Abstract
Infertile men have higher levels of semen reactive oxygen species (ROS) than do fertile men. High levels of semen ROS can cause sperm dysfunction, sperm DNA damage, and reduced male reproductive potential. These observations suggest that oxidative stress (OS) is an important cause of male infertility. This has led clinicians to treat infertile men with the aim of reducing seminal OS (e.g., with antioxidant supplements). The purpose of this review is to discuss the role of ROS in male infertility and the rationale for antioxidant therapy in these men. To date, most clinical studies suggest that dietary and in vitro antioxidant supplements are beneficial in terms of improving sperm function. However, the exact mechanism of action of dietary antioxidants and the optimal dietary supplement has not yet been established.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
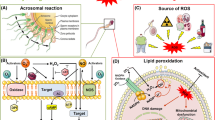
Reactive oxygen species (ROS) are ubiquitous in aerobic biologic systems and are formed as a byproduct of oxygen metabolism. Small amounts of semen ROS are necessary for the initiation of critical sperm functions, including capacitation and the acrosome reaction (de Lamirande and Gagnon 1993; Griveau et al. 1994). High ROS levels produce a state known as oxidative stress that can lead to biochemical or physiologic abnormalities with subsequent cell dysfunction or cell death (Aitken and Fisher 1994). Seminal oxidative stress (OS) is believed to be one of the main factors in the pathogenesis of sperm dysfunction and sperm DNA damage in male infertility (Chen et al. 2012; Aitken et al. 2010; Aitken and Roman 2008; Iwasaki and Gagnon 1992). Several intrinsic (testicular) and extrinsic factors (external stressors) can promote reactive oxygen species (ROS) generation in the testis and in the post-testicular (e.g., epididymal) environment, resulting in defective spermatogenesis and sperm dysfunction (see Fig. 124.1) (Aitken and Roman 2008). Indeed, it is estimated that 25 % of infertile men possess high levels of semen ROS, whereas fertile men do not have high levels of semen ROS (Chen et al. 2012; Iwasaki and Gagnon 1992; Zini and Sigman 2009; Agarwal et al. 2004). The detrimental effects of reactive oxygen species (ROS) on spermatozoa were suggested more than 60 years ago with the demonstration that exposing sperm to oxygen results in sperm toxicity (MacLeod 1943). Later studies confirmed that human spermatozoa and semen leukocytes have the capacity to generate ROS (Iwasaki and Gagnon 1992; Alvarez et al. 1987; Aitken et al. 1989; Zini et al. 1993).
Several intrinsic (developmental, genetics, age) and extrinsic factors (e.g., heat, hormonal imbalance, toxins) can promote reactive oxygen species (ROS) generation in the testis and in the post-testicular (e.g., epididymal) environment. In the testis, ROS can interfere with normal spermatogenesis resulting in the generation of abnormal or immature sperm. In the post-testicular environment, ROS produced from various sources (epithelium, leukocytes, immature spermatozoa) can cause defective sperm function and DNA damage. Antioxidants may act at several sites to neutralize some of these ROS-mediated effects. AOX antioxidants, ROS reactive oxygen species, WBC white blood cell
ROS such as the superoxide anion (O2 •−) are generated as a byproduct of aerobic metabolism (Grisham and McCord 1986). The superoxide anion can then either spontaneously dismutate or be converted to H2O2 by the enzyme superoxide dismutase (SOD). The enzymes catalase or glutathione peroxidases will then convert H2O2 to water and oxygen, eradicating the ROS. Recently, investigators in our laboratory have detected active peroxiredoxins (a new family of antioxidant enzymes) in human semen and have shown that the low levels of these antioxidants in spermatozoa of infertile men is associated with impaired sperm motility, lipid peroxidation, and sperm DNA damage (O’Flaherty and de Souza 2011; Gong et al. 2012). These studies have also shown that unlike what is observed in fertile men, the peroxiredoxins in spermatozoa of infertile men are mostly oxidized, suggesting that the redox state of peroxiredoxins is important in the protection of human spermatozoa against oxidative stress (Alvarez and Storey 1983). Alternatively, both superoxide anion and hydrogen peroxide can be neutralized by nonenzymatic antioxidants such as albumin, glutathione, hypotaurine, taurine, as well as vitamins C and E (Alvarez and Storey 1983).
Seminal OS results from an imbalance between ROS production and ROS scavenging by seminal antioxidants. Although a controlled production of these ROS is required for sperm physiology (sperm hyperactivation, capacitation, and acrosome reaction) and for natural fertilization (Aitken et al. 1995a; Griveau and Le Lannou 1997; de Lamirande et al. 1997), the excessive production of ROS by immature germ cells and leukocytes causes sperm dysfunction (lipid peroxidation, loss of motility, and sperm DNA damage) (Aitken and Clarkson 1987; de Lamirande and Gagnon 1992a; de Lamirande and Gagnon 1992b). Spermatozoa are particularly susceptible to oxidative injury due to the abundance of plasma membrane polyunsaturated fatty acids (de Lamirande et al. 1997; Aitken and Clarkson 1987; de Lamirande and Gagnon 1992a; de Lamirande and Gagnon 1992b; Zini et al. 2000). These unsaturated fatty acids provide fluidity that is necessary for membrane fusion events (e.g., the acrosome reaction and sperm-egg interaction) and for sperm motility. However, the unsaturated nature of these molecules predisposes them to free radical attack and ongoing lipid peroxidation throughout the sperm plasma membrane. Once lipid peroxidation has been initiated, accumulation of lipid peroxides occurs on the sperm surface with ensuing sperm dysfunction (loss of sperm motility, sperm DNA damage) and sperm death (Alvarez et al. 1987; Aitken et al. 1989; Twigg et al. 1998; Aitken et al. 1998 new Aitken). Recently, investigators have shown that the products of lipid peroxidation can themselves stimulate free radical generation by the sperm mitochondria, thus creating an ongoing cycle of ROS production and cellular damage (Aitken et al. 2012).
Seminal Antioxidant Capacity and Sperm Dysfunction
Both seminal plasma and spermatozoa contain antioxidants designed to protect spermatozoa from OS, especially, at the post-testicular level (Agarwal et al. 2004; Tremellen 2008; Saleh and Agarwal 2002). Seminal plasma is a rich source of high molecular weight enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidases, and peroxiredoxins), and a deficiency in these enzymes has been reported to cause sperm DNA damage and male infertility (O’Flaherty and de Souza 2011; Gong et al. 2012; de Lamirande et al. 1997; Chabory et al. 2009; Weir and Robaire 2007; Jelezarsky et al. 2008). Seminal fluid also contains nonenzymatic antioxidants (ascorbic acid, α-tocopherol, pyruvate, glutathione, L-carnitine, taurine, hypotaurine) that constitute the bulk of seminal antioxidant capacity (Zini et al. 1993; Smith et al. 1996; Kobayashi et al. 1991; Zini and Schlegel 1996). In addition, urate, pyruvate, albumin, beta-carotenes, and ubiquinol have been detected in seminal plasma, and these are believed to protect spermatozoa from oxidative injury (Aitken and Fisher 1994; Thiele et al. 1995; Kalthur et al. 2008; Bilodeau et al. 2002; Tauber et al. 1975).
A number of investigators have shown that seminal antioxidant capacity is suppressed in infertile men with high ROS levels compared to men with normal levels of ROS (Smith et al. 1996; Lewis et al. 1997; Sanocka et al. 1996). However, it is unclear whether reduced semen antioxidant capacity necessarily causes sperm dysfunction (including sperm DNA damage) (Aitken and Roman 2008; Appasamy et al. 2007; Verit et al. 2006). Indeed, there is some controversy as to whether the high ROS levels detected in the semen of infertile men are due to increased ROS production, decreased ROS scavenging capacity, or both (Zini et al. 1993; Lewis et al. 1995). If the high semen ROS levels are due (at least in part) to a decreased ROS scavenging capacity of semen, it would support the use of dietary antioxidant supplementation (Zini et al. 1993; Lewis et al. 1995).
Although a relationship between male infertility and systemic antioxidant deficiency has not been reported to date, it is possible that a subset of infertile men may be at risk for antioxidant deficiency, particularly vitamin C deficiency (Hampl et al. 2004). We suspect that infertile men with specific lifestyles (e.g., smoking, increased alcohol intake, dieting) may be at high risk for antioxidant or vitamin deficiency, but this remains to be tested (Jacob 1990; Ryle and Thomson 1984). Investigators have evaluated dietary antioxidant intake (vitamins C and E or ß-carotene) and sperm DNA damage in a cohort of fertile men but failed to identify any relationships between these parameters (Silver et al. 2005).
Detection of Seminal ROS
Semen ROS are generally measured by detection of chemiluminescence. Briefly, this is done by incubating fresh semen or sperm suspensions with a redox-sensitive, light-emitting probe (e.g., luminol) and by measuring the light emission over time with a light meter (luminometer). Sperm-specific (e.g., PMA) or leukocyte-specific (e.g., FMLP) stimulants can enhance the chemiluminescent signal (Krausz et al. 1994). Sperm ROS can also be measured by using cellular probes coupled with flow cytometry (Marchetti et al. 2002).
Several independent researchers have assessed the clinical value of semen ROS determination. Aitken et al. observed an inverse relationship between semen ROS levels and spontaneous pregnancy outcome in a cohort of 139 untreated infertile couples (Aitken et al. 1991). However, no other studies have supported these findings.
Semen ROS determination possesses some inherent weaknesses as a clinical test. Firstly, there is no established cutoff or threshold semen ROS level or value that can be used to predict reproductive outcomes. Secondly, semen ROS determination requires the use of fresh semen samples, making it impossible to send samples to a distant reference laboratory for ROS determination or assessment of interlaboratory variability. Finally, the protocol for ROS determination varies from laboratory to laboratory.
Several studies have evaluated the relationship between semen ROS and fertilization at IVF (Krausz et al. 1994; Marchetti et al. 2002; Yeung et al. 1996; Sukcharoen et al. 1996; Moilanen et al. 1998; Zorn et al. 2003; Saleh et al. 2003; Hammadeh et al. 2006). The value of semen ROS determination in the context of IVF is inconclusive (only half of the studies support its application). Some of these studies have reported a significant inverse relationship between sperm ROS levels and fertilization rate at conventional IVF. However, an equal number of studies have failed to demonstrate a significant relationship between ROS levels and fertilization rates (Table 124.1). Therefore, the clinical value of semen ROS determination in predicting IVF outcome remains unproven.
The available studies fail to demonstrate that semen ROS determination is of any significant clinical value. Semen ROS levels may be useful in predicting spontaneous pregnancy outcome, but there is only one supporting study in this respect. Although additional studies would help define the clinical utility of semen ROS testing, it is unlikely that such studies will be conducted because the test is labor intensive and requires testing fresh semen. Other surrogate tests of semen oxidative stress (e.g., 8-hydroxy-2-deoxyguanosine – a measure of oxidative DNA damage) may be better candidate tests of semen OS as they can be automated (e.g., flow cytometry) and can be applied to cryopreserved samples.
Treatment of Oxidative Stress
Treatment of oxidative stress should first involve strategies to reduce or eliminate stress-provoking conditions including smoking, varicocele, genital infection, gonadotoxins, and hyperthermia. The rationale for treating infertile men with oral antioxidants is based on the premise that seminal oxidative stress (common in infertile men) is due in part to a deficiency in seminal antioxidants. The practice of prescribing oral antioxidant is supported by the lack of serious side effects related to antioxidant therapy, although few studies have carefully evaluated the risk of overtreatment with antioxidants (Henkel 2011). Ideally, an oral antioxidant should reach high concentrations in the reproductive tract and replete a deficiency of vital elements important for spermatogenesis. Additionally, the antioxidant supplement should augment the scavenging capacity of seminal plasma and reduce the levels of semen ROS (Chen et al. 2012). However, the levels of semen ROS should not be entirely suppressed (by oral antioxidants) as this may impair normal sperm functions (e.g., sperm capacitation and hyperactivation) that normally require low levels of ROS (Aitken et al. 1995a; de Lamirande et al. 1997).
To date, over 100 clinical and experimental studies have examined the effect of antioxidants on sperm parameters. Despite this large body of literature, it is not possible to establish firm conclusions regarding the optimal antioxidant treatment for infertile men because the published studies report on different types and doses of antioxidants, the studies are small, the end points vary, and few of the studies are placebo controlled (Agarwal et al. 2004; Tremellen 2008). Moreover, the presumed mechanism of action of antioxidants in the treatment of male infertility (i.e., suppression of seminal OS) has not been confirmed because few studies have evaluated seminal OS and/or antioxidant capacity before and after treatment (Comhaire et al. 2005).
Effect of Oral (Dietary) Antioxidants on Sperm Dysfunction and DNA Damage
While there is a good body of literature on the effect of oral antioxidants on sperm parameters (including sperm DNA integrity), no study has established the optimal dose, duration of treatment, or subpopulation of infertile patients who might benefit most from antioxidant therapy (isolated asthenozoospermia, oligoasthenoteratozoospermia, sperm DNA damage, or all) (Lombardo et al. 2011). Many small, uncontrolled studies have shown a significant improvement in semen parameters following different doses and types of antioxidant therapy (Agarwal et al. 2004; Tremellen 2008). The most commonly studied oral antioxidants (or antioxidant enzyme cofactors) include vitamin C, vitamin E, selenium, zinc, glutathione, L-carnitine, and N-acetylcysteine.
The randomized controlled trials (RCTs) on antioxidant therapy for male infertility generally demonstrate that treatment with antioxidants has a beneficial effect (in terms of semen parameter improvements), whereas no significant effect is seen in the placebo group (see Tables 124.2 and 124.3) (Comhaire et al. 2005; Suleiman et al. 1996; Lenzi et al. 1993; Keskes-Ammar et al. 2003; Balercia et al. 2005; Scott et al. 1998; Cavallini et al. 2004; Ciftci et al. 2009; Dawson et al. 1992; Ebisch et al. 2006; Hawkes et al. 2009; Lenzi et al. 2003; Mahajan et al. 1982; Omu et al. 2008; Omu et al. 1998; Piomboni et al. 2008a; Galatioto et al. 2008; Safarinejad and Safarinejad 2009; Wong et al. 2002; Kessopoulou et al. 1995; Moilanen et al. 1993; Rolf et al. 1999; Greco et al. 2005a; Sigman et al. 2006; Paradiso Galatioto et al. 2008). The variable treatment outcomes in the different studies could be due to differences in antioxidant supplements (e.g., vitamins C and E, selenium, zinc, L-carnitine), dosages, duration of treatment, and patient population (Agarwal et al. 2004; Tremellen 2008). This topic has recently been evaluated in a systematic fashion and published in a Cochrane Review (Showell et al. 2011). The results of the systematic review indicate that oral antioxidant supplements (for male infertility) can help increase pregnancy rates.
One randomized controlled trial (RCT) evaluated the effects of vitamin C alone and reported a significant improvement in sperm parameters in the treatment arm only (Dawson et al. 1992). Six RCTs evaluated the effects of vitamin E alone or in combination with vitamin C or selenium. Two of these studies reported a significant improvement in sperm motility (Suleiman et al. 1996; Keskes-Ammar et al. 2003), and one reported a significant improvement in sperm DNA integrity (Greco et al. 2005a) in the treatment arm only. In contrast, three RCTs reported no significant improvement in sperm parameters after vitamin E ± C treatment (Kessopoulou et al. 1995; Moilanen et al. 1993; Rolf et al. 1999) although sperm-zona binding improved in one of these studies (Kessopoulou et al. 1995). Five RCTs evaluated the effects of zinc alone or in combination with folic acid, and all five reported a significant improvement in sperm parameters in the treatment arm only (Ebisch et al. 2006; Mahajan et al. 1982; Omu et al. 2008; Omu et al. 1998; Wong et al. 2002). Three RCTs evaluated the effects of selenium alone or in combination with N-acetylcysteine, and two of the three studies reported a significant improvement in sperm parameters in the treatment arm only (Scott et al. 1998; Hawkes et al. 2009; Safarinejad and Safarinejad 2009). Four RCTs evaluated the effects of L-carnitine alone or in combination with L-acetylcarnitine, and three of the four reported a significant improvement in sperm parameters in the treatment arm only (Balercia et al. 2005; Cavallini et al. 2004; Lenzi et al. 2003; Sigman et al. 2006). Three RCTs evaluated the effects of N-acetylcysteine alone or in combination with selenium, and all three reported a significant improvement in sperm parameters in the treatment arm only (Ciftci et al. 2009; Safarinejad and Safarinejad 2009; Paradiso Galatioto et al. 2008).
Several investigators have examined the effect of antioxidant therapy on sperm DNA integrity because sperm DNA damage may be caused, at least in part, by oxidative stress (Tremellen 2008; Zini and Schlegel 1996; Appasamy et al. 2007; Piomboni et al. 2008a; Gil-Villa et al. 2009; Greco et al. 2005b; Greco et al. 2005c; Kodama et al. 1997; Menezo et al. 2007; Tremellen et al. 2007; Tunc et al. 2009; Piomboni et al. 2008b). However, it is not possible to determine if sperm damage is due to increase seminal ROS production or deficient seminal antioxidant capacity unless one measures both ROS and total antioxidant capacity – TAC (Mahfouz et al. 2010). Nonetheless, sperm DNA damage is a more reliable outcome measure than sperm concentration or motility because measures of sperm DNA damage exhibit a lower degree of biological variability than conventional semen parameters (Zini et al. 2001; Evenson et al. 1991). Moreover, it is easier to implement testing of sperm DNA damage because the test can be applied to cryopreserved samples. Treatment with oral antioxidants has generally been associated with improvement in sperm DNA integrity and in some cases pregnancy rates after assisted reproduction, although most of these studies are small and few are randomized placebo-controlled trials (see Table 124.4) (Chen et al. 2012). To date, none of the studies on sperm DNA damage and oral antioxidants have estimated seminal oxidative stress, seminal vitamin levels, or used oxidative DNA damage (e.g., by estimation of 8-hydroxy-2′-deoxyguanosine [8OHdG]) as a selection criterion for monitoring the response to antioxidant treatment (Chen et al. 2012; Aitken et al. 2010; De Iuliis et al. 2007). As such, the precise mechanism of action of these antioxidant supplements on sperm DNA quality is unknown.
Effect of In Vitro ROS on Sperm Dysfunction and DNA Damage
The addition of ROS to sperm preparations (exogenous ROS) or the activation of intrinsic sperm ROS production (endogenous ROS) in vitro has been associated with the development of lipid peroxidation, sperm dysfunction, and sperm DNA damage (Alvarez et al. 1987; Aitken et al. 1989; Twigg et al. 1998; Aitken et al. 1998; Aitken and Baker 1995; Aitken et al. 1995b; Anderson et al. 2003). This is particularly important in the context of in vitro fertilization where seminal plasma is removed during semen processing and the toxic oxygen metabolites (generated by immature spermatozoa and leukocytes) are able to attack spermatozoa without being protected by seminal plasma antioxidants. In addition, the detrimental effect of oxidative stress on sperm functional competence can be exaggerated by the in vitro sperm processing techniques (centrifugation, prolonged incubation) that usually precede assisted reproductive techniques (Chen et al. 2012; Twigg et al. 1998; Aitken and Baker 1995; Aitken and Clarkson 1988).
Role of In Vitro Antioxidants in Protecting Spermatozoa from Exogenous and Endogenous ROS
Attenuating the effects of exogenous ROS is clinically relevant as many of the semen samples from infertile men contain abnormal spermatozoa and leukocytes, and these cells have the potential to generate exogenous ROS (Aitken et al. 1995b). Antioxidants such as vitamin E, catalase, and glutathione have been shown to protect sperm motility from the effects of exogenous ROS (see Table 124.5) (Griveau et al. 1994). In contrast, superoxide dismutase is less effective in preventing the loss of motility due to exogenous oxidants (Griveau et al. 1994). Altogether, these data suggest that hydrogen peroxide (H2O2) is the most sperm-toxic ROS. Antioxidants have also been shown to protect the sperm DNA from the effects of exogenous ROS (see Table 124.5) (Lopes et al. 1998; Potts et al. 2000; Russo et al. 2006; Sierens et al. 2002). This is highly relevant as sperm DNA damage may impact on reproductive outcomes after ARTs (Zini and Sigman 2009). Indeed, sperm DNA damage has been associated with reduced pregnancy rates with IUI and to a lesser extent with conventional IVF (Zini and Sigman 2009; Bungum et al. 2007; Collins et al. 2008).
Spermatozoa can be stimulated to generate ROS using a variety of agents (e.g., NADPH, estrogens), and this endogenous ROS production can potentially impair sperm function (Aitken et al. 1997). In contrast to the beneficial effect of antioxidants in protecting spermatozoa from exogenous ROS, antioxidants appear to be of limited value in protecting spermatozoa from endogenous ROS production (Twigg et al. 1998). Twigg et al. demonstrated that SOD, catalase, or both are ineffective, whereas albumin is effective in protecting spermatozoa from loss of motility due to endogenous ROS generation (Twigg et al. 1998). These studies stress the importance of using gentle semen processing protocols (e.g., low centrifugation force) so as to minimize the production and adverse impact of endogenous ROS.
Similarly, antioxidants appear to be of limited value in protecting the DNA of normal spermatozoa (with normal chromatin compaction) from endogenous ROS production (e.g., NADPH induced or centrifugation induced) (Twigg et al. 1998; Anderson et al. 2003; Cemeli et al. 2004; Dobrzynska et al. 2004). In samples with poor morphology and poor sperm chromatin compaction, antioxidants may protect the sperm DNA from endogenous ROS production, as these samples are more vulnerable to oxidative stress (Muratori et al. 2000; Said et al. 2005). In support of these clinical observations, experimental (animal) studies suggest that the spermatozoa of infertile men may be more susceptible to oxidative injury in vitro but benefit more so from antioxidants than the spermatozoa of fertile men (Libman et al. 2010).
Summary
Oxidative stress plays an important role in the pathophysiology of male infertility. High levels of semen ROS can cause sperm dysfunction and reduced male reproductive potential. Studies have shown that dietary antioxidants generally have a beneficial effect on sperm function and male fertility potential. However, the mechanism of action of these oral antioxidants as well as the optimal type and dosage of antioxidant are unknown. The study of in vitro antioxidants is highly relevant in the era of assisted reproduction because of the susceptibility of human spermatozoa to oxidative injury and the vulnerability of these cells during semen processing. Most studies have demonstrated a beneficial effect of in vitro antioxidant supplements in protecting spermatozoa from exogenous oxidants but not from endogenous ROS.
Competing Financial Interests
Dr. Armand Zini is a shareholder in YAD technologies Inc. (a nutraceutical supplement company).
References
Agarwal A, Nallella KP, Allamaneni SS, Said TM (2004) Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 8:616–627
Aitken RJ, Baker HW (1995) Seminal leukocytes: passengers, terrorists or good Samaritans? Hum Reprod 10:1736–1739
Aitken RJ, Clarkson JS (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81:459–469
Aitken RJ, Clarkson JS (1988) Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl 9:367–376
Aitken JR, Fisher H (1994) Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 16:259–267
Aitken RJ, Roman SD (2008) Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev 1:15–24
Aitken JR, Clarkson JS, Fishel S (1989) Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol Reprod 40:183–197
Aitken RJ, Irvine DS, Wu FC (1991) Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol 164(2):542–551
Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M (1995a) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108(Pt 5):2017–2025
Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HW, Irvine DS (1995b) Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod 10:2061–2071
Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, Lewis B et al (1997) Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev 47:468–482
Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS (1998) Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 59:1037–1046
Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI (2010) Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 25:2415–2426
Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA (2012) Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 287:33048–33060
Alvarez JG, Storey BT (1983) Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod 29:548–555
Alvarez JG, Touchstone JC, Blasco L et al (1987) Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: superoxide dismutase as a major enzyme protectant against oxygen toxicity. J Androl 8:338–348
Anderson D, Schmid TE, Baumgartner A, Cemeli-Carratala E, Brinkworth MH, Wood JM (2003) Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the comet assay). Mutat Res 544:173–178
Appasamy M, Muttukrishna S, Pizzey AR, Ozturk O, Groome NP, Serhal P et al (2007) Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online 14:159–165
Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M (2005) Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril 84:662–671
Bilodeau JF, Blanchette S, Cormier N, Sirard MA (2002) Reactive oxygen species-mediated loss of bovine sperm motility in egg yolk tris extender: protection by pyruvate, metal chelators and bovine liver or oviductal fluid catalase. Theriogenology 57:1105–1122
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J et al (2007) Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 22:174–179
Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G (2004) Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl 25:761–770; discussion 71–72
Cemeli E, Schmid TE, Anderson D (2004) Modulation by flavonoids of DNA damage induced by estrogen-like compounds. Environ Mol Mutagen 44:420–426
Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, Zevnik B et al (2009) Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 119:2074–2085
Chen H, Zhao HX, Huang XF, Chen GW, Yang ZX, Sun WJ, Tao MH, Yuan Y, Wu JQ, Sun F, Dai Q, Shi HJ (2012) Does high load of oxidants in human semen contribute to male factor infertility? Antioxid Redox Signal 16:754–759
Ciftci H, Verit A, Savas M, Yeni E, Erel O (2009) Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology 74:73–76
Collins JA, Barnhart KT, Schlegel PN (2008) Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 89:823–831
Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F (2005) Combined conventional/antioxidant “astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl 7:257–262
Dawson EB, Harris WA, Teter MC, Powell LC (1992) Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril 58:1034–1039
De Iuliis GN, Thomson L, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, Nixon B, Aitken RJ (2007) DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod 81:517–524
de Lamirande E, Gagnon C (1992a) Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl 13:379–386
de Lamirande E, Gagnon C (1992b) Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 13:368–378
de Lamirande E, Gagnon C (1993) A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl 16:21–25
de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C (1997) Reactive oxygen species and sperm physiology. Rev Reprod 2:48–54
Dobrzynska MM, Baumgartner A, Anderson D (2004) Antioxidants modulate thyroid hormone- and noradrenaline-induced DNA damage in human sperm. Mutagenesis 19:325–330
Ebisch IM, Pierik FH, Dej FH, Thomas CM, Steegers-Theunissen RP (2006) Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J Androl 29:339–345
Evenson DP, Jost LK, Baer RK, Turner TW, Schrader SM (1991) Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 5:115–125
Galatioto GP, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G et al (2008) May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol 26(1):97–102
Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A (2009) Role of male factor in early recurrent embryo loss: do antioxidants have any effect? Fertil Steril 92:565–571
Gong S, San Gabriel MC, Zini A, Chan P, O’Flaherty C (2012) Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J Androl 33:1342–1351
Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J (2005a) Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 26:349–353
Greco E, Romano S, Iacobelli M, Ferrero S, Baroni E, Minasi MG et al (2005b) ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod 20:2590–2594
Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S et al (2005c) Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 20:226–230
Grisham MB, McCord JM (1986) Chemistry and cytotoxicity of reactive oxygen metabolites. In: Taylor AE, Matalon S, Ward P (eds) Biology of oxygen radicals. American Physiological Society, Bethesda, pp 1–18
Griveau JF, Le Lannou D (1997) Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 20:61–69
Griveau JF, Renard P, Le Lannou D (1994) An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int J Androl 17:300–307
Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, Schmidt W (2006) Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online 13(5):696–706
Hampl JS, Taylor CA, Johnston CS (2004) Vitamin C deficiency and depletion in the united states: the third national health and nutrition examination survey, 1988 to 1994. Am J Public Health 94:870–875
Hawkes WC, Alkan Z, Wong K (2009) Selenium supplementation does not affect testicular selenium status or semen quality in north American men. J Androl 30:525–533
Henkel RR (2011) Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl 13:43–52
Iwasaki A, Gagnon C (1992) Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 57:409–416
Jacob RA (1990) Assessment of human vitamin C status. J Nutr 120(Suppl 11):1480–1485
Jelezarsky L, Vaisberg C, Chaushev T, Sapundjiev E (2008) Localization and characterization of glutathione peroxidase (GPx) in boar accessory sex glands, seminal plasma, and spermatozoa and activity of GPx in boar semen. Theriogenology 69:139–145
Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P (2008) Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril 89:1723–1727
Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S et al (2003) Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl 49:83–94
Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID et al (1995) A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril 64:825–831
Kobayashi T, Miyazaki T, Natori M, Nozawa S (1991) Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod 6:987–991
Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T (1997) Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 68:519–524
Krausz C, Mills C, Rogers S, Tan SL, Aitken RJ (1994) Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: relationships with motility and fertilization in vitro. Fertil Steril 62(3):599–605
Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F (1993) Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Hum Reprod 8:1657–1662
Lenzi A, Lombardo F, Sgro P, Salacone P, Caponecchia L, Dondero F et al (2003) Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril 79:292–300
Lewis SE, Boyle PM, McKinney KA, Young IS, Thompson W (1995) Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril 64:868–870
Lewis SE, Sterling ES, Young IS, Thompson W (1997) Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril 67:142–147
Libman J, Gabriel MS, Sairam MR, Zini A (2010) Catalase can protect spermatozoa of FSH receptor knock-out mice against oxidant-induced DNA damage in vitro. Int J Androl 33(6):818–822
Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, Lenzi A (2011) The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl 13:690–697
Lopes S, Jurisicova A, Sun JG, Casper RF (1998) Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13:896–900
MacLeod J (1943) The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol 138:512–518
Mahajan SK, Abbasi AA, Prasad AS, Rabbani P, Briggs WA, McDonald FD (1982) Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Ann Intern Med 97:357–361
Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, Agarwal A (2010) Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil Steril 94:2141–2146
Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P (2002) Study of mitochondrial membrane potential, reactive oxygen species. DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod 17:1257–1265
Menezo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P et al (2007) Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online 14:418–421
Moilanen J, Hovatta O, Lindroth L (1993) Vitamin E levels in seminal plasma can be elevated by oral administration of vitamin E in infertile men. Int J Androl 16:165–166
Moilanen JM, Carpen O, Hovatta O (1998) Flow cytometric light scattering analysis, acrosome reaction, reactive oxygen species production and leukocyte contamination of semen preparation in prediction of fertilization rate in vitro. Hum Reprod 13:2568–2574
Muratori M, Piomboni P, Baldi E, Filimberti E, Pecchioli P, Moretti E et al (2000) Functional and ultrastructural features of DNA-fragmented human sperm. J Androl 21:903–912
O’Flaherty C, de Souza AR (2011) Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod 84:238–247
Omu AE, Dashti H, Al-Othman S (1998) Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod Biol 79:179–184
Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC (2008) Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract 17:108–116
Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G et al (2008) May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol 26:97–102
Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V (2008) Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl 10:201–206
Potts RJ, Notarianni LJ, Jefferies TM (2000) Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res 447:249–256
Rolf C, Cooper TG, Yeung CH, Nieschlag E (1999) Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod 14:1028–1033
Russo A, Troncoso N, Sanchez F, Garbarino JA, Vanella A (2006) Propolis protects human spermatozoa from DNA damage caused by benzo[a]pyrene and exogenous reactive oxygen species. Life Sci 78:1401–1406
Ryle PR, Thomson AD (1984) Nutrition and vitamins in alcoholism. Contemp Issues Clin Biochem 1:188–224
Safarinejad MR, Safarinejad S (2009) Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol 181:741–751
Said TM, Agarwal A, Sharma RK, Thomas AJ Jr, Sikka SC (2005) Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril 83:95–103
Saleh RA, Agarwal A (2002) Oxidative stress and male infertility: from research bench to clinical practice. J Androl 23:737–752
Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A et al (2003) Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 79(3):79–87
Sanocka D, Miesel R, Jedrzejczak P, Kurpisz MK (1996) Oxidative stress and male infertility. J Androl 17:449–454
Scott R, MacPherson A, Yates RW, Hussain B, Dixon J (1998) The effect of oral selenium supplementation on human sperm motility. Br J Urol 82:76–80
Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ (2011) Antioxidants for male subfertility. Cochrane Database Syst Rev 1, CD007411
Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV (2002) In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen 22:227–234
Sigman M, Glass S, Campagnone J, Pryor JL (2006) Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril 85:1409–1414
Silver EW, Eskenazi B, Evenson DP, Block G, Young S, Wyrobek AJ (2005) Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J Androl 26:550–556
Smith R, Vantman D, Ponce J, Escobar J, Lissi E (1996) Total antioxidant capacity of human seminal plasma. Hum Reprod 11:1655–1660
Sukcharoen N, Keith J, Irvine DS, Aitken RJ (1996) Prediction of the in-vitro fertilization (IVF) potential of human spermatozoa using sperm function tests: the effect of the delay between testing and IVF. Hum Reprod 11(5):1030–1034
Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA (1996) Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 17:530–537
Tauber PF, Zaneveld LJ, Propping D, Schumacher GF (1975) Components of human split ejaculates. I. Spermatozoa, fructose, immunoglobulins, albumin, lactoferrin, transferrin and other plasma proteins. J Reprod Fertil 43:249–267
Thiele JJ, Friesleben HJ, Fuchs J, Ochsendorf FR (1995) Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen. Hum Reprod 10:110–115
Tremellen K (2008) Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update 14:243–258
Tremellen K, Miari G, Froiland D, Thompson J (2007) A randomised control trial examining the effect of an antioxidant (menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol 47:216–221
Tunc O, Thompson J, Tremellen K (2009) Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online 18:761–768
Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ (1998) Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 13:1429–1436
Verit FF, Verit A, Kocyigit A, Ciftci H, Celik H, Koksal M (2006) No increase in sperm DNA damage and seminal oxidative stress in patients with idiopathic infertility. Arch Gynecol Obstet 274:339–344
Weir CP, Robaire B (2007) Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the brown Norway rat. J Androl 28:229–240
Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP (2002) Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril 77:491–498
Yeung CH, De Geyter C, De Geyter M, Nieschlag E (1996) Production of reactive oxygen species by and hydrogen peroxide scavenging activity of spermatozoa in an IVF program. Production of reactive oxygen species by and hydrogen peroxide scavenging activity of spermatozoa in an IVF program. J Assist Reprod Genet 13(6):495–500
Zini A, Schlegel PN (1996) Catalase mRNA expression in the male rat reproductive tract. J Androl 17:473–480
Zini A, Sigman M (2009) Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 30:219–229
Zini A, de Lamirande E, Gagnon C (1993) Reactive oxygen species in the semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 16:183–188
Zini A, Garrels K, Phang D (2000) Antioxidant activity in the semen of fertile and infertile men. Urology 55:922–926
Zini A, Kamal K, Phang D, Willis J, Jarvi K (2001) Biologic variability of sperm DNA denaturation in infertile men. Urology 58:258–261
Zorn B, Vidmar G, Meden-Vrtovec H (2003) Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl 26(5):279–285
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Zini, A., Libman, J. (2014). Oxidative Stress and Male Infertility. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_180
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_180
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences