Abstract
Infertility is a universal health problem affecting 15% of couples, out of which 20–30% cases are due to male infertility. The leading causes of male infertility include hormonal defects, physical reasons, sexual problems, hazardous environment, stressful lifestyle, genetic factors, epigenetic factors, and oxidative stress. Various physiological functions involve reactive oxygen species (ROS) and nitrogen species at appropriate levels for proper smooth functioning. ROS control critical reproductive processes such as capacitation, acrosomal reaction, hyperactivation, egg penetration, and sperm head decondensation. The excessive free radicals or imbalance between ROS and endogenous antioxidant enzymes damages sperm membrane by inducing lipid peroxidation causing mitochondrial dysfunction and DNA damage that eventually lead to male infertility. Numerous synthetic products are available in the market to treat infertility problems, largely ending in side effects and repressing symptoms. Ayurveda contains a particular group of Rasayana herbs, called vajikarana, that deals with nourishment and stimulation of sexual tissues, improves male reproductive vitality, and deals with oxidative stress via antioxidant mechanism. The present study aims to describe oxidative stress and the role of herbal drugs in treating male infertility.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Infertility (or subfertility) is defined as the failure of couples to establish a clinical pregnancy after 1 year of consistent unprotected sexual intercourse (Zegers-Hochschild et al. 2017). According to the WHO, about 50–80 million people suffer from infertility worldwide. Globally, infertility affects approximately 13–15% of all couples, and out of all cases of infertility, the male is responsible in 20–30% of cases. Male infertility is widespread not only in developing countries but also in developed ones. The exact figure is unpredictable because majority of cases are not registered often due to the scarcity and high cost of medical resources and treatment, respectively, sociocultural phobia, humiliations, etc. (Leslie et al. 2021). The leading causes of male infertility include hormonal defects, physical reasons like blockage of the ejaculatory pathway, sexual problems like erectile dysfunction or impotence, hazardous environment and stressful lifestyle, genetic factors like chromosomal abnormalities, single-gene mutations, and epigenetic factors (Babakhanzadeh et al. 2020; Iammarrone et al. 2003). Such factors can be broadly categorized (Table 9.1) in primary gonadal and hypothalamic-pituitary disorders which can be both, congenital and acquired, and disorders of sperm transport (post-testicular).
9.2 Pathophysiological Factors of Male Infertility
9.2.1 Hormonal Defects
The right concentration of male hormones is required for the proper functioning of the testes and sexual development. They are produced by hypothalamic-pituitary-gonadal axis. Decrease or lack of release of gonadotropic-releasing hormone (GnRH) by the brain produces less testosterone and reduced sperm production, resulting in disorders like Kallmann syndrome (Monaco et al. 2015). Furthermore, the insufficient release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland causes failure to stimulate the testes, decrease in testosterone and sperm production, and defects in spermatogenesis (Wdowiak et al. 2014). Elevated prolactin concentrations also reduce sperm production and impotence (Marrag et al. 2015). The treatment of such disorders includes the use of long-term hormonal therapy involving the use of sex steroids (testosterone injections) or gonadotropin-releasing hormone (Schagen et al. 2016). But such treatment regimens lead to other complications such as diabetes mellitus, heart diseases, etc.
9.2.2 Physical Reasons and Sexual Problems
Various physical or anatomical changes, viz., blockage of the ejaculatory pathway, enlargement of sperm vessels (varicocele), testicular torsions, impaired sperm circulation, genital tract infection, obstruction in semen flow by damaged urinary bladder sphincter (retrograde and antegrade ejaculation), and its movement along the path, also lead to infertility. Erectile dysfunction, early ejaculation, and inability to ejaculate are problems related to intercourse that led to the inability of couples to establish a clinical pregnancy or indirectly cause infertility (Babakhanzadeh et al. 2020; Sun et al. 2018).
9.2.3 Lifestyle and Environment
Changing lifestyle or more of a sedentary lifestyle has been considered as one of the major reasons for infertility. Excessive consumption of alcohol, high-fat diet, smoking, prolonged sitting, poor nutrition, repeated use of drugs, high stress (leading to anxiety, depression, and other psychological disturbances), pollution in the air, etc. causes degradation of sperm, reduce sperm count and motility, and increase the chances of impotency in men. Exposure to harmful radiation, hazardous substances, and high temperature can also lead to infertility (Katib 2015; Mustafa et al. 2019).
9.2.4 Genetic and Epigenetic Factors
Among various genetic factors, chromosomal abnormalities and single-gene mutation are one of the leading causes of infertility. Chromosomal defects like microdeletions and disarrangement in chromosome fragments can cause dysfunction in spermatogenesis. Genetic mutations like a defect in ciliary function, congenital bilateral absence of the vas deferens (CBAVD), and testicular dysgenesis syndrome are also responsible for male infertility. Furthermore, epigenetic modifications, like acetylation, methylation, hypermethylation, etc., of the germ cells cause alteration in the expression of the genes that control spermatogenesis and other characters of sperm, leading to the deficiency in semen parameters (Boissonnas et al. 2013; Iammarrone et al. 2003; Neto et al. 2016; Stouffs et al. 2014).
9.2.5 Oxidative Stress (OS)
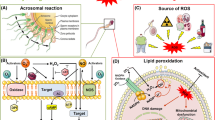
Free radicals, of both oxygen and nitrogen, can act as friend and foe for our reproductive system. These free radicals are generated as by-products of normal physiological effects in different cells including sperm cells. When their concentration is at appropriate levels, they act as friends and help in critical processes like capacitation, hyperactivation, egg penetration, and sperm head decondensation (Barati et al. 2020). Under normal conditions, human sperm generates free radicals (ROS and RNS) by numerous pathways, which instigate tyrosine kinases, and cyclic adenosine monophosphate (cAMP) that increases tyrosine phosphorylation levels (Wagner et al. 2018). Free radicals such as hydrogen peroxide, superoxide anion (O2−), nitric oxide, nicotinamide adenine dinucleotide (NADH), and nicotinamide adenine dinucleotide phosphate (NADPH) activate phosphorylation of protein tyrosine (p81 and p105). Furthermore, these phospho-tyrosine proteins are overexpressed during sperm capacitation. In the female genital tract, sperm gets hyperactive due to tyrosine phosphorylation in its tail region that further aids in the acrosome reaction which eventually helps the sperm to get attached to the zona pellucida (Fig. 9.1).
It has also been observed that apart from phosphorylation of tyrosine proteins, free radicals also induce sperm capacitation which is a compulsory process for fertilization (Tremellen 2008; Thundathil et al. 2003), while antioxidants like catalases, superoxide dismutase (SOD), and NADPH oxidase inhibit sperm capacitation (Takeshima et al. 2021). Moreover, Ca2+ also stimulates cyclic adenosine monophosphate (cAMP) during the capacitation process, which further regulates the superoxide anion generation and leads to rise in phosphorylation of p81 and p105 (Leclerc et al. 1998). Moreover, activities of adenylyl cyclase (AC) and phosphodiesterases (PDEs) also regulate the production and degradation of cAMP, thereby regulating its levels (Lefièvre et al. 2002). The above literature showed that many intricate pathways are involved during sperm capacitation and acrosomal reaction, which require ROS for functioning.
Human semen contains antioxidants, which can be categorized into enzymatic and nonenzymatic antioxidants, in the seminal plasma to protect sperm from OS. The former group includes include glutathione peroxidase (GPX), SOD, glutathione S-transferase, catalase, etc., whereas the latter one includes ascorbic acid, alpha-tocopherol, coenzyme Q10, myoinositol, astaxanthin, taurine, transferrin, L-carnitine, urate, melatonin, and lactoferrin. Under normal conditions, these antioxidants scavenge free radicals and preserves redox homeostasis in the sperm so that the sperm delivers the healthy and intact DNA to the oocyte during fertilization. Disproportion amid production and scavenging or neutralization of these free radicals heads to oxidative stress and acts as foe for our system (Fig. 9.1).
Various conditions that lead to such an oxidative stress include both endogenous [immature sperm (Sabeti et al. 2016), leukocytospermia (Fariello et al. 2009), metabolic syndromes, etc.]and exogenous [smoking (Aboulmaouahib et al. 2018), alcohol (Akang et al. 2017), radiations, environmental causes, etc.] factors. Free radicals during OS also have an indirect effect on the production of male reproductive hormones which leads to increased production of immature sperm (Barati et al. 2020). Immature sperm are the ones that have excess cytoplasmic residues, and because of this, they develop mitochondrial dysfunction which eventually leads to depletion of energy required for sperm motility and also produce excess of ROS (Sabeti et al. 2016). Leukocytes, though few, are found in normal semen, but when their number is more than 10,00,000/ml, the condition is known as leukocytospermia. Infection or inflammation of the reproductive tract is the main reason for leukocytospermia which also increase the levels of ROS in the semen leading to OS (Fariello et al. 2009). OS leads to various pathological and biochemical changes in sperm such as damage to axoneme, decrease in ATP levels, and generation of 4-hydroxynonenal and malondialdehyde (MDA) which further initiates lipid peroxidation and DNA and mitochondrial damage (Fang and Zhong 2019).
During OS, ROS affects the double bonds present in the structure of the membrane lipids, thereby initiating lipid peroxidation of sperm membrane that changes its structure, dynamics, and fluidity (Gaschler and Stockwell 2017). ROS also cause oxidation of sulfhydryl groups, along with the change in membrane structure, which also decrease the sperm motility (Saleh and Agarwal 2002). Moreover, lipid peroxidation of membranes also initiates a series of reduction-oxidation reactions of mutagenic and genotoxic electrophiles causing sperm damage (Bui et al. 2018). Furthermore, hydrogen peroxide (H2O2), a non-radical ROS, easily passes through the sperm membrane and enters into its cytoplasm. It inhibits glucose-6-phosphate dehydrogenase (G6PD) enzyme, thereby reducing the levels of NADPH, a molecule required for the activity of glutathione peroxidase, an antioxidant enzyme (Said et al. 2004). Due to the reduction of NADPH, the activity of glutathione peroxidase also reduces, thus weakening the antioxidant defense against OS (Walczak-Jedrzejowska et al. 2013). All these events eventually lead to sperm damage or sperm with poor motility. High levels of ROS also cause damage to mitochondrial membranes resulting in the activation of caspases which finally initiate apoptosis in sperm (Wagner et al. 2018).
ROS also attacks the guanine base of the DNA, thereby converting it to 8-hydroxyguanine, and under stress conditions, these further get oxidized to 8-hydroxy-2-deoxyguanosine (8-OHdG) (Noblanc et al. 2013). Glycosidase enzyme present in sperm acts on 8-OHdG and releases another base compound, thereby causing further damage to DNA which can further lead to other changes like changes in single- or double-strand fragment, DNA fragmentation, base pair configuration, etc. OS is considered as on the major reasons for sperm DNA fragmentation (Muratori et al. 2015). Such changes eventually lead to genomic instability that effect the production or genesis of sperm (Barati et al. 2020).
Numerous synthetic products are available globally to treat infertility problems, largely ending in side effects and repressing only symptoms. Thus, once again, the focus has been shifted on herbal and Ayurvedic treatments for curing male reproductive problems (Dutta and Sengupta 2018). Ayurveda contains a particular group of Rasayana herbs, called vajikarana, that deals with nourishment and stimulation of sexual tissues and improves male reproductive vitality. In Sanskrit, vaji means “horse,” and karana means “power,” giving the idea of horse’s strength. Vajikarana herbs revitalize the seven dhatus (body elements) and restore equilibrium in the body. They act on the hypothalamus and limbic system and modulate the neuroendocrine immune system. Besides, another category of herbs known as shukrala increases spermatogenesis and can improve sperm count, quality, and motility (Dalal et al. 2013). The herbs can also be classified on the basis of their beneficial effects on the male reproductive health, such as herbs that (i) enhance or stimulate semen production (Mucuna pruriens, Asparagus racemosus, etc.), (ii) improve semen quality (Vetiveria zizanioides, Sesamum indicum, etc.), (iii) revitalize ejaculatory functions (Strychnos nux-vomica, Cannabis sativa, etc.), (iv) improve nourishment and ejaculatory performance (Cinnamomum tamala, Asparagus racemosus, etc.), and (v) increase libido (Asparagus racemosus, Withania somnifera, etc.) (Chauhan et al. 2014).
9.3 Some Common Plants Used to Treat Male Infertility
9.3.1 Nigella sativa (Family: Ranunculaceae)
Nigella sativa, also known as black cumin, black seed, Habbatus sauda, and kalonji, is a medicinal plant widely used traditionally in Ayurveda, Unani Tibb, and Siddha systems of medicine. In Islamic literature, it is considered as Tibb-e-Nabawi (Prophetic medicine) and is believed to be the most extraordinary form of healing medicine (Shuid et al. 2012; Yimer et al. 2019). It has been extensively used as anti-inflammatory, spasmolytic, bronchodilator, immunomodulator, antioxidant, antidiabetic, antihypertensive, liver tonics, diuretics, digestive, and analgesics and in skin disorders (Yimer et al. 2019). The main active compounds isolated from the seeds are thymoquinone (30–48%), thymohydroquinone, dithymoquinone, p-cymene (7–15%), carvacrol (6–12%), 4-terpineol (2–7%), anethole (1–4%), longifolene (1–8%), α-pinene and thymol, and alkaloids nigellimine and nigellimine-N-oxide, nigellidine, and nigellicine (Fig. 9.2). Furthermore, the seeds also contain a water-soluble pentacyclic triterpene alpha-hederin, used in treating cancer; unsaturated fatty acids, mainly dihomolinoleic (10%), linoleic (50–60%), eicosadienoic (3%), and oleic acid (20%); saturated fatty acids mainly palmitic and stearic acid; and α-sitosterol (44–54%) followed by stigmasterol (6–20%). Most of the beneficial properties of N. sativa are attributed due to the presence of thymoquinone (Fig. 9.2), the major bioactive component of the essential oil (Ahmad et al. 2013).
Alcoholic extract of N. sativa increases the production of sperm cells, increases sperm motility, and improves epididymal sperm reservation, gonadotropin content, testosterone levels, and fertility indexes in male rats. Black cumin seeds induce a rise in spermatogenesis, levels of hormones like testosterone and luteinizing hormone (LH), and an increase in the weight of reproductive organs (Parandin et al. 2012). The treatment with seeds also increased the sperm count, fertility index, and overall characters of sperm. A randomized, double-blind, placebo-controlled clinical study conducted on 68 infertile Iranian men revealed that 2.5 mL of black seed oil twice daily for 2 months significantly improved the sperm count and motility (Kolahdooz et al. 2014). Thymoquinone (TQ) at 50 mg/kg p.o. protected the testicular tissue by alleviating inflammation and apoptosis and by restoring the average balance of sex hormones (Alyoussef and Al-Gayyar 2016). Moreover, TQ treatments significantly increase testosterone level in serum, testicular GSH, and SOD activity and lower MDA and nitric oxide activity when compared with the control group (Fouad et al. 2014). It also increases the mean volumes of testis and seminiferous tubules, count of spermatogenic cells, and Leydig cells (Darand et al. 2019; Yimer et al. 2019). These studies indicate that N. sativa/TQ can be used as an alternate source for developing natural aphrodisiac agents.
9.3.2 Mucuna pruriens (Family: Fabaceae)
It is also known as velvet bean or Kapikacchu or Konch and is an underutilized wild legume that is spread throughout the tropical and subtropical regions across the globe. It is largely utilized as fodder, forage, and green manure crop. It is mentioned in Rigveda as a Balavardhaka aushadhi (strength-promoting medicine) (Pandey and Lalitha 2018). The plant is used to treat impotence and diabetes mellitus, whereas the seeds are used to manage Parkinson’s, diabetes, arthritis, atherosclerosis, and analgesic, antipyretic, and antioxidant activities. It is a common ingredient in itching powder due to the presence of 5-hydroxytryptamine (serotonin) in the seed pods which causes severe itching (Yadav et al. 2017).
The seeds of velvet beans contain beta-sitosterol, 3-(3,4- dihydroxyphenyl)-l-alanine (L-DOPA), gallic acid, and glutathione. It also contains alkaloids like mucunadine, mucunine, pruriendine, and prurienine, 3-methoxy-1,1-dimethyl-6,7-dihydroxy-1,2,3.4-tetrahydroquinoline, and 3-methoxy-1,1-dimethyl-7,8-dihydroxy-1,2,3.4-tetrahydroquinoline (Fig. 9.3). A β-carboline alkaloid, 6-methoxyharman, has also been isolated from leaves. Seeds also contain oil rich in palmitic, stearic, oleic, and linoleic acids (Sathiyanarayanan and Arulmozhi 2007; Yadav et al. 2017).
Alkaloids present in seeds of M. pruriens are considered as main bioactive compounds as they trigger spermatogenesis and increase the weight of the testes in male rats (Saksena and Dixit 1987). The plant escalates mounting frequency, duration to ejaculate, and intromission frequency in male rats (Amin et al. 1996; Suresh et al. 2010). It also significantly recovers the spermatogenic loss induced by ethinyl estradiol administration in male rats. The plant showed beneficial effects by reducing ROS, regulating apoptosis, and increasing germ cell number. Aphrodisiac activity of M. pruriens can also be due to L-DOPA, the principal constituent of the plant, which accounted for pro-spermatogenic properties (Singh et al. 2013). The L-DOPA metabolite, dopamine, may further stimulate the hypothalamus and anterior pituitary to secrete GnRH, FSH and LH, which improves testosterone synthesis in the Leydig cells of the testicles (Sriraman et al. 2003). The ethanolic extract of seeds exhibited noticeable improvement in sexual potency, libido, sperm parameters, and endocrine levels (Suresh and Prakash 2012). M. pruriens improves semen quality by attenuating OS-induced lipid peroxidation in the seminal vesicles and reinstating GSH, ascorbic acid, catalase, and SOD levels (Ahmad et al. 2008; Shukla et al. 2010). The plant also raises LH, adrenaline, testosterone, noradrenaline, and dopamine levels and lowers FSH and prolactin levels in infertile men. It also improves steroidogenesis and semen quality in infertile males (Ahmad et al. 2008). Many drugs like “Tentex forte,” “Speman,” and “Confido” by Himalaya Drug Company contain Mucuna for sexual well-beings (Bhagwati and Singh 2017).
9.3.3 Asparagus racemosus (Family: Asparagaceae)
Asparagus racemosus, commonly known as Shatavari or Shatavar or Shatmul, is an ingredient of Vajikarana Rasayana in Ayurveda for its aphrodisiac role. It acts as a vitalizer and regulates hormone imbalance. The primary chemical constituents present in the plant are steroidal saponins, shatavarin I to IV, sarsasapogenin, adscendin (A, B, C), and asparanin (A, B, C); alkaloid, asparagine; isoflavones, 8-methoxy-5,6,4′-trihydroxyisoflavone-7-O-β-d-glucopyranoside; flavonoids, quercetin and rutin (Fig. 9.4); and sterols, sitosterol, 4,6-dihydroxy-2-O-(2′-hydroxyisobutyl). Unani traditional system of medicine used shatavari as Mwallide mani (ovulation-inducing), Mwallide labn (galactogogue), Mugallize mani and Muqawwie bah (aphrodisiac), Dafe jiryan (prevent spermaturia), Dafe sailanur rehm (prevent leucorrhoea), Muqawwie qalb (cardiac tonic), and Muhallile warm (anti-inflammatory) (Shameem and Majeedi 2020). The cooling property of the herb controls pitta in the small intestine and balances the heating effect of herbs like garlic, onion, and ashwagandha, which improve sperm count. The effect of Shatavari on pitta also helps in preventing the sperm damage, thereby improving/maintaining the sperm count. It can also be given in combination with Brahmi (Centella asiatica also known as gotu kola) to boost libido and benefit in overcoming emotions like anger and irritability (Dutta and Sengupta 2018).
Shatavari root extract significantly increased the number of mounts and mounting frequency as well as mating performance in adult male albino rats (Mishra et al. 2010; Wani et al. 2011). The plant has shown to enhance sexual activity and treat numerous sexual disorders like lack of sexual desire, erectile failure, and premature ejaculation in males. Moreover, the aqueous extract of A. racemosus rich in steroidal saponins and fructo-oligosaccharides restores the sexual functions deteriorated by alloxan or streptozotocin treatment and can be used to treat sexual dysfunctions related to diabetes due to hyperglycemia (Thakur et al. 2009). Shatavari (at 100 mg/kg dose) along with three other herbs increases sperm count and nitric oxide release and improves penile erection in male albino rats (Thakur et al. 2011). A herbo-mineral preparation including Shatavari and Gokshura (50 g each) along with 5 g of Anhraka bhasma has shown to increase the production and quality of sperm, thereby improving the fertility of a 28-year-old male (Kumar and Venkatesh 2020).
9.3.4 Withania somnifera (Family: Solanaceae)
Withania somnifera, also known as Ashwagandha, Indian ginseng, and winter cherry, is a well-known Ayurvedic Rasayana drug widely used for increasing energy and longevity (Winters 2006). The plant has been reported for its effects against inflammation, as an antioxidant, immune-modulating, antistress, memory enhancer, and anticonvulsant activities. Phytochemical studies on Ashwagandha revealed the presence of various steroidal lactones (collectively known as withanolides) and alkaloids (withanine, somniferine, somnine, somniferinine). The prime withanolides isolated from the plant include withaferin A, withanone, withanolide A–Q, sitoindoside VII–X, and withanamides A–I (Fig. 9.5) (Mirjalili et al. 2009; Singh et al. 2010).
Studies exhibited that ashwagandha roots increase sperm motility, semen volume, and sperm count, stabilize testosterone production in oligospermic male, and, therefore, it can be used as a valuable treatment for infertility (Pathak et al. 2020). The plant acts via oxidative and non-oxidative process to exert its effect on male fertility. In oxidative mechanism, W. somnifera maintains antioxidant enzymes and the cofactors responsible for antioxidant activity. Non-oxidative mechanism includes regulation of the hypothalamus-pituitary-gonadal axis and hypothalamus-pituitary-adrenal axis for the proper functioning of reproductive organs. It regulates endocrine homeostasis, reduces the stress response, and normalizes the cortisol levels to improve male fertility (Sengupta et al. 2018). Moreover, the roots of the plants hold considerable amounts of lactate and lactate dehydrogenase (LDH) which stimulate Krebs cycle and increase ATP and cAMP levels, improving sperm concentration, quality, and motility (Gupta et al. 2013; Teixeira and de Araujo 2019).
W. somnifera exhibited enhancement in spermatozoa factors in males who have idiopathic infertility (Azgomi et al. 2018). W. somnifera, along with Cynomorium coccineum, showed testosterone-like effect and influence spermatogenesis in the seminiferous tubules of immature rats (Abdel-Magied et al. 2001). Treatment with the plant in infertile males has also improved semen quality by inhibiting lipid peroxidation, restoring antioxidant enzymes, increasing testosterone and LH levels, and reducing FSH and prolactin levels (Ahmad et al. 2010). Moreover, withanone exhibited GABA-mimetic action which regulates gonadotropin-releasing hormone (GnRH) at cellular levels which support the claims of Ashwagandha extracts in improving sexual function and testosterone production in male rats (Kataria et al. 2015). Some of the market formulations for male infertility problems, containing W. somnifera, by Himalaya Drug Company, are “Speman,” “Himplasia,” “Confido,” and “Tentex Forte.”
9.3.5 Panax ginseng (Family: Araliaceae)
Ginseng is one of the ancient herbs used in traditional Chinese medicine. The most common varieties of ginseng are Asian ginseng (Panax ginseng), American ginseng (Panax quinquefolius), and Japanese ginseng (Panax japonicus) (Leung and Wong 2013). Based on their structural differences, the plant consists of three tetracyclic dammarane triterpenoid saponin glycosides (called as ginsenosides): panaxadiols (e.g., Rb1-Rb3, Rc, Rd., Rg3, Rh2, and Rs1), panaxatriols (e.g., Re, Rf, Rg1–2, and Rh1), and oleanolic acid derivatives (e.g., Ro) (Fig. 9.6). Ginseng has been used mainly as a tonic to rejuvenate fragile bodies and restore proper metabolism in the body. It possesses antioxidant, anti-apoptotic, anti-inflammatory, immune-stimulatory activities and showed beneficial effects on aging and neurodegenerative diseases. The plant and its various chemical constituents reduce lipid peroxidation, maintain cellular ATP levels, and inhibit excitotoxicity and Ca2+ over-influx into neurons (Choudhary et al. 2013).
Ample studies have exhibited the importance of ginseng in boosting of sex hormone levels, sperm numbers, and testicular antioxidants, restoring Leydig cells, and improving spermatogenesis and sperm motility (Kopalli et al. 2015; Ku et al. 2020). Many ginsenosides especially Rg1 (10 mg/kg) help in treating erectile dysfunction by inducing synthesis of nitric oxide (NO) in endothelial cells by glucocorticoid receptor-dependent, non-genomic mechanisms. NO release causes smooth muscle relaxation which allows more blood to enter into the corpus cavernosum, causing an erection (Leung and Wong 2013). Experimental models revealed that the intake of Panax ginseng (5%), ginsenoside Rg1 (10 mg/kg), and ginsenoside Rb1 (10 μg/kg) increases serum testosterone levels, improves copulatory behavior, and increases LH secretion (Fahim et al. 1982; Tsai et al. 2003; Wang et al. 2010). Apart from this, saponins from the stem and leaves of P. ginseng also reduce the oxidative stress linked with hyperthermia and heat stress. They also inhibit the activation of mitogen-activated protein kinase (MAPK) signaling pathways and the expression of apoptotic proteins (Liu et al. 2021). Rg3-enriched extract of Korean red ginseng attenuates heat stress-induced testicular damage and change in expression of sex hormone receptors that affect spermatogenesis (Kopalli et al. 2019). Long-term administration of its aqueous extract significantly delays the aging-induced testicular dysfunction by modulating the expression of enzymes that regulate oxidation, acetylation, and growth-related activities linked with spermatogenesis (Kopalli et al. 2017; Kopalli et al. 2015). It also prevents or treats psychological stress-induced male infertility by increasing antioxidant enzyme expression, sex hormone receptor expression, and functioning of spermatogenesis-related proteins (Lee et al. 2019). The plant also prevents oxidative stress and apoptosis linked with monobutyl phthalate in human Sertoli cells by increasing the expression of nuclear factor erythroid 2-related factor 2 (NRF-2), sirtuin (SIRT-1), and antioxidant enzymes (De Freitas et al. 2019).
9.3.6 Trigonella foenum-graecum (Family: Fabaceae)
Trigonella foenum-graecum Linn, also known as fenugreek, is an aromatic annual plant widely grown in Egypt, India, China, France, Spain, and Turkey. The plant comprises of active constituents, such as alkaloids (choline, trigonelline, carpaine), saponins (yamogenin, fenugrin, gitogenin, yuccagenin, tigonenin, diosgenin, neotigogenin, sarsasapogenin), flavonoids (luteolin, kaempferol, naringenin, quercetin, tricin 7-O-D glucopyranoside, iso-vitexin, vitexin), coumarins (methyl coumarin, trigocoumarin, trimethyl coumarin), steroids, and phenolics like gallic acid, catechin, chlorogenic acid, vanillic acid, and syringic acid (Fig. 9.7) (Dini 2018). The seeds contain diosgenin which is an essential precursor for synthesizing several sex hormones including testosterone and estrogen. Traditionally, fenugreek seeds were given to lactating females as a stimulant for milk production (El-Hak and Elrayess 2018).
Fenugreek seed aqueous extract improved the sperm damage caused by bisphenol by reducing MDA levels, decreasing the expression of apoptotic markers, increasing levels of antioxidant enzymes and thereby improving sperm parameters (Kaur and Sadwal 2020). Furosap™, a patented 20% protodioscin-enriched seed extract of fenugreek, increases free testosterone levels, sperm count, and sperm motility and causes significant alleviation in mood, reflex erection, and overall performance in male subjects (Maheshwari et al. 2017; Swaroop et al. 2017). Testofen®, a patented formulation by Gencor Pacific Lifestage Solutions, containing standardized extract of Trigonella foenum-graecum boosts male libido and maintains prolactin, testosterone, and prostate-specific antigen levels (Rao et al. 2016). A 12-week, single-site, double-blind, randomized clinical trial, on 120 male subjects, aged 43–70, showed that Testofen® effectively improved sexual health by remarkably increasing sexual desire, arousal, and testosterone levels (Rao et al. 2016). The mixed extract of fenugreek seeds and Lespedeza cuneata exhibited significant improvement in testosterone deficiency syndrome (Park et al. 2018). Moreover, consumption of aqueous extract of fenugreek seeds improves fertility and reproductive function in male rats (Hind et al. 2017).
9.3.7 Allium sativum (Family: Liliaceae)
Allium sativum, also known as garlic, is an intensely aromatic bulb crop that is cultivated across the globe. Traditionally, garlic is used as an aphrodisiac, to relieve cough problems, prevent graying of hair, lower cholesterol, and treat eczema, rheumatism, and high blood pressure. The Kashyapa Samhita contains a special chapter called “Lasunkalpa Addhyaya,” which deals with the uses and pharmaceutical preparations containing garlic for treating infertility in males and females (Vaijnath and Manikrao 2018). Fresh garlic bulb contains water (65%), carbohydrates (28%), organosulfur compounds (2.3%), proteins (2%), fiber (1.5%), amino acids (1.2%), saponins, and phenolics (Nouroz et al. 2015). Many sulfur-containing compounds include alliin, diallyl polysulfides, S-allylcysteine, diallyl sulfide, diallyl disulfide, diallyl trisulfide, S-allyl mercaptocysteine, and vinyldithiins (formed in the breakdown of allicin) (Fig. 9.8). Saponins reported from Allium sativum extract include proto-eruboside B; eruboside B; voghierosides A1, A2, B1, B2, C1, C2, D1, D2, E1, and E2; gitogenin 3-O-tetrasaccharide; and agigenin 3-O-trisaccharide. The phenolics isolated from garlic extract contain caffeic, ferulic, vanillic, p-hydroxybenzoic, and p-coumaric acid (Fig. 9.8) (Kuete 2017).
Many studies revealed the importance of garlic in the treatment of male infertility due to sulfur compounds that directly affect the uptake of CYP450 and glutathione S-transferase and protect spermatogenesis. It inhibits caspase-3 and CYP450 enzymes, which had a deleterious effect on the testicles (El-Akabawy and El-Sherif 2016). Moreover, garlic has antioxidant properties which reduce lipid peroxidation and increase fertility in men (Hammami and El May 2013). It also acts as a precursor to testosterone production, which further stimulates sexual cells and testosterone secretion from the testicles, boosts LH from the pituitary gland, and improves spermatogenesis. Garlic also inhibits reactive oxygen species and increases sperm motility and survival (Musavi et al. 2018). It also increases the flow of blood in the testis. Fresh garlic juice (120 mg/kg) protects semen oxidation by decreasing MDA levels and increasing antioxidant activity in rat testes (Ghalehkandi 2014). The aged garlic extract (250 mg/kg, 14 days) causes an increase in sperm count, motility, testis weight, recuperation of seminiferous tubules, and decreased sperm abnormality and death via antioxidant mechanism (Nasr 2017).
Similarly, treatment with single bulb garlic (250 mg/kg) improves sperm count, sperm motility, and sperm normality in male mice with hyperlipidemia (Qadariah et al. 2020). Also, aqueous garlic extract enhances spermatogenesis and ameliorates testicular and hematological alterations induced by cadmium poisoning in male rats (Mbegbu et al. 2021). The primary protective mechanism of garlic for testicular damage is to combat oxidative stress by decreasing free radicals and increasing antioxidant parameters in the semen (Adeyemi et al. 2021; Alsenosy and El-Aziz 2019; Eric et al. 2020; Ifeoma et al. 2018; Nasr et al. 2017).
9.3.8 Shilajit (Asphaltum, Mineral Pitch)
Shilajit, also known as salajit, shilajatu, mimie, or mummiyo, is a drug of mineral origin. It occurs as a blackish-brown powder or an exudate from high mountain rocks between India and Nepal. It is known as a rejuvenator and as an antiaging compound in Ayurveda. Shilajit is composed of humic substances, mainly fulvic acid (60–80%) (Fig. 9.9) plus some oligo-elements, including selenium. The composition of the phytocomplex varies from region to region (Carrasco-Gallardo et al. 2012).
The pure extract of shilajit and processed shilajit capsules improve spermatogenesis and male fertility and have beneficial effects on oligozoospermia (Biswas et al. 2010; Chouhan et al. 2018). It decreases oxidative stress in sperm and improves sperm quality parameters, such as motility, plasma membrane integrity, etc., in the semen of buffalo (Sultan et al. 2021). Furthermore, when those sperm were stored under cryogenic conditions, shilajit preserves their viability, livability, and DNA integrity, indicating its beneficial effects on sperm quality. In another study, shilajit increases the serum LH and testosterone levels and the number of seminiferous tubular cell layers in the testes and improves spermatogenesis in the treated rats (Park et al. 2006). Moreover, treatment with shilajit also increases the weight of reproductive organs and sperm production, enhances activities of testicular enzymes, and reverts the adverse effects of cadmium on motility and concentration of spermatozoa (Mishra et al. 2018). Inhibition of phosphodiesterase 5A (PDE5A) has become the first-line therapy for treatments of erectile dysfunction. Fulvic acid, the main constituent of shilajit, binds to the catalytic site of PDE5A and blocks the degradative action of cGMP for penile erection (Bhavsar et al. 2016). Shilajit displayed a peripheral parasympathomimetic effect for endothelium-dependent relaxation of corpus cavernosum smooth muscles, which supports the traditional claims of shilajit on libido and fertility (Kaur et al. 2013).
Apart from these extensive studied plants to treat male infertility, there are numerous other plants that have exhibited beneficial effects against male infertility. Some of the plants/phytoconstituents responsible for enhancing male fertility and fertilization process are mentioned in Table 9.2.
9.4 Conclusion
OS is considered as one of the major causes of male infertility. It leads to increase in lipid peroxidation and DNA damage which disturbs sperm functions making early diagnosis of infertility essential to avoid permanent impairment in the long run. Proper diet, exercise, reduced smoking, moderate consumption of alcohol, and non-exposure to radiation have substantial effects on lowering OS levels, thus improving male fertility. Besides lifestyle changes, antioxidant therapy is also used to prevent OS in the body, but additional studies are required for modulating their doses and duration.
References
Abarikwu SO, Onuah CL, Singh SK. Plants in the management of male infertility. Andrologia. 2020;52:e13509. https://doi.org/10.1111/and.13509.
Abbasi Z, Tabatabaei SRF, Mazaheri Y, Barati F, Morovvati H. Effects of sesame oil on the reproductive parameters of diabetes mellitus-induced male rats. World J Mens Health. 2013;31:141–9.
Abdel-Magied EM, Abdel-Rahman HA, Harraz FM. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J Ethnopharmacol. 2001;75:1–4. https://doi.org/10.1016/s0378-8741(00)00348-2.
Abo-Ghanema II, El-Nasharty M, El-Far A, Ghonium HA. Effect of ginger and L-carnitine on the reproductive performance of male rats. World Acad Sci Eng Technol. 2012;64:980–6.
Aboulmaouahib S, et al. Impact of alcohol and cigarette smoking consumption in male fertility potential: looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia. 2018;50 https://doi.org/10.1111/and.12926.
Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, Anuar MNN. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9. https://doi.org/10.3390/diseases5010009.
Adeyemi AA, Yekini SD, Oloyede OJ. Antioxidant impact of Zingiber officinale and Allium sativum on rabbit semen. J Vet Androl. 2021;5:18–22.
Ahmad A, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337–52. https://doi.org/10.1016/S2221-1691(13)60075-1.
Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90:627–35.
Ahmad MK, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94:989–96.
Akang EN, Oremosu AA, Osinubi AA, James AB, Biose IJ, Dike SI, Idoko KM. Alcohol-induced male infertility: is sperm DNA fragmentation a causative? J Exp Clin Anat. 2017;16:53–9.
Al-Olayan EM, El-Khadragy MF, Metwally DM, Abdel Moneim AE. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014;14:164. https://doi.org/10.1186/1472-6882-14-164.
Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res. 2018;32:514–21. https://doi.org/10.1002/ptr.5998.
Alsenosy AE-WA, El-Aziz AHA. Effect of garlic supplementation to rabbit semen extender on semen metabolic and oxidative markers. Alexandria J Vet Sci. 2019;60:94–101.
Alyoussef A, Al-Gayyar MMH. Thymoquinone ameliorated elevated inflammatory cytokines in testicular tissue and sex hormones imbalance induced by oral chronic toxicity with sodium nitrite. Cytokine. 2016;83:64–74. https://doi.org/10.1016/j.cyto.2016.03.018.
Amin YMN, Rehman ZS, Khan NA. Sexual function improving effect of M. pruriens in sexually normal male rats. Fitoterapia. 1996;67:53–8.
Ashamu E, Salawu E, Oyewo O, Alhassan A, Alamu O, Adegoke A. Efficacy of vitamin C and ethanolic extract of Sesamum indicum in promoting fertility in male Wistar rats. J Hum Reprod Sci. 2010;3:11–4. https://doi.org/10.4103/0974-1208.63115.
Ateşşahin A, Karahan I, Türk G, Gür S, Yilmaz S, Ceribaşi AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol (Elmsford, NY). 2006;21:42–7. https://doi.org/10.1016/j.reprotox.2005.05.003.
Azgomi NDR, et al. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: a triple-blind randomised clinical trial. Andrologia. 2018;50:e13041. https://doi.org/10.1111/and.13041.
Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med. 2020;13:29–41.
Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. 2020;77:93–113.
Bayatli F, Akkuş D, Kilic E, Saraymen R, Sönmez MF. The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: an experimental study. World J Urol. 2013;31:615–22. https://doi.org/10.1007/s00345-013-1049-8.
Bhagwati S, Singh R. Plant products in the management of male infertility. In: Singh R, Singh K, editors. Male infertility: understanding, causes and treatment. Springer Singapore; 2017. p. 381–99.
Bhavsar SK, Thaker AM, Malik JK. Shilajit. In: Gupta RC, editor. Nutraceuticals. Boston: Academic Press; 2016. p. 707–16. https://doi.org/10.1016/B978-0-12-802147-7.00051-6.
Biswas TK, et al. Clinical evaluation of spermatogenic activity of processed Shilajit in oligospermia. Andrologia. 2010;42:48–56. https://doi.org/10.1111/j.1439-0272.2009.00956.x.
Boissonnas CC, Jouannet P, Jammes H. Epigenetic disorders and male subfertility. Fertil Steril. 2013;99:624–31. https://doi.org/10.1016/j.fertnstert.2013.01.124.
Bordbar H, Esmaeilpour T, Dehghani F, Panjehshahin MR. Stereological study of the effect of ginger's alcoholic extract on the testis in busulfan-induced infertility in rats. Iran J Reprod Med. 2013;11:467–72.
Bui A, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. J Andro. 2018;50:e13012. https://doi.org/10.1111/and.13012.
Carrasco-Gallardo C, Guzmán L, Maccioni RB. Shilajit: a natural phytocomplex with potential procognitive activity. Int J Alzheimers Dis. 2012;2012:1–4. https://doi.org/10.1155/2012/674142.
Chauhan NS, Sharma V, Dixit V, Thakur M. A review on plants used for improvement of sexual performance and virility. Biomed Res Int. 2014;2014 https://doi.org/10.1155/2014/868062.
Chenniappan K, Murugan K. Therapeutic and fertility restoration effects of Ionidium suffruticosum on sub-fertile male albino Wistar rats: effects on testis and caudal spermatozoa. Pharm Biol. 2017;55:946–57. https://doi.org/10.1080/13880209.2016.1278453.
Choudhary S, Kumar P, Malik J. Plants and phytochemicals for Huntington’s disease. Pharmacog Rev. 2013;7:81–91.
Chouhan BS, Rajput SS, Dwivedi R, Singh A. A review on Ayurveda perspective and therapeutic consideration of oligozoospermia. J Drug Deliv Ther. 2018;8:55–8.
Clément C, Kneubühler J, Urwyler A, Witschi U, Kreuzer M. Effect of maca supplementation on bovine sperm quantity and quality followed over two spermatogenic cycles. Theriogenology. 2010;74:173–83. https://doi.org/10.1016/j.theriogenology.2010.01.028.
Dalal P, Tripathi A, Gupta S. Vajikarana: treatment of sexual dysfunctions based on Indian concepts. Indian J Psychiatry. 2013;55:S273–6.
Darand M, Hajizadeh M, Mirmiran P, Mokari-Yamchi A. The effect of Nigella sativa on infertility in men and women: a systematic review. Prog Nutr. 2019;21:33–41.
De Freitas ATAG, Figueiredo Pinho C, de Aquino AM, Fernandes AAH, Fantin Domeniconi R, Justulin LA, Scarano WR. Panax ginseng methabolit (GIM-1) prevents oxidative stress and apoptosis in human Sertoli cells exposed to monobutyl-phthalate (MBP). Reprod Toxicol. 2019;86:68–75. https://doi.org/10.1016/j.reprotox.2019.02.008.
Del Prete C, et al. Influences of dietary supplementation with Lepidium meyenii (Maca) on stallion sperm production and on preservation of sperm quality during storage at 5 °C. Andrology. 2018;6:351–61. https://doi.org/10.1111/andr.12463.
Dini I. Spices and herbs as therapeutic foods. In: Holban AM, Grumezescu AM, editors. Food quality: balancing health and disease. Academic Press; 2018. p. 433–69. https://doi.org/10.1016/B978-0-12-811442-1.00014-6.
Dutta S, Sengupta P. Medicinal herbs in the management of male infertility. J Preg Reprod. 2018;2:1–6.
El-Akabawy G, El-Sherif NM. Protective role of garlic oil against oxidative damage induced by furan exposure from weaning through adulthood in adult rat testis. Acta Histochem. 2016;118:456–63.
El-Hak HNG, Elrayess RA. Evaluation of Trigonella foenum graecum L. (Fenugreek) seeds efficiency in enhancing male reproductive health. Kenkyu J Pharm Pract Health Care. 2018;4:1–3.
Eric E, Eseimo N, Josiah V. Evaluation of aluminium phosphide induced testicular toxicity in wistar rat: the role of Allium sativum. Asian J Adv Res Reports. 2020;11:1–9.
Fahim MS, Fahim Z, Harman JM, Clevenger TE, Mullins W, Hafez ES. Effect of Panax ginseng on testosterone level and prostate in male rats. Arch Androl. 1982;8:261–3. https://doi.org/10.3109/01485018208990207.
Fang Y, Zhong R. Effects of oxidative stress on spermatozoa and male infertility. In: Das K, Das S, Biradar MS, Bobbarala V, Tata SS, editors. Free radical medicine and biology. London: IntechOpen; 2019. p. 73–95.
Fariello RM, Del Giudice PT, Spaine DM, Fraietta R, Bertolla RP, Cedenho AP. Effect of leukocytospermia and processing by discontinuous density gradient on sperm nuclear DNA fragmentation and mitochondrial activity. J Assist Reprod Genet. 2009;26:151–7. https://doi.org/10.1007/s10815-008-9288-0.
Fouad AA, Albuali WH, Jresat I. Protective effect of thymoquinone against arsenic-induced testicular toxicity in rats. Int J Pharmacol Pharm Sci. 2014;8:98–101.
Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419–25. https://doi.org/10.1016/j.bbrc.2016.10.086.
Ghalehkandi JG. Garlic (Allium sativum) juice protects from semen oxidative stress in male rats exposed to chromium chloride. Anim Reprod. 2014;11:526–32.
Giribabu N, Kumar KE, Rekha SS, Muniandy S, Salleh N. Chlorophytum borivilianum (Safed Musli) root extract prevents impairment in characteristics and elevation of oxidative stress in sperm of streptozotocin-induced adult male diabetic Wistar rats. BMC Complement Altern Med. 2014;14:291. https://doi.org/10.1186/1472-6882-14-291.
Grami D, et al. Protective action of Eruca sativa leaves aqueous extracts against bisphenol A-caused in vivo testicular damages. J Med Food. 2020;23:600–10. https://doi.org/10.1089/jmf.2019.0170.
Grami D, et al. Aqueous extract of Eruca sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod Toxicol. 2018;82:103–10. https://doi.org/10.1016/j.reprotox.2018.10.008.
Gupta A, Mahdi AA, Shukla KK, Ahmad MK, Bansal N, Sankhwar P, Sankhwar SN. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: a proton NMR study at 800 MHz. J Ethnopharmacol. 2013;149:208–14.
Hala A, Khattab Z, Abdallah G, Kamel M. Grape seed extract alleviate reproductive toxicity caused by aluminium chloride in male rats. J Am Sci. 2010;6:352–61.
Hamden K, Carreau S, Jamoussi K, Ayadi F, Garmazi F, Mezgenni N, Elfeki A. Inhibitory effects of 1alpha, 25dihydroxyvitamin D3 and Ajuga iva extract on oxidative stress, toxicity and hypo-fertility in diabetic rat testes. J Physiol Biochem. 2008;64:231–9. https://doi.org/10.1007/bf03216108.
Hammami I, El May M. Impact of garlic feeding (Allium sativum) on male fertility. Andrologia. 2013;45:217–24.
Hammoda HM, Ghazy NM, Harraz FM, Radwan MM, ElSohly MA, Abdallah II. Chemical constituents from Tribulus terrestris and screening of their antioxidant activity. Phytochemistry. 2013;92:153–9. https://doi.org/10.1016/j.phytochem.2013.04.005.
Han C, Liu C, Geng J, Tang Y, Li Y, Wang Y, Xie Z. Black and green tea supplements ameliorate male infertility in a murine model of obesity. J Med Food. 2020;23:1303–11. https://doi.org/10.1089/jmf.2020.4784.
Heydari M, Rezanezhadi JB, Delfan B, Birjandi M, Kaviani H, Givrad S. Effect of saffron on semen parameters of infertile men. Urol J. 2008;5:255–9.
Hind B, Zineb M, Elbachir H, Najat EA, Siham A, Driss R. Evaluation of potential effects of the aqueous extract of fenugreek seeds on fertility in male rats. J Ayurvedic Herb Med. 2017;3:210–5.
Iammarrone E, Balet R, Lower AM, Gillott C, Grudzinskas JG. Male infertility. Best Pract Res Clin Obstet Gynaecol. 2003;17:211–29. https://doi.org/10.1016/S1521-6934(02)00147-5.
Ifeoma OR, Edmund M, Ekere SO, Amaeze C. Hypoglycemic profile and ameliorative potential of aqueous garlic extract on sperm characteristics in glibenclamide treated diabetic male rats. African J Pharm Pharmacol Ther. 2018;12:356–60.
Jayaganthan P, Perumal P, Balamurugan TC, Verma RP, Singh LP, Pattanaik AK, Kataria M. Effects of Tinospora cordifolia supplementation on semen quality and hormonal profile in rams. Anim Reprod Sci. 2013;140:47–53. https://doi.org/10.1016/j.anireprosci.2013.05.003.
Kang HR, et al. Petasites japonicus stimulates the proliferation of mouse spermatogonial stem cells. PLoS One. 2015;10:e0133077. https://doi.org/10.1371/journal.pone.0133077.
Kataria H, Gupta M, Lakhman S, Kaur G. Withania somnifera aqueous extract facilitates the expression and release of GnRH: in vitro and in vivo study. Neurochem Int. 2015;89:111–9. https://doi.org/10.1016/j.neuint.2015.08.001.
Katib A. Mechanisms linking obesity to male infertility. Cent Europ J Urol. 2015;68:79–85. https://doi.org/10.5173/ceju.2015.01.435.
Kaur S, Kumar P, Kumar D, Kharya M, Singh N. Parasympathomimetic effect of shilajit accounts for relaxation of rat corpus cavernosum. Am J Mens Health. 2013;7:119–27.
Kaur S, Sadwal S. Studies on the phytomodulatory potential of fenugreek (Trigonella foenum-graecum) on bisphenol-A induced testicular damage in mice. Andrologia. 2020;52:e13492.
Khaki A, Fathi AF, Nouri M, Khaki AA, Ozanci CC, Ghafari NM, Hamadeh M. The effects of ginger on spermatogenesis and sperm parameters of rat. Iran J Reprod Med. 2009;7:7–12.
Khaki A, Nouri M, Fathi AF, Khaki AA. Evaluation of Zingiber officinalis and Allium cepa on spermatogenesis in rat. Med J Tabriz Uni Med Sci. 2008;30:53–8.
Khani B, Bidgoli SR, Moattar F, Hassani H. Effect of sesame on sperm quality of infertile men. J Res Med Sci. 2013;18:184–7.
Kolahdooz M, Nasri S, Modarres SZ, Kianbakht S, Huseini HF. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2014;21:901–5. https://doi.org/10.1016/j.phymed.2014.02.006.
Kopalli SR, Cha K-M, Hwang S-Y, Jeong M-S, Kim S-K. Korean red ginseng (Panax ginseng Meyer) with enriched Rg3 ameliorates chronic intermittent heat stress–induced testicular damage in rats via multifunctional approach. J Ginseng Res. 2019;43:135–42. https://doi.org/10.1016/j.jgr.2018.06.004.
Kopalli SR, et al. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis related molecules. Exp Gerontol. 2017;90:26–33. https://doi.org/10.1016/j.exger.2017.01.020.
Kopalli SR, et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol. 2015;69:94–102. https://doi.org/10.1016/j.exger.2015.05.004.
Ku JY, Park MJ, Park HJ, Park NC, Joo BS. Combination of Korean red ginseng extract and hydrogen-rich water improves spermatogenesis and sperm motility in male mice. Chin J Integr Med. 2020;26:361–9.
Kuete V. Allium sativum. In: Kuete V, editor. Medicinal spices and vegetables from Africa. Academic Press; 2017. p. 363–77. https://doi.org/10.1016/B978-0-12-809286-6.00015-7.
Kumar R, Venkatesh S. Ayurvedic management of male infertility (oligo astheno teratozoospermia) due to varicocele; a single case study. Int J Resn AYUSH Pharm Sci. 2020;4:420–30.
Leclerc P, De Lamirande E, Gagnon C. Interaction between Ca2+, cyclic 3 ‘,5’ adenosine monophosphate, the superoxide anion, and tyrosine phosphorylation pathways in the regulation of human sperm capacitation. J Androl. 1998;19:434–43.
Lee S-H, et al. Protective effects of Korean Red Ginseng against sub-acute immobilization stress-induced testicular damage in experimental rats. J Ginseng Res. 2019;43:125–34. https://doi.org/10.1016/j.jgr.2017.09.002.
Lefièvre L, Jha KN, de Lamirande E, Visconti PE, Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl. 2002;23:709–16.
Leslie S, Siref L, Soon-Sutton T, Khan MA. Male infertility. Treasure Island (FL): StatPearls Publishing; 2021; https://www.ncbi.nlm.nih.gov/books/NBK562258/
Leung KW, Wong AST. Ginseng and male reproductive function. Spermatogenesis. 2013;3:e26391. https://doi.org/10.4161/spmg.26391.
Liu W, et al. Saponins derived from the stems and leaves of Panax ginseng attenuate scrotal heat-induced spermatogenic damage via inhibiting the MAPK mediated oxidative stress and apoptosis in mice. Phytother Res. 2021;35:311–23. https://doi.org/10.1002/ptr.6801.
Maheshwari A, Verma N, Swaroop A, Bagchi M, Preuss HG, Tiwari K, Bagchi D. Efficacy of Furosap (TM), a novel Trigonella foenum-graecum seed extract, in enhancing testosterone level and improving sperm profile in male volunteers. Int J Med Sci. 2017;14:58–66. https://doi.org/10.7150/ijms.17256.
Marrag I, Hajji K, Braham MY, Dhifallah M, Nasr M. Antipsychotics and hyperprolactinemia: prevalence and risk factors. Ann Psychiatr Ment Health. 2015;3:1047–53.
Mbegbu EC, Odo RI, Ozioko PT, Awachie ME, Nwobi LG, Obidike IR. Aqueous Allium sativum (garlic) extract ameliorated CdCl2-induced alterations in blood formation and spermatogenesis in albino rats. Trop J Pharm Res. 2021;20:309–14.
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–93.
Mishra RK, Jain A, Singh SK. Profertility effects of Shilajit on cadmium-induced infertility in male mice. Andrologia. 2018;50:e13064.
Mishra VK, Sheikh S, Agnihotri V, Chourey N. Effects of Asparagus racemosus, (shatavari) on mounting behaviour of male rats. Int J Pharm Life Sci. 2010;1:30–4.
Monaco D, Fatnassi M, Padalino B, Aubé L, Khorchani T, Hammadi M, Lacalandra GM. Effects of a GnRH administration on testosterone profile, libido and semen parameters of dromedary camel bulls. Res Vet Sci. 2015;102:212–6. https://doi.org/10.1016/j.rvsc.2015.08.011.
Muratori M, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21:109–22.
Musavi H, Tabnak M, Sheini FA, Bezvan MH, Amidi F, Abbasi M. Effect of garlic (Allium sativum) on male fertility: a systematic review. J Herbmed Pharmacol. 2018;7:306–12.
Mustafa M, Sharifa A, Hadi J, IIIzam E, Aliya S. Male and female infertility: causes, and management. IOSR- J Dent Med Sci. 2019;18:27–32.
Nasr AY. The impact of aged garlic extract on adriamycin-induced testicular changes in adult male Wistar rats. Acta Histochem. 2017;119:648–62. https://doi.org/10.1016/j.acthis.2017.07.006.
Nasr NE, Elmadawy MA, Almadaly EA, Abdo W, Zamel MM. Garlic powder attenuates apoptosis associated with lead acetate-induced testicular damage in adult male rats. Alexandria J Vet Sci. 2017;54:70–8.
Neto FTL, Bach PV, Najari BB, Li PS, Goldstein M. Genetics of male infertility. Curr Urol Rep. 2016;17:70. https://doi.org/10.1007/s11934-016-0627-x.
Noblanc A, et al. DNA oxidative damage in mammalian spermatozoa: where and why is the male nucleus affected? Free Radic Biol Med. 2013;65:719–23. https://doi.org/10.1016/j.freeradbiomed.2013.07.044.
Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33:3203–11. https://doi.org/10.1002/ptr.6493.
Nouroz F, Mehboob M, Noreen S, Zaidi F, Mobin T. A review on anticancer activities of garlic (Allium sativum L.). Middle East J Sci Res. 2015;23:1145–51.
Pandey N, Lalitha BR. Phyto-pharmacognostic and pharmaco-therapeutic review of kapikacchu (Mucuna pruriens). Ayurpub. 2018;3:1094–104.
Parandin R, Yousofvand N, Ghorbani R. The enhancing effects of alcoholic extract of Nigella sativa seed on fertility potential, plasma gonadotropins and testosterone in male rats. Iran J Reprod Med. 2012;10:355–62.
Park HJ, Lee KS, Lee EK, Park NC. Efficacy and safety of a mixed extract of Trigonella foenum-graecum seed and Lespedeza cuneata in the treatment of testosterone deficiency syndrome: a randomized, double-blind, placebo-controlled clinical trial. World J Mens Health. 2018;36:230–8.
Park J-S, Kim G-Y, Han K. The spermatogenic and ovogenic effects of chronically administered Shilajit to rats. J Ethnopharmacol. 2006;107:349–53. https://doi.org/10.1016/j.jep.2006.03.039.
Pathak M, Sharma S, Kushwaha PP, Kumar S. Functional lead compounds and targets for the development of drugs for the treatment of male infertility. In: Egbuna C, Kumar S, Ifemeje JC, Ezzat SM, Kaliyaperumal S, editors. Phytochemicals as lead compounds for new drug discovery. Elsevier; 2020. p. 333–45.
Qadariah N, Lestari SR, Rohman F. Single bulb garlic (Allium sativum) extract improve sperm quality in hyperlipidemia male mice model. Jurnal Kedokteran Hewan. 2020;14:7–11.
Rao A, Steels E, Inder WJ, Abraham S, Vitetta L. Testofen, a specialised Trigonella foenum-graecum seed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. 2016;19:134–42. https://doi.org/10.3109/13685538.2015.1135323.
Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed. 2016;14:231–40.
Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, Thomas AJ Jr. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil Steril. 2004;82:871–7. https://doi.org/10.1016/j.fertnstert.2004.02.132.
Saksena S, Dixit VK. Role of total alkaloids of Mucuna pruriens Baker in spermatogenesis in albino rats. Indian J Nat Prod. 1987;3:3–7.
Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52.
Santiago J, Silva JV, Santos MA, Fardilha M. Fighting bisphenol A-induced male infertility: the power of antioxidants. Antioxidants. 2021;10:289. https://doi.org/10.3390/antiox10020289.
Sathiyanarayanan L, Arulmozhi S. Mucuna pruriens Linn. – a comprehensive review. Pharmacog Rev. 2007;1:157–62.
Schagen SE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016;13:1125–32. https://doi.org/10.1016/j.jsxm.2016.05.004.
Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online. 2018;36:311–26. https://doi.org/10.1016/j.rbmo.2017.11.007.
Shalaby MA, Hammouda AA. Assessment of protective and anti-oxidant properties of Tribulus terrestris fruits against testicular toxicity in rats. J Intercultural Ethnopharmacol. 2014;3:113–8. https://doi.org/10.5455/jice.20140627123443.
Shameem I, Majeedi SF. A review on potential properties and therapeutic application of Asparagus racemosus Wild. World J Pharm Res. 2020;9:2532–40.
Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–9. https://doi.org/10.1089/109662003772519831.
Shuid AN, et al. Nigella sativa: A potential antiosteoporotic agent. Evid Based Complement Altern Med. 2012;2012:696230. https://doi.org/10.1155/2012/696230.
Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Altern Med. 2010;7:137–44. https://doi.org/10.1093/ecam/nem171.
Singh AP, Sarkar S, Tripathi M, Rajender S. Mucuna pruriens and its major constituent L-DOPA recover spermatogenic loss by combating ROS, loss of mitochondrial membrane potential and apoptosis. PLoS One. 2013;8:e54655. https://doi.org/10.1371/journal.pone.0054655.
Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1:56–63.
Sriraman V, Sairam MR, Rao AJ. Evaluation of relative roles of LH and FSH in regulation of differentiation of Leydig cells using an ethane 1,2-dimethylsulfonate-treated adult rat model. J Endocrinol. 2003;176:151–61. https://doi.org/10.1677/joe.0.1760151.
Stouffs K, Seneca S, Lissens W. Genetic causes of male infertility. Ann Endocrinol. 2014;75:109–11. https://doi.org/10.1016/j.ando.2014.03.004.
Sultan J, et al. Asphaltum improves the post-thaw quality and antioxidant status of Nili Ravi buffalo bull sperm. Biopreserv Biobank. 2021; https://doi.org/10.1089/bio.2020.0033.
Sun XL, et al. Bilateral is superior to unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a prospective randomized controlled study. Int Urol Nephrol. 2018;50:205–10. https://doi.org/10.1007/s11255-017-1749-x.
Suresh S, Prakash S. Effect of Mucuna pruriens (Linn.) on sexual behavior and sperm parameters in streptozotocin-induced diabetic male rat. J Sex Med. 2012;9:3066–78. https://doi.org/10.1111/j.1743-6109.2010.01831.x.
Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33:22–32. https://doi.org/10.1111/j.1365-2605.2008.00949.x.
Swaroop A, et al. A novel protodioscin-enriched fenugreek seed extract (Trigonella foenum-graecum, family Fabaceae) improves free testosterone level and sperm profile in healthy volunteers. Funct Foods Health Dis. 2017;7:235–45.
Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, Yumura Y. Oxidative stress and male infertility. Reprod Med Biol. 2021;20:41–52.
Teixeira MYP, de Araujo COD. Effect of Withania somnifera in the treatment of male infertility: a literature review. J Med Plant Res. 2019;13:473–9.
Thakur M, Bhargava S, Dixit VK. Effect of Asparagus racemosus on sexual dysfunction in hyperglycemic male rats. Pharm Biol. 2009;47:390–5. https://doi.org/10.1080/13880200902755234.
Thakur M, Thompson D, Connellan P, Deseo MA, Morris C, Dixit VK. Improvement of penile erection, sperm count and seminal fructose levels in vivo and nitric oxide release in vitro by Ayurvedic herbs. Andrologia. 2011;43:273–7. https://doi.org/10.1111/j.1439-0272.2010.01068.x.
Thundathil J, de Lamirande E, Gagnon C. Nitric oxide regulates the phosphorylation of the threonine-glutamine-tyrosine motif in proteins of human spermatozoa during capacitation. Biol Reprod. 2003;68:1291–8. https://doi.org/10.1095/biolreprod.102.008276.
Tremellen K. Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update. 2008;14:243–58. https://doi.org/10.1093/humupd/dmn004.
Tsai SC, Chiao YC, Lu CC, Wang PS. Stimulation of the secretion of luteinizing hormone by ginsenoside-Rb1 in male rats. Chin J Physiol. 2003;46:1–7.
Türk G, Sönmez M, Ceribaşi AO, Yüce A, Ateşşahin A. Attenuation of cyclosporine A-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int Immunopharmacol. 2010;10:177–82. https://doi.org/10.1016/j.intimp.2009.10.013.
Vaijnath YV, Manikrao YV. Role of garlic as a fertility enhancer–a review. Int J Res Ayurveda Med Sci. 2018;1:145–9.
Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2018;16:35–43. https://doi.org/10.1016/j.aju.2017.11.001.
Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–7.
Wang X, Chu S, Qian T, Chen J, Zhang J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. J Sex Med. 2010;7:743–50. https://doi.org/10.1111/j.1743-6109.2009.01482.x.
Wani JA, Achur RN, Nema RK. Phytochemical screening and aphrodisiac activity of Asparagus racemosus. Int J Pharm Sci Drug Res. 2011;3:112–5.
Wdowiak A, Raczkiewicz D, Stasiak M, Bojar I. Levels of FSH, LH and testosterone, and sperm DNA fragmentation. Neuro Endocrinol Lett. 2014;35:73–9.
Winters M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern Med Rev. 2006;11:269–77.
Yadav MK, Upadhyay P, Purohit S, Pandey BL, Shah H. Phytochemistry and pharmacological activity of Mucuna pruriens: a review. Int J Green Pharm. 2017;11:69–73.
Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar F. Nigella sativa L. (Black cumin): a promising natural remedy for wide range of illnesses. Evid Based Compl Alter Med. 2019:2019:1528635. https://doi.org/10.1155/2019/1528635.
Yüce A, Türk G, Çeribaşi S, Sönmez M, Çiftçi M, Güvenç M. Effects of cinnamon (Cinnamomum zeylanicum) bark oil on testicular antioxidant values, apoptotic germ cell and sperm quality. Andrologia. 2013;45:248–55. https://doi.org/10.1111/and.12000.
Zahedi A, Khaki A, Ahmadi AH, Rastgar H, Rezazadeh S. Zingiber officinale protective effects on gentamicin’s toxicity on sperm in rats. J Med Plant Res. 2010;9:93–8.
Zegers-Hochschild F, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32:1786–801.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Malik, J., Choudhary, S., Mandal, S.C., Sarup, P., Pahuja, S. (2022). Oxidative Stress and Male Infertility: Role of Herbal Drugs. In: Roychoudhury, S., Kesari, K.K. (eds) Oxidative Stress and Toxicity in Reproductive Biology and Medicine. Advances in Experimental Medicine and Biology, vol 1391. Springer, Cham. https://doi.org/10.1007/978-3-031-12966-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-12966-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12965-0
Online ISBN: 978-3-031-12966-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)