Abstract
Purpose of Review
Atrial fibrillation (AF) and heart failure (HF) are commonly encountered clinical disorders that often co-exist, accelerating disease progression and adverse outcomes. It is known that restoration of sinus rhythm positively impacts this population; however, the complex comorbidity profile associated with HF introduces intricacies not encountered in other patient populations. The current review focuses on the safety and efficacy of an interventional-based management for atrial tachyarrhythmias in HF.
Recent Findings
While pharmacotherapy has been the standard treatment of cardiac dysrhythmias in the HF population, recent evidence suggests catheter ablation is more effective and causes less harm than antiarrhythmic drugs (AADs) in the HF population.
Summary
For the maintenance of sinus rhythm, catheter ablation results in improved freedom from recurrent arrhythmia, with secondary benefit on mortality and hospitalization in those with HF and reduced ejection fraction. For those with permanent AF, cardiac resynchronization therapy and atrioventricular junction ablation result in improved quality of life, physical functioning, and cardiac function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are colliding global cardiovascular epidemics. AF and HF are individually responsible for impaired quality of life, risk of hospitalization, and mortality. However, when AF and HF co-exist, disease progression accelerates and the adverse outcomes are magnified, leading to incrementally higher healthcare expenditure [1,2,3,4]. Given the complexities of this unique population, optimal patient management requires a comprehensive, patient-centered approach including optimization of co-morbidities, goal-directed HF therapies, stroke prevention, and heart rhythm management. While restoration of sinus rhythm positively impacts this population, the comorbidity profile introduces complexities not experienced in other patient populations. Catheter ablation is more effective and causes less harm than antiarrhythmic drugs (AADs) in the HF population in multiple randomized trials and meta-analyses [5,6,7,8,9,10,11,12,13,13,14, 15••]. The current review focuses on the safety and efficacy of an interventional-based management for atrial tachyarrhythmias in HF.
Atrial Fibrillation and Heart Failure—Epidemiology
AF is the most common sustained cardiac arrhythmia, affecting approximately 3% of the general population [16]. HF similarly affects approximately 2% of the overall population, with approximately half of those affected by HF with reduced ejection fraction (HFrEF) and half having HF with preserved ejection fraction (HFpEF) [17]. AF and HF coexist through varying degrees of shared risk factors (e.g., hypertension, diabetes) and interacting causal mechanisms (e.g., tachycardia-induced cardiomyopathy). For both conditions, the incidence is greater among men and increases significantly with age [16,17,18]. The incidence of HF in patients with primary AF is estimated at 3.3% per year [19]. Conversely, the incidence of AF in patients with primary HF is estimated at 5.8% per year [19]. While the incidence of AF and HF have individually been relatively stable over time, the overall prevalence of each disease has been increasing due to population-aging (AF) or the improved survival associated with goal-directed medical therapy (HF).
In the large clinical trials in patients with HF, the prevalence of AF increases with HF severity, from < 5% in New York Heart Association (NYHA) functional class I–II, 10–30% in NYHA functional class II–III, and 30–50% in patients with NYHA functional class III–IV symptoms. Similarly, large, randomized AF trials have observed a prevalence of HF ranging from 22 to 63%.
Atrial Fibrillation and Heart Failure—Pathophysiology
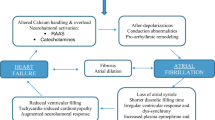
The heterogeneous conditions of AF and HF often represent the zenith of several adverse cardiovascular conditions and potentially represent “chamber-specific expressions” of a more diffuse myopathic process. In other words, the multifactorial cellular abnormalities may manifest as predominantly electrical abnormalities (e.g., fibrillation) in the atria and structural/functional abnormalities (e.g., systolic/diastolic dysfunction) in the ventricles. In addition, there are several mechanisms through which these disorders may perpetuate each other in reciprocal fashion (Fig. 1). AF impairs cardiac function through a combination of uncontrolled ventricular rate (predisposing to tachycardia-mediated cardiomyopathy), suboptimal ventricular filling which is exaggerated by limited ventricular compliance, and irregularity of contraction. Vis-à-vis, HF induces AF predominantly through left atrial dilation and structural remodeling (atrial fibrosis induced by elevated ventricular filling pressures, functional valvular regurgitation, and renin–angiotensin–aldosterone system-induced volume retention), with a lesser contribution from electrical remodeling (anisotropy, conduction delay, and attenuation of action-potential duration) and alteration in cellular calcium handling. In aggregate, these changes lead to a combination of arrhythmogenic triggered activity, AF perpetuation (e.g., re-entry), and contractile dysfunction.
Pathophysiological interactions between AF and HF. Atrial fibrillation (AF) and heart failure (HF) commonly co-exist, with interacting causal mechanisms driving the occurrence of each condition. Shared risk factors include advanced age, hypertension, diabetes, obesity, sleep apnea, and chronic kidney disease. Legend: atrial fibrillation (AF); atrioventircular (AV); heart failure (HF); pulmonary vein (PV)
Rate Versus Rhythm Control in AF and HF
Given the poor outcomes in patients with concomitant HF and AF, it seemed intuitive that restoration of sinus rhythm would improve outcomes. However, eight large clinical trials including 7499 patients have not proven pharmacologic rhythm control to be superior to rate control. Specifically, these trials demonstrated no significant difference in all-cause mortality (RR 0.95, CI 0.86–1.05), cardiovascular mortality (RR 0.99, CI 0.87–1.13), or arrhythmic/sudden death (RR 1.12, CI 0.91–1.38) when patients were randomized to pharmacologic rate control versus pharmacologic rhythm control [20]. However, only one of these trials, the Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure (AF-CHF) trial, specifically examined the HF population. This trial enrolled a population of 1376 patients with nonvalvular AF (67% persistent, 33% paroxysmal) and HFrEF (left ventricular ejection fraction (LVEF) ≤ 35% and NYHA class II–IV symptoms in the past 6 months) [21]. Medical therapy for HF and AF was consistent with the era, with > 90% use of ACE-inhibitors, beta-blockers, and warfarin. The predominant AAD was amiodarone. After a mean follow-up of 37 months, rhythm control did not reduce the primary endpoint of cardiovascular mortality (HR 1.06, 95% CI 0.86–1.30; P = 0.59) or the secondary outcomes of worsening heart failure (HR 0.87, 95% CI 0.72–1.06, P = 0.17), or all-cause mortality (HR 0.97, 95% CI 0.80–1.17, P = 0.73).
While pharmacologic rhythm control had little benefit in these trials, several key features merit consideration. First, the trial populations were minimally symptomatic, limiting generalizability to a symptomatic HF population. Second, these trials evaluated AADs as the means to maintain sinus rhythm. These agents are relatively ineffective at maintaining sinus rhythm, particularly for more advanced forms of AF such as patients with concomitant HF. As such, the lower cumulative person-time in sinus rhythm would serve to diminish the apparent treatment effect associated with sinus rhythm maintenance. The net benefit is further reduced by the non-cardiac and cardiac toxicities of AADs, including proarrhythmia, conduction block, and negative inotropy, all of which pose a relatively greater risk in patients with HF. Conceptually, this was demonstrated in the time-dependent “on-treatment” analysis of Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, in which sinus rhythm was associated with a lower risk of death (hazard ratio 0.53; 95% CI 0.42–0.70; p < 0.001) but the use of pharmacologic rhythm control was associated with increased risk of death (hazard ratio 1.50; 95% CI 1.18–1.89; p < 0.001) [22]. Maintenance of sinus rhythm was also associated with significantly lower mortality in Danish Investigators of Arrhythmia and Mortality on Dofetilide (DIAMOND; RR = 0.44 for all-cause mortality; 95% CI 0.30–0.64; P < 0.0001), Veterans Affairs Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT; 62.5% vs. 34.2% 1 year survival in SR vs. AF; P = 0.04), and the Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation (ATHENA; HR for cardiovascular mortality 0.71; 95% CI 0.51–0.98; P = 0.03) trials [23,24,25]. However, it is possible that these associations may represent unadjusted confounding, as maintenance of sinus rhythm may be a marker of less severe underlying pathology.
Catheter Ablation to Maintain Sinus Rhythm
The options for AAD therapy in patients with HF are limited due to the increased mortality associated with the use of class Ic agents and dronedarone in this population [26, 27]. Moreover, multiple randomized controlled trials in the non-HF population have demonstrated catheter ablation to be superior to AAD therapy as both an initial (“first-line”) and “second-line” therapy, suggesting that ablation may be the preferred therapeutic option to maintain sinus rhythm [28, 29•]. A general approach to AF management in patients with HF is presented in Fig. 2.
Treatment options for patients with concomitant AF and HF. The approach to managing atrial fibrillation (AF) and heart failure (HF) includes management of precipitants and co-morbid risk conditions, stroke prevention therapies, and optimization of goal-directed heart failure therapies. The rhythm management in an HF patient depends on the prevention (acute vs. chronic), the specific arrhythmia (atrial flutter vs. fibrillation), and the clinical categorization (paroxysmal vs. persistent vs. permanent). In general, sinus rhythm maintenance is preferred in newly diagnosed AF, and symptomatic patients with HF. When sinus rhythm is desired catheter ablation to achieve pulmonary vein isolation (PVI) is the preferred therapeutic strategy, being strongly preferred in those with tachycardia-induced cardiomyopathy and in those where antiarrhythmic drugs have failed. Atrioventricular junction (AVJ) ablation is a reasonable strategy for those with a pre-existing biventricular pacemaker-defibrillator or in those with permanent AF when heart rate (HR) cannot be controlled. Legend: AF, atrial fibrillation; AFL, atrial flutter; CV, cardioversion; DCCV, electrical cardioversion; LVEF, left ventricular ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ND-CCB, non-dihydropyridine calcium channel blocker; OAC, oral anticoagulation
Catheter Ablation in Patients with HFrEF
In patients with HF and AF, several randomized trials have demonstrated clinically important improvements in QoL, exercise tolerance, and LV function with catheter ablation compared to pharmacological rate-control or rhythm-control strategies (Table 1) [5,6,7,8,9,10,11,12,13,14, 15••]. Collectively these studies demonstrate a single procedure success (e.g., elimination of any atrial tachyarrhythmia episode lasting more than 30 s) of 40–69% [5,6,7,8,9,10,11,12,13,14, 15••]. Beyond arrhythmia recurrence, catheter ablation of AF in patients with HFrEF was associated with improvement in LVEF (mean improvement 4.5–18%), exercise performance (mean improvement in VO2 max 3 ml/kg/min), 6-min walk distance (mean improvement of 20–70 m), quality of life (33% average improvement in Minnesota Living with Heart Failure [MLHF] Questionnaire score), and biomarkers (e.g., B-type natriuretic peptide) [5,6,7,8,9,10,11,12,13,14, 15••]. For each outcome, patients who maintained sinus rhythm experienced improved outcomes relative to those with arrhythmia recurrence.
However, a truer measure of the utility of catheter ablation in HFrEF patients with AF is objective mortality and hospitalization outcomes. Recently, these outcomes have been evaluated in three large randomized controlled trials [9, 13, 31]. The Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device (AATAC) trial randomized 203 patients with New York Heart Association functional class II to III heart failure and an LVEF < 40% to catheter ablation (n = 102) or amiodarone rhythm control (n = 101) [9]. After a minimum follow-up of 24 months, patients in the ablation group had a significantly greater freedom from recurrent AF (70% vs. 34%; P < 0.001). In addition, the secondary endpoints of unplanned hospitalization and all-cause mortality were both significantly reduced (8% vs. 18%; P = 0.037, and 31% vs. 57%; P < 0.001, respectively), corresponding to a NNT of 3.8 for unplanned hospitalization and 10 for all-cause mortality. The Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) trial randomized patients with symptomatic paroxysmal or persistent AF, New York Heart Association class II–IV heart failure, and an LVEF ≤ 35% to catheter ablation (179 patients) or medical therapy (184 patients) [13]. All patients had a cardiac implantable device (ICD or CRT-ICD). After a median follow-up of 37.8 months, patients in the ablation group were significantly less likely to meet the primary composite end point of all-cause mortality or heart failure admission (16.1% absolute reduction; HR 0.62; 95% CI 0.43–0.87; p = 0.006). Similar to AATAC, all-cause mortality (HR 0.53; 95% CI 0.32–0.86) and HF hospitalization (HR 0.56; 95% CI 0.37–0.83) were significantly reduced. In contrast, the Randomized Ablation-based atrial Fibrillation rhythm control versus rate control Trial in patients with heart failure and high burden Atrial Fibrillation (RAFT-AF) trial enrolled patients with AF and HFpEF as well as HFrEF [31]. A total of 411 patients were randomized to catheter ablation (n = 214) or rate-control (n = 197). At a median follow-up duration of 37.4 months, there was no significant difference in the primary composite outcome of death and HF events (23.4% for rhythm-control vs. 32.5% for rate-control respectively; HR 0.71, 95% CI 0.49–1.03, p = 0.066). In contrast to AATAC and CASTLE-AF, there was no difference in all-cause mortality (13.6% vs. 17.3%; p = 0.35), or HF events (17.8% vs. 24.4%; p = 0.12). However, in aggregate, the AATAC, CASTLE-AF, and RAFT-AF studies demonstrate that catheter ablation is associated with a consistent and significant reduction in mortality (RR 0.61; 95% CI 0.45, 0.82; P < 0.001) and HF events (RR 0.61; 95% CI 0.50, 0.75; P < 0.001).
Importantly, the use of invasive arrhythmia monitoring has demonstrated that the clinical benefit associated with catheter ablation is not dependent on the “complete” elimination of AF. For example, in the CASTLE-AF Study an AF burden (percentage time in AF) of < 5% was associated with a more than 3 times significantly greater freedom from all-cause mortality or HF hospitalization at 1 year, when compared to greater AF burdens. It is hypothesized that the reduction in AF burden facilitates improvement in cardiac hemodynamics (e.g., cardiac output), autonomic nervous system performance, and reverse remodeling. It is possible that this observation may also explain the lack of benefit observed with pharmacological rate control, as this therapeutic approach does not impact AF burden.
Catheter Ablation in Patients with HFpEF
There is relatively limited evidence supporting catheter ablation in the HFpEF population. The Study Using Invasive Haemodynamic Measurements Following Catheter Ablation For AF And Early HFpEF (STALL AF-HFpEF) evaluated symptomatic AF patients with HFpEF (defined LVEF ≥ 50% with a resting pulmonary capillary wedge pressure (PCWP) of ≥ 15 mmHg or exercise PCWP of ≥ 25 mmHg) [32]. At 12 ± 6 months post-ablation 9 of the 20 HFpEF patients undergoing AF ablation no longer fulfilled the criteria for HFpEF. Patients remaining arrhythmia free showed significant improvements in peak exercise PCWP, serum BNP, and quality of life scores (MLHF and Atrial Fibrillation Effect on QualiTy-of-Life questionnaire [AFEQT]). Conversely, those with recurrence did not demonstrate improvements in peak exercise PCWP or MLHF score. Similar findings were observed in a retrospective analysis from Rattka et al., whereby catheter ablation was associated with symptomatic improvement (average NYHA class improved from 2.6 ± 0.7 to 1.7 ± 0.9; P < 0.001), reduction in mean NT-proBNP (1840 ± 2115 pg/mL to 824 ± 1095 pg/mL), and regression of echocardiographic diastolic dysfunction [33].
The multinational, randomized Catheter Ablation vs. Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, which was the largest randomized trial of catheter ablation versus medical therapy, observed no significant difference in the primary composite endpoint of death, stroke, bleeding, and cardiac arrest (hazard ratio 0.86; 95% CI, 0.65–1.15; P = 0.30), nor the individual endpoints of mortality (hazard ratio 0.85; 95% CI 0.60–1.21; P = 0.38) or stroke (hazard ratio 0.42; 95% CI 0.11–1.62; P = 0.19) [34]. However, pre-specified subgroup analysis of 778 patients with clinical heart failure (defined as ≥ NYHA Class II symptoms) observed a significant reduction in the primary composite endpoint (HR 0.64; 95% CI, 0.41–0.99) and all-cause mortality (HR 0.50, 95% CI 0.33–0.96) with ablation, as compared to medical therapy [14]. In addition, patients undergoing catheter ablation had a lower rate of arrhythmia recurrence, lower non-invasive AF burden, and improved QoL and symptom score. While this pre-specified substudy did not specifically examine the HFpEF population, it is worth noting that > 90% of the included patients had a LVEF of ≥ 40% (~ 80% having an LVEF ≥ 50%). Contrariwise, in the RAFT-AF study, which used a more stringent definition of HFpEF, the primary outcome was reduced less in those with HFpEF (HR 0.88, 95% CI 0.48–1.61; p = 0.67), compared to HFrEF (HR 0.63, 95% CI 0.39–1.02; p = 0.059).
Catheter Ablation in Patients with HF—Summary
In aggregate, the evidence suggests that patients with AF and HFrEF have a better clinical outcome with catheter ablation rather than with pharmacological rhythm or rate control, with a consistent and significant reduction in arrhythmia recurrence, mortality, and hospitalizations. Conversely, while it is reasonable to conclude that catheter ablation in the HFpEF population is effective in reducing arrhythmia recurrence, the effect of catheter ablation on mortality outcomes or HF hospital admissions is less clear for those with HFpEF [5].
Catheter Ablation for Rate Control
Pharmacotherapy for long-term control of ventricular rate revolves around agents with negative dromotropic properties such as beta-blockers and non-dihydropyridine calcium channel blockers (ND-CCBs, verapamil, and diltiazem). Beta-blockers and ND-CCBs are first-line options in patients with LVEF > 40%, but contraindicated in those with HFrEF due to their negative inotropic properties [35]. In the population with AF and HFrEF, maximally-tolerated doses of evidence-based beta-blockers (extended-release metoprolol succinate, bisoprolol, carvedilol) remain first-line therapy for rate-control, although the benefits of beta-blockade, beyond ventricular rate control, are uncertain [36, 37]. Moreover, almost all of the evidence guiding clinical decision-making for ventricular rate-control targets has been acquired in patients with preserved LVEF. In unselected patients with AF and HFrEF, it is reasonable to target a resting heart rate of < 100 bpm, although this target should be individualized to symptoms and hemodynamics [38].
For AF patients with a cardiac resynchronization (CRT) device, the goal is to maximize biventricular pacing rather than to target a specific heart rate [35]. In the AF population, the irregularity of the ventricular response interferes with the ability to deliver optimal resynchronization, even at “controlled” heart rates, with a consequent increase in mortality [39]. Rhythm control (as above) or atrioventricular junction (AVJ) ablation should be considered in those unable to achieve a high percentage of biventricular pacing despite medical optimization. In addition to optimizing CRT delivery, AVJ ablation ensures reliable ventricular rate control and regularization of the RR intervals. The observational CERTIFY study examined patients with permanent AF and HF (LVEF ≤ 35%, NYHA III or ambulatory IV, QRS ≥ 120 ms) undergoing CRT plus AVJ ablation (n = 443) or CRT plus pharmacological rate-control (n = 895), and compared them to patients undergoing CRT in sinus rhythm [40]. Over a median follow-up of 37 months, the primary endpoint of all-cause mortality was similar for the AVJ ablation group and patients in sinus rhythm (6.8 vs 6.1 per 100-person-years, P = NS), but higher with pharmacological rate-control (11.3, p < 0.0001). A meta-analysis of 6 observational studies including 768 patients with AF, left ventricular dysfunction (LVEF ≤ 35%), symptomatic HF, and a CRT device reported that AVJ ablation was associated with lower all-cause (RR 0.42; 95% CI, 0.26–0.68; P < 0.001) and cardiovascular mortality (RR 0.44; 95% CI, 0.24–0.81; P = 0.008), and a greater improvement in NYHA class (− 0.34; 95% CI, − 0.56 to − 0.13; P = 0.002), when compared to medical rate-control [41]. Similarly, a pooled analysis of two randomized trials and one prospective observational study observed reductions in all-cause hospitalizations (incidence rate ratio 0.57 (0.41–0.79), P < 0.001) and ICD therapies (incidence rate ratio of 0.18 (0.10–0.32), P < 0.001 for appropriate and inappropriate defibrillator shocks) with AVJ ablation vs. medical rate control [42]. However, the predominantly observational nature of these studies renders them at risk of significantly confounding (e.g., significant differences were noted in patients selected for AVJ ablation vs. alternate therapy).
In those without a traditional indication for CRT (i.e., a narrow QRS), AVJ ablation and pacemaker implantation significantly improved symptoms and QOL compared to medical therapy, despite no significant changes in exercise capacity or functional status (e.g., treadmill exercise or VO2 max) [43, 44]. The APAF-CRT trial compared AVJ ablation plus CRT to pharmacological rate control (target resting heart rate < 110 bpm) in 102 patients with symptomatic permanent AF (> 6 months), narrow QRS (≤ 110 ms), and at least one hospitalization for HF in the previous year [45]. The primary composite outcome of death due to HF, hospitalization due to HF, or worsening HF occurred in 10 patients (20%) of the AVJ ablation group vs. 20 patients (38%) in the pharmacotherapy group (HR 0.38, 95% CI 0.18–0.81, P = 0.013). The individual endpoint of all-cause mortality occurred in 7 patients (11%) in the AVJ ablation group vs. 20 patients (29%) in the pharmacotherapy group [HR 0.26, 95% CI 0.10–0.65; P = 0.004] [46]. In addition, the AVJ ablation group showed a 36% decrease in symptoms and physical limitations of AF at 1-year follow-up (P = 0.004). Patients with a baseline EF < 35% and those with more symptomatic AF were more likely to benefit from AVJ ablation combined with CRT.
Taken together, these studies suggest that it is reasonable to focus on rhythm control in those patients capable of maintaining sinus rhythm, as outlined in the PABA-CHF trial, which demonstrated AF ablation to be superior to AVJ ablation at improving quality of life, physical functioning, and LVEF [8]. However, in those with treatment-resistant or permanent AF, AVJ ablation combined with cardiac resynchronization therapy is a reasonable treatment option [47].
Atrial Flutter
While both AF and atrial flutter (AFL) may co-exist in the same patient, more than 70% of patients with atrial flutter do not experience AF, and less than 10% with AF are also diagnosed with atrial flutter. Atrial flutter is pathophysiologically distinct from AF, usually involving a single macroreentrant circuit rotating around a large central obstacle (classified as “typical” if counter-clockwise cavo-tricuspid isthmus-dependent, or “atypical” when non-counterclockwise cavo-tricuspid isthmus-dependent or arising from scar related to prior heart surgery or catheter ablation). Furthermore, in contrast to AF, the incidence and prevalence of atrial flutter in the HF population is less well described.
A recent systematic review examined the relationship between atrial flutter and HF [48]. The authors noted that none of the 65 studies study included in the review described the incidence or prevalence of atrial flutter in unselected patients with HF. Conversely, in patients with atrial flutter, the prevalence of HF ranges from 6 to 56%, with the prevalence being higher in patients with established (14 to 56%) compared with newly diagnosed atrial flutter (6 to 28%).
For atrial flutter, the preferred therapeutic intervention is catheter ablation given the relatively high success rate and relatively low rate of peri-procedural complications. In unselected patients, the acute and long-term success rate is above 90%, which is significantly more effective than pharmacological rhythm control. In those with HF, the immediate procedural success has been observed to be 87 to 100%, with longer-term rates of atrial flutter recurrence between 5 and 30% [48]. In addition, catheter ablation of atrial flutter reduces the risk of developing AF and improves symptom status and QOL, with observational evidence suggesting that catheter ablation of atrial flutter is associated with significant mortality benefit [48]. Specifically, an analysis from the Loire Valley AF project demonstrated that patients with atrial flutter undergoing cavotricuspid isthmus ablation (n = 875) had a lower risk of all-cause mortality (HR 0.55, 95% CI 0.36 to 0.84, p = 0.006) and stroke/systemic embolism (HR 0.53, 95% CI 0.30 to 0.92, p = 0.02) [49•]. Similar results were observed in a matched cohort of 3784 patients with atrial flutter from Taiwan’s National Health Insurance database and Death Registry [50]. After a mean follow-up of 8 years, patients undergoing catheter ablation for atrial flutter had a significantly lower adjusted risk of all-cause mortality (HR 0.68, P < 0.001), cardiovascular death (HR 0.78, P = 0.001), HF hospitalization (HR 0.84, P = 0.01), and stroke (HR 0.80, P = 0.01).
Conclusion
AF and HF are common chronic conditions. The evolution of our understating of how these entities influence and interact has influenced proposed therapies. In this patient population, catheter ablation has become an increasingly relevant therapy, improving LVEF, quality of life, and exercise tolerance, while reducing hospitalizations, arrhythmia recurrence, and mortality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mogensen UM, Jhund PS, Abraham WT, Desai AS, Dickstein K, Packer M, et al. Type of atrial fibrillation and outcomes in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017;70(20):2490–500.
Dries D, Exner D, Gersh B, Domanski M, Waclawiw M, Stevenson L. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. J Am Coll Cardiol. 1998;32(3):695–703.
Heist EK, Ruskin JN. Atrial fibrillation and congestive heart failure: risk factors, mechanisms, and treatment. Prog Cardiovasc Dis. 2006;48(4):256–69.
Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482.
Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, et al. Meta-analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;142:66–73.
Elgendy AY, Mahmoud AN, Khan MS, Sheikh MR, Mojadidi MK, Omer M, et al. Meta-analysis comparing catheter-guided ablation versus conventional medical therapy for patients with atrial fibrillation and heart failure with reduced ejection fraction. Am J Cardiol. 2018;122(5):806–13.
MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97(9):740–7.
Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–85.
Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133(17):1637–44.
Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7(1):31–8.
Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61(18):1894–903.
Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70(16):1949–61.
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–27.
Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143(14):1377–90.
Malhi N, Hawkins NM, Andrade JG, Krahn AD, Deyell MW. Catheter ablation of atrial fibrillation in heart failure with reduced ejection fraction. J Cardiovasc Electrophysiol. 2018;29(7):1049–58. Meta-analysis demonstrating the value of catheter ablation in patients with heart failure, in terms of improving LVEF, mortality, and hospitalization.
Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–68.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202.
Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20(6):447–55.
Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6, Supplement 1):2–8.
Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis. 2012;105(4):226–38.
Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–77.
Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–13.
Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360(7):668–78.
Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104(3):292–6.
Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98(23):2574–9.
Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):2678–87.
Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–8.
Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12(9):e007414.
Andrade JG, Wazni OM, Kuniss M, Hawkins NM, Deyell MW, Chierchia GB, et al. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(9):914–30. Meta-analysis demonstrating the benefit of a first-line catheter ablation, in terms of improving arrhythmia outcomes, patient-reported outcomes, and healthcare resource utilization.
Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schluter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731.
Parkash R, Wells G, Rouleau J, Talajic M, Essebag V, Skanes A, et al. A randomized ablation-based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation: The RAFT-AF trial rationale and design. Am Heart J. 2021;234:90–100.
Sugumar H, Nanayakkara S, Vizi D, Wright L, Chieng D, Leet A, et al. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF-HFpEF. Eur J Heart Fail. 2021;23(5):785–96.
Rattka M, Pott A, Kuhberger A, Weinmann K, Scharnbeck D, Stephan T, et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace. 2020;22(9):1328–36.
Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261–74.
Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847–948.
Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, et al. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017;69(24):2885–96.
Cadrin-Tourigny J, Shohoudi A, Roy D, Talajic M, Tadros R, Mondesert B, et al. Decreased mortality with beta-blockers in patients with heart failure and coexisting atrial fibrillation: an AF-CHF substudy. JACC Heart Fail. 2017;5(2):99–106.
Andrade JG, Roy D, Wyse DG, Tardif JC, Talajic M, Leduc H, et al. Heart rate and adverse outcomes in patients with atrial fibrillation: a combined AFFIRM and AF-CHF substudy. Heart Rhythm. 2016;13(1):54–61.
Brenyo A, Link MS, Barsheshet A, Moss AJ, Zareba W, Wang PJ, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;58(16):1682–9.
Gasparini M, Leclercq C, Lunati M, Landolina M, Auricchio A, Santini M, et al. Cardiac resynchronization therapy in patients with atrial fibrillation: the CERTIFY study (Cardiac Resynchronization Therapy in Atrial Fibrillation Patients Multinational Registry). JACC Heart Fail. 2013;1(6):500–7.
Ganesan AN, Brooks AG, Roberts-Thomson KC, Lau DH, Kalman JM, Sanders P. Role of AV nodal ablation in cardiac resynchronization in patients with coexistent atrial fibrillation and heart failure a systematic review. J Am Coll Cardiol. 2012;59(8):719–26.
Gasparini M, Kloppe A, Lunati M, Anselme F, Landolina M, Martinez-Ferrer JB, et al. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations. Eur J Heart Fail. 2018;20(10):1472–81.
Kay GN, Ellenbogen KA, Giudici M, Redfield MM, Jenkins LS, Mianulli M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol. 1998;2(2):121–35.
Chatterjee NA, Upadhyay GA, Ellenbogen KA, McAlister FA, Choudhry NK, Singh JP. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012;5(1):68–76.
Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39(45):3999–4008.
Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021;42(46):4731–9.
Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–85.
Diamant MJ, Andrade JG, Virani SA, Jhund PS, Petrie MC, Hawkins NM. Heart failure and atrial flutter: a systematic review of current knowledge and practices. ESC Heart Fail. 2021;8(6):4484–96.
Clementy N, Desprets L, Pierre B, Lallemand B, Simeon E, Brunet-Bernard A, et al. Outcomes after ablation for typical atrial flutter (from the Loire Valley Atrial Fibrillation Project). Am J Cardiol. 2014;114(9):1361–7. Large study demonstrating the benefit of atrial flutter ablation in improving survival, and preventing stroke/hospitalization.
Yugo D, Chen YY, Lin YJ, Chien KL, Chang SL, Lo LW, et al. Long-term mortality and cardiovascular outcomes in patients with atrial flutter after catheter ablation. Europace. 2021;euab308. https://doi.org/10.1093/europace/euab308.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
JG has nothing to declare. NH received speakers’ fees from Novartis. MD reports grants and personal fees from Biosense-Webster and personal fees from Abbott, Boston Scientific, and Medtronic. JA received grants and personal fees from Medtronic, Bayer, BMS-Pfizer, and Servier and personal fees from Biosense-Webster.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Devices
Rights and permissions
About this article
Cite this article
Gencher, J.A., Hawkins, N.M., Deyell, M.W. et al. Management of Atrial Tachyarrhythmias in Heart Failure—an Interventionalist’s Point of View. Curr Heart Fail Rep 19, 126–135 (2022). https://doi.org/10.1007/s11897-022-00543-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00543-4