include stroke risk reduction and improved rate control. Once achieved, attention should focus on rhythm management. Based upon her age, sex, and past medical history, her CHA2DS2-VASc score is 3 (Female; age 73, and history of hypertension) which warrants initiation of anticoagulation. She does not have any active contraindications to anticoagulation and apixaban 5mg BID will be initiated for stroke risk reduction. Dosing of apixaban based upon her age <80, body weight of >60 kg, and normal renal function. Due to concerns of worsening left ventricular dysfunction, selection of rate control medication should not include nondihydropyridine calcium channel blockers. Jean will be initiated on metoprolol succinate 50 mg once daily every evening. A common side effect of metoprolol succinate is somnolence and evening dosing may ameliorate those complaints and improve adherence.
Due to noted hyperthyroid state on pre-visit labs, levothyroxine dose will be lowered to 50 mcg twice daily with plans for repeat TSH and free T4 in 6 weeks.
She should return to the clinic in 1 week for follow-up and repeat ECG to evaluate effectiveness of rate control. If her rates are better controlled and she still exhibits lower extremity edema, consider initiation of low-dose diuretic therapy based upon proBNP result and blood pressure response to beta blocker initiation. If rates are not controlled, consider further titration of beta blocker or if patient is becoming hypotensive, initiate low dose of digoxin at 0.125mg daily and another 1 week follow-up for ECG and further lab work including BMP and digoxin level.
Once Jean is adequately rate controlled, obtain echocardiogram to evaluate left ventricular function and left atrial size. At this point, it would be appropriate to engage in co-management of the patient with cardiology.
After 4 weeks of therapeutic anticoagulation, proceed with cardioversion. If she becomes unstable during those four weeks, would proceed with transesophageal echocardiogram to rule out left atrial thrombus and proceed with cardioversion to sinus rhythm if no thrombus is found.
Considering female sex and complaints of exertional dyspnea and fatigue, as well as family history of ischemic heart disease, obtain a nuclear stress test to evaluate for ischemia once patient has returned to sinus rhythm. As she is an avid walker, exercise nuclear stress test would be recommended over pharmacologic stress test.
Her blood pressure is currently in an acceptable range but may lower with the addition of beta blocker therapy.
Due to increased risk of atrial fibrillation with untreated or suboptimal treated sleep apnea, she will follow up with her sleep medicine specialist for further titration and adjustment of her CPAP.
9.5 Clinical Pearls
Though her AF may be induced from her hyperthyroid state, the persistent nature of her atrial fibrillation likely initiated atrial remodeling and she will be significantly more prone to recurrent and more persistent AF in the future. Consideration should be made to referring her for more advanced therapies for her atrial fibrillation including initiation of antiarrhythmic drug therapy and catheter ablation.
Patients who present with newly diagnosed atrial fibrillation in the setting of heart failure exacerbation often require close monitoring and follow-up. While a thorough evaluation of risk factors is imperative to the long-term success of rhythm management, these conversations may be delayed to subsequent follow-ups once the patient is stable. It is helpful to provide the patient with education materials to review once they get home, as the quantity of material covered may be difficult to maintain. One strategy is to provide the patient with a simple one-page document outlining common modifiable risk factors for atrial fibrillation and what items require more focus (Fig. 10.3).
References
Colilla S, Crow A, Petkun W, Singer D, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–7.
Cha Y, Redfield M, Shen W, Gersh B. Atrial fibrillation and ventricular dysfunction; a vicious electromechanical cycle. Circulation. 2004;109(23):2839.
Santhanakrishnan R, Wang N, Larson M, Magnani J, McManus D, Lubitz S, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484.
Howard R, Stopps T, Moe G, Gotlieb A, Armstrong P. Recovery from heart failure: structural and functional analysis in a canine model. Can J Physiol Pharmacol. 1988;66(12):1505.
Morgan D, Tomlinson C, Qayumi A, Toleikis P, McConville B, Jamieson WR. Evaluation of ventricular contractility indexes in the dog with left ventricular dysfunction induced by rapid atrial pacing. J Am Coll Cardiol. 1989;14(2):489.
Gopinathannair R, Etheridge S, Marchlinski F, Spinale F, Lakkireddy D, Olshansky B. Arrhythmia-induced cardiomyopathies; mechanisms, recognition, and management. J Am Coll Cardiol. 2015;66(15):1714–28.
Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101(22):2612.
Goette A, Arndt M, Röcken C, Spiess A, Staack T, Geller J, et al. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;101(23):2678.
Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62.
Kalifa J, Jalife J, Zaitsev A, Bagwe S, Warren M, Moreno J, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108(6):668.
Yoneda Z, Anderson K, Quintana J, O’Neill M, Sims R, Glazer A, et al. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. 2021;6(12):1371.
Chugh S, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin E, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47.
Lau D, Nattel S, Kalman J, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–96.
Gosselink A, Crijns H, Hamer H, Hillege H, Lie K. Changes in left and right atrial size after cardioversion of atrial fibrillation: role of mitral valve disease. J Am Coll Cardiol. 1993;22(6):1999–672.
Jagadish P, Kabra R. Stroke risk in atrial fibrillation: beyond the CHA2DS2-VASc Score. Curr Cardiol Rep. 2019;21:95.
Lip G, Nieuwlaat R, Pisters R, Lane D, Crijns H. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest. 2010;137(2):263–72.
Lee C, Toft-Petersen A, Ozenne B, Phelps M, Olesen J, Ellinor P, et al. Assessing absolute stroke risk in patients with atrial fibrillation using a risk factor-based approach. Eur Heart J Cardiovasc Pharmacother. 2021;7(FI1):f3–f10.
Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137(8):832.
Olesen J, Lip G, Lindhardsen J, Lane D, Ahlehoff O, Hansen M, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a 'real world' nationwide cohort study. Thromb Haemost. 2011;106(4):739.
January C, Wann L, Calkins H, Chen L, Cigarroa J, Cleveland Jr J, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. 2019. https://doi.org/10.1016/j.hrthm.2019.01.024.
Pisters R, Lane D, Nieuwlaat R, de Vos C, Crijns H, Lip G. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest. 2010;138(5):1093–100.
Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38(9):555–61.
Kotecha D, Piccini J. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36(46):3250–7.
Crijns H, Van Den Berg M, Van Gelder I, Van Veldhuisen D. Management of atrial fibrillation in the setting of heart failure. Eur Heart J. 1997;18(Supplement C):C45–9.
Manning W, Silverman D, Katz S, Riley M, Come P, Doherty R, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23(7):1535.
Klein A, Murray R, Grimm R. Role of transesophageal echocardiography-guided cardioversion of patients with atrial fibrillation. J Am Coll Cardiol. 2001;37(3):691–704.
Van Gelder I, Rienstra M, Crijns H, Olshansky B. Rate control in atrial fibrillation. Lancet. 2016;388:818–28.
Dixon K, Thanavaro J, Thais A, Lavin M. Amiodarone surveillance in primary care. J Nurse Pract. 2003;9(1):46–54.
Piccini J, Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388:829–40.
Kelly J, DeVore A, Wu J, Hammill B, Sharma A, Cooper L, et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines – heart failure. J Am Heart Assoc. 2019;8(24):e011560. https://doi.org/10.1161/JAHA.118.011560.
Patel R, Vaduganathan M, Shah S, Butler J. Atrial fibrillation in heart failure with preserved ejection fraction: insights into mechanisms and therapeutics. Pharmacol Ther. 2017;176:32–9.
Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L. Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol. 2021;7(5):594–603.
Joseph P, Swedberg K, Leong D, DPhil S. The evolution of β-blockers in coronary artery disease and heart failure (part 1/5). J Am Coll Cardiol. 2019;74(5):672–82.
Boriani G, Biffi M, Diemberger I, Martignani C, Branzi A. Rate control in atrial fibrillation; choice of treatment and assessment of efficacy. Drugs. 2003;63:1489–509.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mudd, T.U. (2023). Atrial Fibrillation and Heart Failure. In: Hayes, K.M.S., Dellise, N.R. (eds) Managing Heart Failure in Primary Care: A Case Study Approach. Springer, Cham. https://doi.org/10.1007/978-3-031-20193-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-20193-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20192-9
Online ISBN: 978-3-031-20193-6
eBook Packages: MedicineMedicine (R0)